Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic hereditary kidney disease characterized by progressive enlargement of renal cysts. The incidence is 1-2‰ worldwide. Mutations in two genes (PKD1 and PKD2) cause ADPKD. Currently, there is no pharmaceutical treatment available for ADPKD patients in China. Summary: This review focused on advances in clinical manifestation, gene diagnosis, risk factors, and management of ADPKD in China. There is an age-dependent increase in total kidney volume (TKV) and decrease in renal function in Chinese ADPKD patients. ADPKD is more severe in males than in females. Great progress has been made in molecular diagnosis in the last two decades. Nephrologists found many novel PKD mutations in Chinese ADPKD patients early through polymerase chain reaction, and then through liquid chromatography in 2000s, and recently through next-generation sequencing. Major predictive factors for ADPKD progression are age, PKD genotype, sex, estimated glomerular filtration rate (eGFR), and TKV. With respect to the management of ADPKD, inhibitors targeting mTOR and cAMP are the focus of clinical trials. Triptolide has been used to treat ADPKD patients in clinical trials in China. Triptolide significantly protected eGFR of ADPKD patients compared with placebo.
Key Messages
ADPKD affects about 1.5 million people in China. An additional PKD gene besides PKD1 and PKD2 was not found in the Chinese. The prevalence of intracranial aneurysm in Chinese ADPKD patients was 12.4%. The predictive factors for eGFR decrease in Chinese ADPKD patients are TKV, proteinuria, history of hypertension, and age. The treatment strategies in clinical trials for ADPKD patients in China are similar to those in the West except for triptolide.
Facts from East and West
(1) ADPKD is diagnosed globally by ultrasound detection of kidney enlargement and presence of cysts. Recent analyses of variants of the PKD1 and PKD2 genes by next-generation sequencing in Chinese and Western ADPKD patients might lead to the development of reliable genetic tests. (2) Besides lifestyle changes (low-salt diet, sufficient fluid intake, and no smoking), blood pressure control is the primary nonspecific treatment recommended by Kidney Disease – Improving Global Outcomes (KDIGO) for ADPKD patients. How low the blood pressure target should be and what the means of achieving it are remain open questions depending on the severity of chronic kidney disease and the age of the patients. In a recent Chinese study, diagnostic needle aspiration and laparoscopic unroofing surgery successfully improved infection, pain, and hypertension. Peritoneal dialysis was found to be a feasible treatment for most Chinese ADPKD patients with end-stage renal disease. In most Western centers, patients without contraindication are selected for peritoneal dialysis. Kidney transplantation with concurrent bilateral nephrectomy was successful in relieving hypertension and infection in Chinese ADPKD patients. In Western countries, sequential surgical intervention with kidney transplantation after nephrectomy, or the other way round, is preferred in order to reduce risks. (3) The vasopressin 2 receptor antagonist tolvaptan was approved in Europe, Canada, Japan, and Korea to slow down progression of kidney disease in ADPKD patients. Tolvaptan is not yet approved in the USA or in China. mTOR pathway-targeting drugs are currently under evaluation: mTOR inhibitors could slow down the increase in total kidney volume in a cohort of Western and Japanese ADPKD patients. Western studies as well as an ongoing study in China failed to show benefit from rapamycin. A study performed in Italy indicates protective effects of the somatostatin analog octreotide in ADPKD patients. Western and Chinese studies revealed a potential beneficial effect of triptolide, the active substance of the traditional Chinese medicine Tripterygium wilfordii (Lei Gong Teng) to prevent worsening in ADPKD patients.
Key Words: Autosomal dominant polycystic kidney disease, Clinical characteristics, End-stage renal disease, Transplantation, Gene
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic hereditary kidney disease characterized by the progressive enlargement of renal cysts [1]. ADPKD affects about 1.5 million people in China [2], and the incidence of this disease is estimated to be from 1 to 2‰ worldwide. ADPKD is the fourth leading cause of renal replacement therapy, and accounts for 4.7% of the patients with end-stage renal disease (ESRD) according to registry data from Shanghai [2]. Moreover, ADPKD is a systemic life-threatening disease with several extrarenal manifestations including hepatic cysts, pancreatic cysts, intracranial aneurysms, colonic diverticulosis, and cardiac vascular defects.
Mutations in two genes (PKD1 and PKD2) cause ADPKD. The PKD1 gene mapped to chromosome16p13.3 accounts for 80-85% of cases and PKD2 gene mapped to 4q21-22 accounts for the remainder 15-20% [1]. The additional PKD3 gene was almost excluded by reanalysis of designated families [3]. Significant phenotypic variability among ADPKD patients exists because of different environmental factors and genetic backgrounds (modifier genes, variant, and mutation loci). However, ADPKD shares several common characteristics in cellular levels (e.g. abnormal cell proliferation, apoptosis, dedifferentiation, polarity disturbance, interstitial fibrosis, and enhanced transepithelial fluid secretion) [4]. Identification of the PKD1 and PKD2 genes in the 1990s were landmark events in ADPKD research field; however, there is still no pharmaceutical agents to halt ADPKD progression in China, although the vasopressin 2 (V2) receptor antagonist tolvaptan has been recently approved to treat ADPKD patients in Japan and Europe. This review focuses on advances in clinical manifestation, diagnosis, risk factors, and management of ADPKD in China.
