Abstract
Background
The emerging role of stem cell technology and transplantation has helped scientists to study their potential role in neural repair and regeneration. The fate of stem cells is determined by their niche, consisting of surrounding cells and the secreted trophic growth factors. This interim report evaluates the safety, feasibility and efficacy (if any) of bone marrow-derived mononuclear stem cells (BM-MNC) in chronic ischemic stroke by studying the release of serum vascular endothelial growth factor (VEGF) and brain-derived neurotrophic growth factor (BDNF).
Methods
Twenty stroke patients and 20 age-matched healthy controls were recruited with the following inclusion criteria: 3 months to 1.5 years from the index event, Medical Research Council (MRC) grade of hand muscles of at least 2, Brunnstrom stage 2-5, conscious, and comprehendible. They were randomized to one group receiving autologous BM-MNC (mean 60-70 million) and to another group receiving saline infusion (placebo). All patients were administered a neuromotor rehabilitation regime for 8 weeks. Clinical assessments [Fugl Meyer scale (FM), modified Barthel index (mBI), MRC grade, Ashworth tone scale] were carried out and serum VEGF and BDNF levels were assessed at baseline and at 8 weeks.
Results
No serious adverse events were observed during the study. There was no statistically significant clinical improvement between the groups (FM: 95% CI 15.2-5.35, p = 0.25; mBI: 95% CI 14.3-4.5, p = 0.31). VEGF and BDNF expression was found to be greater in group 1 compared to group 2 (VEGF: 442.1 vs. 400.3 pg/ml, p = 0.67; BDNF: 21.3 vs. 19.5 ng/ml) without any statistically significant difference.
Conclusion
Autologous mononuclear stem cell infusion is safe and tolerable by chronic ischemic stroke patients. The released growth factors (VEGF and BDNF) in the microenvironment could be due to the paracrine hypothesis of stem cell niche and neurorehabilitation regime.
Key Words: Intravenous bone marrow-derived mononuclear stem cells, Chronic ischemic stroke, Autologous mononuclear stem cell infusion
Introduction
The development of regenerative medicine has enthralled researchers to study and exploit its usage and therapeutic effects [1,2]. Different types of stem cells exhibit a potential that has helped improving symptoms of various intractable neuronal diseases, such as stroke [3,4]. Bone marrow-derived mononuclear stem cells (BM-MNC) have been used in preclinical studies suggesting increased angiogenesis in penumbral tissue following CD34+ cell transplantation, whether given systemically (intra-arterial, intravenous or intrathecal) or by the intracerebral route [5,6].
Stem cells actively contribute to their environment by secreting cytokines, growth factors and extracellular matrix molecules that act either by themselves (autocrine actions) or on human body and other tissues (paracrine) for regeneration [7]. In addition, these cells secrete angiogenic factors, antifibrotic factors, extracellular matrix homeostasis proteins such as collagens, matrix metalloproteinases and other inhibitors [8]. Brain-derived neurotrophic growth factor (BDNF) crucially promotes the synaptic and axonal plasticity associated with learning, memory and sensorimotor recovery [9]. It stimulates neuronal differentiation in vitro. It has also been used to induce neurogenesis after focal ischemia, thereby increasing the number of newborn neurons in several regions of the brain enhancing neural structural plasticity [10]. Vascular endothelial growth factor (VEGF) is a dimeric glycoprotein mitogenic for endothelial cells. It has been shown to increase vascular permeability; it can induce chemotaxis in monocytes in pathological conditions [11] as well as inhibit endothelial cell apoptosis. Recently, it was shown that both VEGF and its receptor Flt-1 are upregulated in both neurons and blood vessels in the penumbra after transient or permanent occlusion of the middle cerebral artery in the rat [12].
Cell treatment or treatment with a stem cell-containing population is nascent in the current stage and has met enormous skepticism in the field of cell therapeutics. Since the realization that the beneficial effects of stem cells may be due to localized or generalized release of trophic factors, and not attributed (in part or entirely) to stem cell transdifferentiation or homing in to the lesioned cortex, many scientists have focused on harnessing the paracrine actions of stem cells to enhance therapeutic efficacy [13,14].
The adult brain can regenerate neurons lost after brain ischemia. Repair mechanisms in stroke are related to acute injury (first epoch) and they are said to occur in the initial few hours after the acute event when changes in blood flow, metabolism and ischemic cascade are most active. A second epoch is related to the upregulation of growth factors which continues for days to weeks and is referred to as endogenous repair-related events. A third epoch occurs weeks to months after stroke when spontaneous recovery mechanisms plateau representing a stable but modifiable early and late chronic phase. The objectives were (a) to study safety, feasibility and efficacy (if any) of BM-MNC infusion in chronic ischemic stroke; (b) to study early/late upregulation of serum growth factors (VEGF and BDNF) after BM-MNC and neuromotor rehabilitation after stroke, and (c) to study the correlation of serum VEGF and BDNF with functional recovery after stroke.