History of Clinical ADPKD Study in China
Chinese nephrologists started ADPKD clinical research in the late 1970s. Early published studies mainly focused on clinical manifestations of ADPKD and outcomes of cyst decortication surgery. In the early 1980s, a case series study found that cyst decortication could relieve abdominal pain and improve renal function [5]. Liu et al. [6] reported clinical manifestations of 205 ADPKD patients of the Han ethnic group in 1995. Since the late 1990s, there were many studies of mutation analysis through polymerase chain reaction-related techniques and microsatellite linkage analysis in Chinese ADPKD families after the PKD1 and PKD2 genes were identified [7,8,9,10,11,12]. Ding et al. [12] first published a paper about three novel PKD1 mutations in the Chinese. Since 2010, there have been clinical studies conducted for Chinese ADPKD patients. Li et al. [13] published the first treatment research about peritoneal dialysis (PD) in ADPKD patients. Chen et al. [14] published the largest-scale Chinese cohort study (541 cases) about characteristics and predictors of ADPKD patients. Recently, Chen et al. [15] reported the effect of a triptolide-containing formulation in ADPKD patients with proteinuria. The aforementioned studies could be regarded as landmarks in the Chinese ADPKD study field, but there are still huge gaps in ADPKD research between Western countries and China. The fact is that many Chinese physicians have not paid enough attention to ADPKD, and very few hospitals in China focus on ADPKD research, which makes it hard to organize high-quality and large-scale clinical trials in China.
Molecular Diagnosis
The standard diagnosis of ADPKD is based on the family heredity history and age-related diagnostic criteria of cyst numbers by ultrasound. Advances in high-resolution ultrasound, CT and MRI could help monitor disease progression and exclude individuals at risk. Molecular diagnosis plays important roles in neonates of early-onset disease patients (to avoid recurrence in subsequent pregnancy), patients with negative family histories (de novo mutation about 7%), and assessments of potential kidney donors with equivocal or negative scans in clinical practice [16]. Nearly 90% of ADPKD mutations can be identified by a clear molecular diagnosis. However, molecular diagnosis has disadvantages such as technical difficulties and high costs.
Studies in molecular diagnosis have made a great progress in the last two decades. Relevant diagnostic studies about ADPKD are summarized in table 1[7,9,10,11,12,17,18,19,20,21,22,23,24,25,26]. Our center started to detect PKD gene mutations and set up the screening system in the late 1990s. Because the amplification of GC-replicated portion in the PKD1 gene is 98% parallel to PKD1 like pseudogenes [27], it took us a lot of time and numerous setbacks to modify and optimize the system. Then, PKD1 and PKD2 gene sequences were screened by PCR-SSCP (polymerase chain reaction single-strand conformation polymorphism) in 72 ADPKD patients from 24 pedigrees [26]. Fifteen novel mutations (11 at the PKD1 gene, 4 at the PKD2 gene) and 8 novel variants (7 at the PKD1 gene, 1 at the PKD2 gene) were found compared with healthy controls [26]. We did not find potential hot spots of PKD1 and PKD2 mutations, but some G-A or C-T mutations which resulted in hydrophobicity change in relative amino acids. After that, a new denaturing high-performance liquid chromatography (DHPLC) technique which increased the detection rate was applied to mutation analysis [9,11]. The total mutation detection rate increased to 64.5% in 94 patients by DHPLC, which included non-sense, reading frameshift, missense, and splicing variant. Individual detection rates of PKD1 mutations reached 56%, and PKD2 mutations reached 8.5% [9,11]. After Rossetti et al. [28] reported a next-generation sequencing (NGS) strategy for analyzing PKD gene in 2012, Qi et al. [22] first used NGS to analyze PKD genes in 6 Chinese families with ADPKD, which revolutionized the molecular diagnosis field for ADPKD study in China. True-positive variants of ADPKD confirmed by NGS were 50, 69.4 and 100% in the duplicated regions, whole coding region and unduplicated regions without false positives [22]. Screening accuracy was largely improved with reduced cost and turnaround time compared with the former microsatellite linkage analysis.
Table 1.
Novel mutations and polymorphisms of PKD1 and PKD2 in Chinese ADPKD patients
| First author [Ref.] | Year | Pedigrees, n | Patients, n | Method | PKD gene mutated | Mutations, n | Novel mutations, n |
|---|---|---|---|---|---|---|---|
| Ding [12] | 2002 | 60 | 80 | PCR-SSCP | 1 | 3 (2 frameshift mutations, 1 transition mutation) | 3 (12593delA and 12470InsA frameshift mutation in E 45, 11151C>T transition in E 37) |
| Zhang [11] | 2004 | 94 | 94 | PCR, DHPLC | 2 | 8 (2 non-sense mutations, 2 deletion mutations, 1 insertion mutation and 3 missense mutations) | 6 (636-637 ins T in E 2, 2348-2351 del AGAA in E 12, 2401 del A in E 13, 568G>A in E 1, 964C>T in E 4, 1168G>A in E 5) |
| Zhang [26] | 2005 | 24 | 72 | PCR-SSCP | 1, 2 | 17 (12 in PKD1 and 5 in PKD2) | 15 (PKD1: 23692C>T E10, 28042G>A E15, 28095G>A E15, 32818Ins5 E18, 43661G>A E31, 47629C>T E36, 47640G>A E36, 47985G>A E37, 4976del3 E41, 50905C>T E44, 38791C>T IVS24; PKD2: 568G>A E1C, 964C>T E4, 1249C>T E5, 2401delA E13) |
| Zhang [9] | 2006 | 19 | 67 | PCR, DHPLC | 1 | 14 (10 missense, 1 insertion, 1 deletion and 2 non-sense mutations) | 2 (nt32819G>A, nt37137T>C) |
| Gao [7] | 2006 | 21 | 25 | PCR | 1 | 2 | 2 (non-sense mutation C12217T, frameshift 12431delCT) |
| Li [10] | 2007 | 2 | 2 | PCR, DHPLC | 1 | 2 (a non-sense mutation, a missense mutation) | 1 (non-sense mutation C11901A in E 42) |
| Li [25] | 2011 | 1 | 14 | PCR, DHPLC | 1 | 1 (1 insertion) | c.3623-3624insGTGT in E15 |
| Yu [24] | 2011 | 65 | 121 | PCR, DHPLC | 1, 2 | 29 (26 in PKD1 and 3 in PKD2) | 20 (19 in PKD1, 1 in PKD2) |
| Yu [23] | 2011 | 2 | 9 | PCR, DHPLC | 1, 2 | 5 (3 in PKD1 and 2 in PKD2) | 2 (PKD1: c.5014_5015delAG, PKD2: c.2020 1_2020delAG) |
| Qi [22] | 2012 | 2 | 8 | NGS | 1, 2 | 6 (5 in PKD1 and 1 in PKD2) | n.a. |
| Liu [21] | 2014 | 10 | 10 | Touchdown PCR | 1 | 7 | 4 (2 truncated frameshift mutations c.4839het_dupT, c.11329het_dupA, and 2 missense mutations: c.10838T>C, c.7184A>G) |
| Wang [20] | 2014 | 1 | 1 | PCR, NGS | 1 | 1 | 1 (frameshift mutation, c. 12605_12632del28) |
| Yang [19] | 2014 | 5 | n.a. | NGS | 1, 2 | 6 (2 missense mutations, 2 non-sense and 2 insertion/deletion mutations) | 5 (p.G1319R, p.Y3781*, p.W4122*, p. Val700Glyfs*14, and p.Leu3656Trpfs*28) |
| Yu [18] | 2014 | n.a. | n.a. | PCR | 1 | 2 | 2 (PKD1:c.12444G>A and PKD1:c.12444 + 1G>A) |
| Liu [17] | 2015 | 1 | 32 | PCR, ligation-dependent probe amplification | 1, 2 | 32 (10 non-sense, 17 frameshift, 4 splicing and 1 in-frame mutation) | 1 (PKD2: c.595_595 + 14delGGTAAGAGCGCGCGA) |
E = Exon; n.a. = not available.
These studies, which reported screened mutations in the PKD1 and PKD2 genes in Chinese patients, provided benchmarks for comparisons between Han ethnic and Caucasian patients. In the future, we will need more widespread adoption of genetic tests for ADPKD patients to prevent transmission of pathologic mutations through generations. Recently, 1 healthy child of an ADPKD couple by preimplantation genetic diagnosis was born in our hospital, which is the first successful birth in China.
Clinical Characteristics and Disease Progress
The clinical manifestation of patients with ADPKD varies in different ethnicities. There have been several prospective cohorts aimed to demonstrate long-term clinical characteristics and predictive factors for progress of ADPKD, including the CRISP cohort [29,30] and the SUISSE ADPKD cohort [31] in Europe.
In 2014, our center performed a prospective large-scale cohort study of 541 Chinese ADPKD patients with a mean follow-up time of 14.3 months. The mean age was 39.5 years [14]. Three fourths of the patients had a family history of ADPKD. Nearly 75% of the patients had polycystic liver disease; 37% of the patients had chronic pain, and 23% had an episode of gross hematuria [14]. Liu et al. [6] reported a cohort study in 205 ADPKD patients with 76.3% of pain, 47.5% of hematuria, 25.7% of urinary system infection, and 19.7% of stone or calcification. Nearly two thirds of the patients had hypertension and were taking one antihypertensive drug on average (range 0-5). About one half of the patients took angiotensin receptor blockers or angiotensin converting enzyme inhibitors, and about 25% of them took calcium channel blockers [14]. Female patients with ADPKD had a significant lower risk of obesity, hematuria, and hypertension compared with male patients, but more often had pain [14].