Methods
Thirty patients were screened who fulfilled the inclusion criteria; among them, 5 refused to participate in the trial and 5 had deranged baseline laboratory values and were therefore excluded; hence, 20 patients were recruited in the study. Patients diagnosed with ischemic stroke 3 months to 1.5 years after the index event with Medical Research Council (MRC) grade of power for wrist and hand muscles of at least 2, Brunnstrom stage of recovery of 2-5, National Institutes of Health Stroke Scale (NIHSS) score of between 4 and 15, being conscious/comprehendible as well as 20 age-matched healthy controls were recruited. The exclusion criteria were hematological disorders, autoimmune disorders, immunocompromised subjects, chronic liver and renal failure, progressive neurological worsening, unilateral neglect, neoplasia, contraindication to MRI and pregnancy. Twenty patients were randomized (computer-assisted) to two groups. One group was infused with BM-MNC and the other group with placebo (i.e. intravenous saline infusion). All patients were administered a neuromotor rehabilitation regime for 8 weeks. Prior to stem cell therapy, patients were screened and educated about the stem cells and bone marrow aspiration technique. Written informed consent was obtained, complete medical history, examination and baseline laboratory tests were performed. The patients were examined by a neurologist and a neurophysiotherapist for muscle strength/power (MRC grade) and tone (modified Ashworth tone scale), Fugl Meyer (FM) scale for upper limb, the Edinburgh handedness inventory and the modified Barthel index (mBI) [15,16,17]. The study is a randomized placebo-controlled clinical trial which was approved by the IC-SCRT (Institute Committee for Stem Cell Research and Therapy) and is registered with CTRI (CTRI/2014/09/005028). Safety and efficacy end points were assessed at 7 days, 8 weeks, 6 months and 1 year after stem cell transplantation. Serum VEGF and BDNF levels were measured at baseline, 8 weeks, 6 months and 1 year. Functional MRI was also performed at the same time points. The outcome measures were blinded to one of the assessors.
This paper presents an interim analysis of safety, feasibility and efficacy of BM-MNC in chronic ischemic stroke along with the upregulation of the serum growth factors VEGF and BDNF after 2 months of cell infusion.
Bone Marrow Aspiration, Separation and Transplantation
Bone marrow (approximately 40-50 ml) was aspirated under aseptic conditions from the posterior superior iliac crest in 10 chronic stroke patients. Bone marrow aspirate was diluted with phosphate-buffered saline, layered over Ficoll density medium and centrifuged at 1,800 rpm for 25 min. BM-MNC layers were collected, and the number of CD34+ cells (flow cytometry method) was counted for each patient [18,19] (table 1). The cells were manufactured entirely under good manufacturing practice conditions. The mean cell viability of BM-MNC at transplantation was 98%, which was performed with trypan blue stain, and the cells were sterile and endotoxin-free during culture and at transplantation. The whole procedure took approximately 120 ± 10 min. An aseptic technique of infusion was performed with collected BM-MNC in a 50-ml sterile syringe, which was directly dissolved in 250 ml of saline and infused intravenously over 3 h.
Table 1.