Laboratory and Imaging Features
In Chinese ADPKD patients, similar results of estimated glomerular filtration rate (eGFR), total kidney volume (TKV), and yearly changes in respective age ranges were found compared with the CRISP and the SUISSE cohorts [30,31]. In adult patients, there was a gradual positive association between serum markers (creatinine, blood urea nitrogen, cystatin C, β2-microglobulin, protein/creatinine ratio, and uric acid) and age [14]. eGFR was negatively correlated with increasing age, which was especially obvious in older patients with a yearly decline of 3.5 ml/min/1.73 m2[14]. The distribution and changes of eGFR over time in ADPKD patients are depicted in figure 1[14]. Hemoglobin level and blood platelet count decreased obviously in older patients. Blood platelet count was negatively associated with age and TKV, and was positively associated with eGFR. In the study by Chapman et al. [30], there was also a decreased level of thrombocyte count in ADPKD patients compared with healthy controls, indicating that enhanced platelet consumption may exist in ADPKD patients.
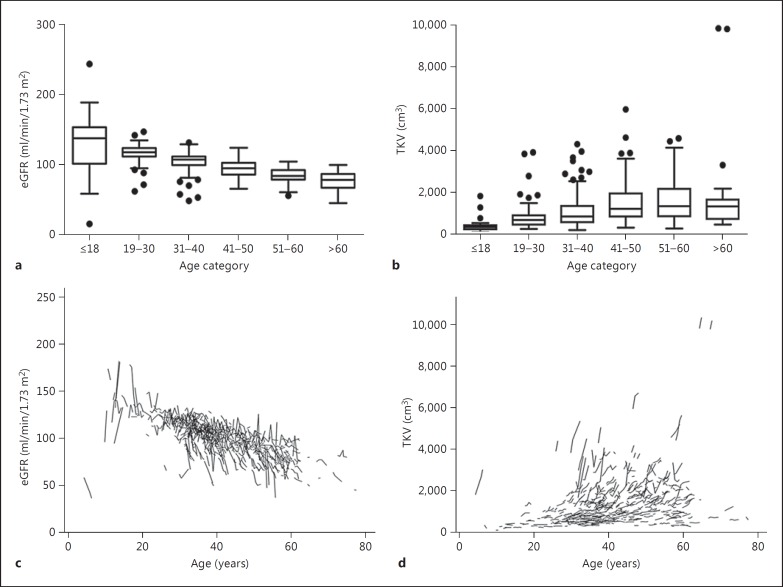
Fig. 1.
eGFR and TKV in different age categories of ADPKD patients. a EGFR for age categories ≤18 years (n = 20), 19-30 years (n = 74), 31-40 years (n = 171), 41-50 years (n = 128), 51-60 years (n = 90), and >60 years (n = 19). b TKV for age categories ≤18 years (n = 23), 19-30 years (n = 81), 31-40 years (n = 180), 41-50 years (n = 134), 51-60 years (n = 95), and >60 years (n = 19). Boxes show the median and the 25th and 75th percentile. Whiskers extend to the farthest points that are not outliers (i.e. that are within 3/2 times the interquartile range), and dots indicate outliers. Spaghetti plots for the course of eGFR (c) and TKV (d) over the entire observation time in individual patients [14].
The average TKV obtained by abdominal MRI scan was 1,265 ± 1,002 cm3 at baseline, and the total cyst volume was 869 ± 929 cm3. The increase rate of TKV was 4.6 ± 10.2% per year. Likewise, the baseline TKV was approximately 1,000 cm3, and the yearly increase of TKV was about 5% in both the CRISP and SUISSE studies. Consistently, both the TKV and yearly TKV growth rates were lower in Chinese female patients than in male patients. Figure 1 shows the distribution and changes of TKV [14]. Baseline TKV was negatively correlated with eGFR (r = −0.596, p < 0.001) [14]. However, poor association was found between TKV changes and yearly eGFR decline.
Intracranial Aneurysm and Carotid Change
The prevalence of intracranial aneurysm in Chinese ADPKD patients was 12.4% (95% CI, 8.95-15.82%), which was reported by Jiang et al. [32] in 355 patients. The prevalence was positively associated with age, and reached 23.3% in the 60- to 69-year-old group. A positive family history of aneurysm or hemorrhagic stroke was the risk factor for aneurysm in ADPKD patients (relative risk = 1.968). The mean diameter of the aneurysm was about 3.9 mm by magnetic resonance angiography. The internal carotid artery was the most frequently affected site of aneurysm [32]. Our center performed a follow-up study of intracranial aneurysms in 40 ADPKD patients for 36 months [33]. Fifty aneurysms were detected. The largest diameter during follow-up was about 9 mm, and the average diameter was 3.6 ± 2.3 mm. Aneurysms enlarged by more than 20% in only 4 patients. No aneurysm ruptured. The chances of rupture and enlargement of intracranial aneurysm in Chinese ADPKD patients were nearly the same as in the general population [33].
In addition, our center performed a study in 60 early ADPKD patients to study carotid vascular wall remodeling using ultrasonography [34]. We found that both carotid intima media thickness and percentage of fibromatosis were the highest in hypertensive ADPKD patients, intermediate in normotensive ADPKD patients, and the lowest in healthy controls. Carotid remodeling happened early in Chinese ADPKD patients, and was more severe in patients with hypertension.
Predictive Factors and Outcome
Although there are thousands of microcysts in the kidneys, ADPKD develops chronically with overall normal renal function because of compensatory hyperfiltration of nephrons. Progressive deterioration (e.g. fibrotic and inflammatory changes) usually happens in the late stages [29]. Major predictive factors for ADPKD progression are age, genotype, sex, eGFR, and TKV [35]. Several cohorts have shown PKD genotypes could predict the age at onset of ESRD [36]. Patients with PKD1 truncating mutations had the worst prognosis, PKD1 nontruncating mutations had the intermediate outcome, and PKD2 mutations had the best prognosis [37]. Environmental factors are also associated with disease progression, but still need to be validated in large cohort studies.
Clinical characteristics of ADPKD patients are important prognostic factors. TKV is an accurate and early measure of cystic volume and trajectory of growth rate in patients with well-preserved renal function (>60 ml/min/1.73 m2) [29]. The baseline of height-adjusted TKV >600 ml/m could predict progression to stage 3 chronic kidney disease within 8 years with high sensitivity and specificity [30]. Therefore, the annual change rate of TKV is a surrogate endpoint for eGFR decline in several studies. In the study by Chen et al. [14], important predictive factors for eGFR decrease in ADPKD patients were log10 TKV, log10 protein/creatinine at baseline, age, blood platelet count, and history of hypertension. For prediction of TKV growth, lower blood platelet count, male sex, and high log10 protein/creatinine level were predictive factors for yearly TKV increase [14].
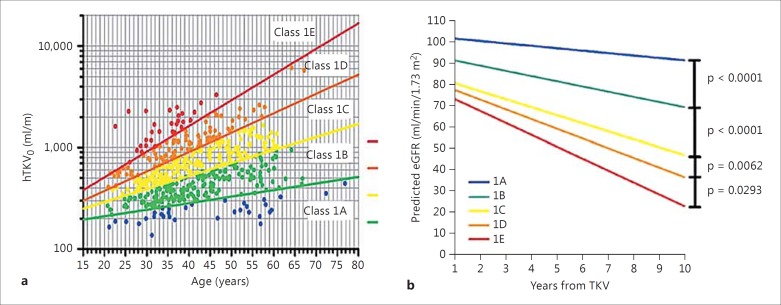
Recently, Irazabal et al. [38] published a model about imaging classification of disease progression in 590 ADPKD patients from the Mayo Clinic Translational PKD Center (MTPC). The model based on height-adjusted TKV ranges for age from 1A to 1E in the increasing order, and predictive eGFR values were significantly distinguished among subclasses. We used ADPKD patients (n = 541) in our center to validate this model. The outcomes of Chinese ADPKD patients were in accord with the MTPC predictive model (fig. 2).
Fig. 2.
Age at height-adjusted TKV₀ (HtTKV₀) and classification by HtTKV₀ predicted the change in eGFR over years in Chinese ADPKD patients. a Subclassification of ADPKD patients at baseline based on HtTKV limits for their age. Limits are defined based on estimated kidney growth rates of 1.5, 3.0, 4.5, and 6.0% [38]. b Slopes of predictive eGFR for Chinese ADPKD patients. Comparative p values between intervals are listed.
Management of ADPKD
Cystic cells in ADPKD are characterized by aberrant changes in apoptosis, proliferation, fluid secretion, cell migration, polarity, matrix abnormality, and primary cilium formation [39]. There are many known abnormal signal pathways which affect cyst growth, especially for mTOR and cAMP pathways [40]. The two pathways have been extensively studied in animal models and in clinics for ADPKD.
Inhibition of the mTOR pathway by everolimus or rapamycin successfully suppressed cyst growth in PKD animal models. Everolimus was found to decrease TKV in patients, but failed to improve eGFR of patients with late ADPKD [41]. In patients with preserved eGFR, rapamycin had no significant effects on both TKV and eGFR [42,43]. We also performed a prospective controlled study in Chinese ADPKD patients with rapamycin or placebo from 2012. The ongoing study shows that the TKV and eGFR of ADPKD patients were not significantly affected by rapamycin.
ADPKD kidneys have significant increased levels of cAMP. At the collecting duct, vasopressin activates V2 receptors to produce cAMP, and V2R antagonists showed beneficial effects in PKD animals. The TEMPO 3/4 trial compared tolvaptan (a V2 receptor inhibitor) with placebo in ADPKD patients with preserved eGFR [44]. Tolvaptan could decrease TKV and slowed the rate of eGFR decline. Tolvaptan may increase the 2.6 years' life expectancy and delay the onset of ESRD by 6.5 years [45]. However, tolvaptan is not yet approved for the management of ADPKD in China.