Demographics, risk factors and baseline characteristics in groups 1 and 2 and healthy controls
| Group 1 (n = 10) | Group 2 (n = 10) | P value | Healthy controls (n = 20) | |
|---|---|---|---|---|
| Male | 10 | 10 | 12 | |
| Female | 2 | 3 | 8 | |
| Age, mean ± SD, years | 48.6±7.1 | 48.1±9.1 | 0.24 | 49.6±5.6 |
| Average time to stroke onset, months | 11.6 | 10.5 | ||
| Risk factors | ||||
| Hypertension | 7 (70%) | 6 (60%) | 0.15 | 12 (60%) |
| Diabetes mellitus | 5 (50%) | 3 (30%) | 0.87 | 6 (30%) |
| Hypercholesterolemia | 6 (60%) | 4 (40%) | 0.56 | 10 (50%) |
| Smoking | 4 (40%) | 4 (40%) | 0.49 | 13 (65%) |
| Alcohol consumption | 3 (30%) | 2 (20%) | 1.0 | 5 (25%) |
| Tobacco abuse | – | – | 0.58 | 3 (15%) |
| Coronary artery disease | 5 (50%) | 6 (40%) | – | 5 (25%) |
| Ischemic stroke (subtype) | ||||
| LA atherosclerosis | 4 (40%) | 4 (40%) | 0.67 | |
| CE | 4 (40%) | 1 (10%) | 0.35 | |
| SA occlusion | 2 (20%) | 3 (30%) | 0.21 | |
| Others | – | 2 (20%) | 0.81 | |
| Laboratory parameters | ||||
| Baseline | ||||
| VEGF | 366.2±78.6 pg/ml | 370.5±91.1ng/ml | 0.42 | |
| BDNF | 18.7±6.6 pg/ml | 16.1±7.6 ng/ml | 0.56 | |
| 8 Weeks | ||||
| VEGF | 453.5±89.1pg/ml | 408.4±98.4ng/ml | 0.96 | |
| BDNF | 32.8±9.2 pg/ml | 27.3±8.9 ng/ml | 0.78 | |
Serum Sampling for VEGF and BDNF
Samples were immediately centrifuged (1,500 g/15 min) and serum was stored at −70°C until assayed. Serum VEGF and BDNF levels were measured by standard quantitative sandwich ELISA (Quantikine) kits, obtained from R&D Systems. Samples from each individual were analyzed in triplicate and subsequently used in all further statistical analysis. The lower limits of detection were 5.0 pg/ml for VEGF and 3 ng/ml for BDNF (information provided by R&D Systems). Twenty age-matched healthy controls were recruited for VEGF and BDNF estimation.
Neurorehabilitation Regime
Motor imagery training using Xbox and mirror therapy were administered to the affected upper limb with bilateral hands focusing on principles of learning. Small hand muscle exercises like ball squeezing, lifting an object, rolling a cylindrical shape, and thrust release (mass finger extension) were included in the protocol. General aerobic exercise training was administered with 10 min of static cycling and brisk walk (the extent the patient can), partial squats (hold for 7 s), and step up and down [20,21].
Statistics
Statistical analysis was performed by SPSS version 17. The comparative data between the two groups were expressed as the mean/median with SD and quartiles. Parametric paired t test was used for intragroup and independent-samples t test for intergroup comparisons with statistically significant p = 0.05. Two-way repeated-measures ANOVA was used to compare motor function scores at different time points. The analysis was two-tailed unless otherwise specified. For correlative analysis, the Spearman rank correlation coefficient (r) was calculated. Analysis was done to identify differences in growth factor expression in chronic stroke, in relation to stroke subtypes and etiology, with clinical disability, including short-term follow-up.
Results
Safety and Feasibility: Monitoring of Infusion-Related Toxicity
Different organ systems were tested through various laboratory tests like percent hemoglobin, total leukocyte count, differential leukocyte count, platelets, prothrombin time, liver and kidney function assessed at the 1st, 3rd and 7th day after infusion. These were within normal limits for all patients. Besides hematological parameters, pulmonary, gastrointestinal, hepatic and renal systems were monitored. Neurological assessment was performed until hospital discharge. The safety outcomes included death, adverse events (fatal or nonfatal myocardial infarction), epileptiform discharges and evidence of any new growth in radiological scans. Cell viability, mononuclear stem cell surface markers, bacteria, syphilis, fungi, viral, mycoplasma, and endotoxin levels were tested in expansion process and transplantation. There were no early or late adverse reactions during and after transplantation observed until 8 weeks. Pulmonary complications like respiratory distress, tachypnea or infections were not observed.
Cell Dosing and Characterization
As this was an amendment of our last trial, dosage was kept the same, i.e., 1 million/kg body weight. CD34+ cells were characterized, with a mean count of 0.31% with 62.8 × 106 cells infused.