PC2 works with PC1 in a complex to mediate increases in cytosolic Ca2+ for signal transduction. In ADPKD, the dysfunction of PC1 or PC2 resulted in exuberant Ca2+-mediated cell proliferation [46]. Triptolide is an active diterpene isolated from the traditional Chinese medicine called Tripterygium wilfordii or Lei Gong Teng. Triptolide could induce Ca2+ release through a PC2- dependent pathway from endoplasmic reticulum in ADPKD [46]. We performed a pilot trial using triptolide-containing formulation to treat 9 ADPKD patients with proteinuria (mean urinary protein excretion = 2.6 ± 1.4 g/day) [15]. After 6 months of treatment with triptolide (1 mg/kg/day), the 24-hour urine protein excretion decreased to 0.7 ± 0.4 g/day. During a follow-up of 6 months, the proteinuria of 8 patients remained stable, while 1 patient relapsed. The eGFR and TKV all remained stable during the treatment, while protective effects were lost after the withdrawal of triptolide [15]. Reversible gonad inhibition was the main side effect in this study. After this study, we performed a randomized controlled trial to test the effects and safety of triptolide compared with placebo in 20 ADPKD patients without overt proteinuria. At 6 months, the eGFR of the triptolide group obviously increased from 77.5 ± 29.1 to 79.2 ± 28.9 ml/min/1.73 m2, while the eGFR of the placebo group decreased from 67.4 ± 21.9 to 63.8 ± 21.4 ml/min/1.73 m2. The rate of eGFR decline was significantly lower in the triptolide group compared with the placebo group (2.5 ± 9.1% vs. −8.6 ± 14.0%, p = 0.046). There was no significant difference in TKV increase between these two groups.
Nonspecific management to slow ADPKD progression is another focus of Chinese studies. Our center has gathered more than 200 ADPKD pedigrees including 560 patients and provided them with medical, lifestyle, and genetic counseling. For patients with the diagnosis of ADPKD, their disease progression was regularly monitored by ultrasound or MRI. Complications such as infection, hypertension, and pain were controlled by relevant prescribed medicine [47]. Sometimes, surgeries and interventional therapies were included. Diagnostic needle aspiration was used for cyst infection. Laparoscopic unroofing or nephrectomy was performed in patients with recurrent hematuria, intractable pain, and severe infection. We have performed 276 laparoscopic unroofing surgeries that resulted in an obvious improvement of pain, renal function, and hypertension. The therapeutic effects on pain and hypertension lasted for more than 1 year.
Renal replacement therapy is necessary for patients who have progressed to ESRD. Most of the Chinese patients choose hemodialysis other than PD, because of excessive abdominal distension and a limited effective peritoneal area in ADPKD. However, we performed a retrospective cohort study about PD in 42 ADPKD patients compared with 84 nondiabetic patients [13]. There were no significant differences in the 5-year survival rate or risk of peritonitis between these two groups. PD was found to be a feasible treatment for most ADPKD patients with ESRD, although ADPKD patients had a higher risk of abdominal wall hernia. Xie et al. [48] obtained similar results to ours in 30 ADPKD patients.
Renal transplantation is another choice for ADPKD patients with ESRD, and there is a good graft survival rate in China. Our center carried out a study comparing 46 ADPKD transplantation patients with 46 patients who underwent transplantation due to nondiabetic nephropathy [49]. There were no significant differences in overall graft and patient survival rates between ADPKD and control group. Female graft patients always had better outcomes than males. Lung and urinary tract infections were the main adverse effects after transplantation. Song et al. [50] found that concurrent bilateral nephrectomies performed during renal transplantation in ADPKD patients could reduce operative procedures, relieve persisting arterial hypertension, and result in a lower risk of urinary tract infection after transplantation.
Conclusion
ADPKD remains a great challenge for nephrologists with no effective treatment in China. Although ADPKD studies from China are limited, more Chinese nephrologists and geneticists will be involved in this promising field. There will be remarkable advances by our efforts in the future.
Conflict of Interest Statement
All authors declare that they have no competing interests.
Acknowledgments
This work was supported by National Nature Science Fund of China (No. 81000281), Chinese Society of Nephrology (No. 13030340419), Major Fundamental Research Program of Shanghai Committee of Science and Technology (No. 12DJ1400300), China Postdoctoral Science Fund (No. 2015M572677), and Key Projects in the National Science & Technology Pillar Program in the Twelfth Five-Year Plan Period (No. 2011BAI10B00).
References
- 1.Ong AC, Devuyst O, Knebelmann B, Walz G. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385:1993–2002. doi: 10.1016/S0140-6736(15)60907-2. [DOI] [PubMed] [Google Scholar]
- 2.Dai B, Mei CL. Research on autosomal dominant polycystic kidney disease in China. Chin Med J (Engl) 2006;119:1915–1924. [PubMed] [Google Scholar]
- 3.Paul BM, Consugar MB, Ryan Lee M, Sundsbak JL, Heyer CM, Rossetti S, et al. Evidence of a third ADPKD locus is not supported by re-analysis of designated PKD3 families. Kidney Int. 2014;85:383–392. doi: 10.1038/ki.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He SZ, An SY, Jiang HM, Yang R, Cao YF. Cyst decapitating decompression operation in polycystic kidney: preliminary report of 52 cases. Chin Med J (Engl) 1980;93:773–778. [PubMed] [Google Scholar]
- 6.Liu Y, Zhang H, Zhong H. Systemic manifestation of adult polycystic kidney disease: an analysis of 205 cases (in Chinese) Chin J Int Med (Chin) 1995;34:612–615. [PubMed] [Google Scholar]
- 7.Gao DX, Cao QW, Ding KJ, Zhao YR, Wang LC, Niu ZH, et al. An analysis for the phenotype and genotype of autosomal dominant polycystic kidney disease from two Chinese families. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2006;23:23–26. [PubMed] [Google Scholar]
- 8.Zhang WL, Mei CL. Genetic heterogeneity and phenotypes of autosomal dominant polycystic kidney disease in Chinese Han nationality. Zhonghua Yi Xue Za Zhi. 2006;86:1516–1521. [PubMed] [Google Scholar]
- 9.Zhang SZ, Zhang YH, Zhang DY, Mei CL. Mutation detection of ADPKD PKD1 gene in Hans by denaturing high-performance liquid chromatography. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2006;23:283–288. [PubMed] [Google Scholar]
- 10.Li L, Li L, Zhong CG, Gao BD, Lu GX. Mutation detection of PKD1 gene in patients with autosomal dominant polycystic kidney diseases. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2007;24:666–669. [PubMed] [Google Scholar]
- 11.Zhang DY, Sun TM, Zhang SZ, Tang B, Dai B, Zhang WL, et al. Mutation detection of PKD2 gene in Chinese by denaturing high-performance liquid chromatograph. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:211–214. [PubMed] [Google Scholar]
- 12.Ding L, Zhang S, Qiu W, Xiao C, Wu S, Zhang G, et al. Novel mutations of PKD1 gene in Chinese patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2002;17:75–80. doi: 10.1093/ndt/17.1.75. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Szeto CC, Kwan BC, Chow KM, Leung CB, Kam-Tao Li P. Peritoneal dialysis as the first-line renal replacement therapy in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2011;57:903–907. doi: 10.1053/j.ajkd.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Ma Y, Wang X, Yu S, Li L, Dai B, et al. Clinical characteristics and disease predictors of a large Chinese cohort of patients with autosomal dominant polycystic kidney disease. PLoS One. 2014;9:e92232. doi: 10.1371/journal.pone.0092232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, Ma Y, Wang X, Yu S, Li L, Dai B, et al. Triptolide-containing formulation in patients with autosomal dominant polycystic kidney disease and proteinuria: an uncontrolled trial. Am J Kidney Dis. 2014;63:1070–1072. doi: 10.1053/j.ajkd.2014.01.418. [DOI] [PubMed] [Google Scholar]
- 16.Xue C, Zhou CC, Sun LJ, He LL, Xu CG, Dai B, et al. Effects of endothelial nitric oxide synthase gene on end stage renal disease progression in autosomal dominant polycystic kidney disease. Nephrology (Carlton) 2014;19:630–637. doi: 10.1111/nep.12310. [DOI] [PubMed] [Google Scholar]
- 17.Liu B, Chen SC, Yang YM, Yan K, Qian YQ, Zhang JY, et al. Identification of novel PKD1 and PKD2 mutations in a Chinese population with autosomal dominant polycystic kidney disease. Sci Rep. 2015;5:17468. doi: 10.1038/srep17468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu C, Li J, Yuan Z, Liu S, Zou L. Two novel mutations affecting the same splice site of PKD1 correlate with different phenotypes in ADPKD. Ren Fail. 2014;36:687–693. doi: 10.3109/0886022X.2014.890010. [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Meng Y, Wei X, Shen J, Zhang M, Qi C, et al. Identification of novel mutations of PKD1 gene in Chinese patients with autosomal dominant polycystic kidney disease by targeted next-generation sequencing. Clin Chim Acta. 2014;433:12–19. doi: 10.1016/j.cca.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Wang Y, Xiong J. A new PKD1 mutation discovered in a Chinese family with autosomal polycystic kidney disease. Kidney Blood Press Res. 2014;39:1–8. doi: 10.1159/000355772. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Chen M, Wei J, He W, Li Z, Sun X, et al. Modification of PCR conditions and design of exon-specific primers for the efficient molecular diagnosis of PKD1 mutations. Kidney Blood Press Res. 2014;39:536–545. doi: 10.1159/000368464. [DOI] [PubMed] [Google Scholar]
- 22.Qi XP, Du ZF, Ma JM, Chen XL, Zhang Q, Fei J, et al. Genetic diagnosis of autosomal dominant polycystic kidney disease by targeted capture and next-generation sequencing: utility and limitations. Gene. 2013;516:93–100. doi: 10.1016/j.gene.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 23.Yu CW, Yang Y, Zhang SZ. Identification of mutations in PKD1 and PKD2 genes in two Chinese families with autosomal dominant polycystic kidney disease. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2011;28:485–489. doi: 10.3760/cma.j.issn.1003-9406.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Yu C, Yang Y, Zou L, Hu Z, Li J, Liu Y, et al. Identification of novel mutations in Chinese Hans with autosomal dominant polycystic kidney disease. BMC Med Genet. 2011;12:164. doi: 10.1186/1471-2350-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Yu C, Tao Y, Yang Y, Hu Z, Zhang S. Putative mutation of PKD1 gene responsible for autosomal dominant polycystic kidney disease in a Chinese family. Int J Urol. 2011;18:240–242. doi: 10.1111/j.1442-2042.2010.02709.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Mei C, Zhang D, Dai B, Tang B, Sun T, et al. Mutation analysis of autosomal dominant polycystic kidney disease genes in Han Chinese. Nephron Exp Nephrol. 2005;100:e63–e76. doi: 10.1159/000084572. [DOI] [PubMed] [Google Scholar]
- 27.Phakdeekitcharoen B, Watnick TJ, Germino GG. Mutation analysis of the entire replicated portion of PKD1 using genomic DNA samples. J Am Soc Nephrol. 2001;12:955–963. doi: 10.1681/ASN.V125955. [DOI] [PubMed] [Google Scholar]
- 28.Rossetti S, Hopp K, Sikkink RA, Sundsbak JL, Lee YK, Kubly V, et al. Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol. 2012;23:915–933. doi: 10.1681/ASN.2011101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 30.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kistler AD, Poster D, Krauer F, Weishaupt D, Raina S, Senn O, et al. Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int. 2009;75:235–241. doi: 10.1038/ki.2008.558. [DOI] [PubMed] [Google Scholar]
- 32.Jiang T, Wang P, Qian Y, Zheng X, Xiao L, Yu S, et al. A follow-up study of autosomal dominant polycystic kidney disease with intracranial aneurysms using 3.0 T three-dimensional time-of-flight magnetic resonance angiography. Eur J Radiol. 2013;82:1840–1845. doi: 10.1016/j.ejrad.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Xu HW, Yu SQ, Mei CL, Li MH. Screening for intracranial aneurysm in 355 patients with autosomal-dominant polycystic kidney disease. Stroke. 2011;42:204–206. doi: 10.1161/STROKEAHA.110.578740. [DOI] [PubMed] [Google Scholar]
- 34.Rong S, Jin X, Ye C, Chen J, Mei C. Carotid vascular remodelling in patients with autosomal dominant polycystic kidney disease. Nephrology (Carlton) 2009;14:113–117. doi: 10.1111/j.1440-1797.2008.01049.x. [DOI] [PubMed] [Google Scholar]
- 35.Schrier RW, Brosnahan G, Cadnapaphornchai MA, Chonchol M, Friend K, Gitomer B, et al. Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol. 2014;25:2399–2418. doi: 10.1681/ASN.2013111184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornec-Le Gall E, Audrezet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24:1006–1013. doi: 10.1681/ASN.2012070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hateboer N, van Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet. 1999;353:103–107. doi: 10.1016/s0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 38.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ong AC, Harris PC. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int. 2005;67:1234–1247. doi: 10.1111/j.1523-1755.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 40.Harris PC, Torres VE. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest. 2014;124:2315–2324. doi: 10.1172/JCI72272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 42.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 43.Xue C, Dai B, Mei C. Long-term treatment with mammalian target of rapamycin inhibitor does not benefit patients with autosomal dominant polycystic kidney disease: a meta-analysis. Nephron Clin Pract. 2013;124:10–16. doi: 10.1159/000354398. [DOI] [PubMed] [Google Scholar]
- 44.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erickson KF, Chertow GM, Goldhaber-Fiebert JD. Cost-effectiveness of tolvaptan in autosomal dominant polycystic kidney disease. Ann Intern Med. 2013;159:382–389. doi: 10.7326/0003-4819-159-6-201309170-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci USA. 2007;104:4389–4394. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue C, Zhou C, Dai B, Yu S, Xu C, Mao Z, et al. Antihypertensive treatments in adult autosomal dominant polycystic kidney disease: network meta-analysis of the randomized controlled trials. Oncotarget. 2015;6:42515–42529. doi: 10.18632/oncotarget.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie XS, Xie ZT, Xiang SL, Yan XQ, Zhang XH, Shou ZF, et al. Peritoneal dialysis for autosomal dominant polycystic kidney disease: a retrospective study. J Zhejiang Univ Sci B. 2016;17:375–381. doi: 10.1631/jzus.B1500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu YH, Cui XG, Mei CL, Gao Y, Wang LM, Wang YW, et al. Clinical study on renal transplantation in polycystic kidney disease. Chin J Organ Transplant (Chin) 2004;25:114–116. [Google Scholar]
- 50.Song WL, Zheng JM, Mo CB, Wang ZP, Fu YX, Feng G, et al. Kidney transplant for autosomal dominant polycystic kidney disease: the superiority of concurrent bilateral nephrectomy. Urol Int. 2011;87:54–58. doi: 10.1159/000324603. [DOI] [PubMed] [Google Scholar]