Clinical Scores
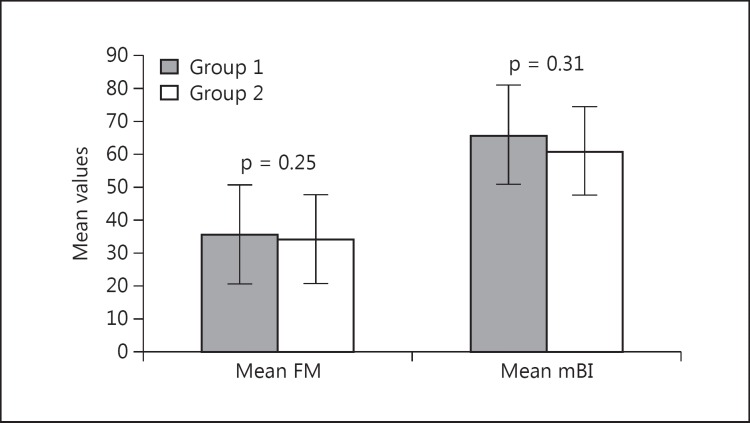
The demographics and baseline characteristics were comparable at baseline. Ischemic stroke classification yielded 8 large-artery (LA), 7 small-artery (SA), 1 cardioembolic (CE) and 4 other (determined and undetermined) strokes. The risk factors were as follows: 55% hypertensive, 25% diabetics, 40% hypercholesterolemia and 4% alcoholism (table 1). In group 1 (male-to-female ratio 8:2, mean age 48.6 ± 7.1 years), the mean FM score at baseline was 23.9 ± 7.5 and at 8 weeks it was 39.7 ± 10.3 (95% CI −24.341 to −7.259, p = 0.002), whereas the mean mBI was 46.7 ± 4.3 at baseline and 65.9 ± 10.5 at 2 months (95% CI −27 to −11.3, p = 0.009). In group 2, the male-to-female ratio was 9:1, the mean age was 48.1 ± 9.1 years, and the mean FM score was 21.4 ± 6.1 and 34.1 ± 10.3 at baseline and 8 weeks, respectively (95% CI −9.7 to −4.3, p = 0.04) (table 2). The mean mBI at baseline was 46.2 ± 7.8 and 61 ± 9.8 at 2 months of follow-up (95% CI −11.4 to −7.8, p = 0.01). The MRC and Ashworth tone scale did not show any significant improvement between baseline and 2 months in all patients.
Table 2.
Mean clinical scores and mononuclear stem cell CD34+ (UD: undisclosed) in groups 1 and 2
| Case No. | Age/sex/weight, kg | Area of infarct, all MCA territory | Time after stroke, months | Cells | CD34+ cells, % | Baseline |
8 weeks |
||
|---|---|---|---|---|---|---|---|---|---|
| FM (/66) | mBI (/100) | FM (/66) | mBI (/100) | ||||||
| Group 1 | |||||||||
| 1 | 55/M/62 | Rt frontotemporal | 12 | UD | UD | 22 | 47 | 36 | 65 |
| 2 | 52/M/70 | Lt corona radiata | 9 | UD | UD | 34 | 53 | 51 | 70 |
| 3 | 45/F/57 | Rt frontal Rt striatocapsular | 5 | UD | UD | 32 | 50 | 50 | 74 |
| 4 | 36/M/71 | Lt temporal parietal | 14 | UD | UD | 25 | 42 | 40 | 53 |
| 5 | 57/M/59 | Rt PLIC | 12 | UD | UD | 18 | 39 | 26 | 53 |
| 6 | 60/M/58 | Lt caudate, parietal lobe | 10 | UD | UD | 12 | 45 | 22 | 55 |
| 7 | 53/M/60 | Lt IC, thalamus | 11 | UD | UD | 20 | 45 | 45 | 60 |
| 8 | 46/M/46 | Rt frontotemporal | 7 | UD | UD | 17 | 47 | 32 | 72 |
| 9 | 40/M/47 | Lt parietal | 8 | UD | UD | 34 | 49 | 49 | 85 |
| 10 | 42/F/63 | Lt PLIC | 9 | UD | UD | 25 | 50 | 46 | 72 |
| Mean | 48.6/61.3 | 9.7 | UD | UD | 23.9 | 46.7 | 39.7 | 65.9 | |
| Group 2 | |||||||||
| 1 | 33/M | Rt lacunar | 5 | UD | UD | 18 | 40 | 20 | 55 |
| 2 | 45/M | Lt frontotemporal | 9 | UD | UD | 20 | 52 | 31 | 62 |
| 3 | 58/M | Rt temporoparietal | 15 | UD | UD | 11 | 42 | 20 | 50 |
| 4 | 56/M | Lt caudate, putamen, temporal | 9 | UD | UD | 24 | 55 | 36 | 65 |
| 5 | 42/M | Rt frontoparietal | 18 | UD | UD | 23 | 50 | 30 | 68 |
| 6 | 60/M | Rt lentiform, Rt parietal | 8 | UD | UD | 30 | 58 | 49 | 71 |
| 7 | 61/M | Lt temporal, corona radiata | 18 | UD | UD | 20 | 39 | 45 | 58 |
| 8 | 60/M | Rt putamen | 6 | UD | UD | 31 | 50 | 51 | 78 |
| 9 | 47/F | Lt frontotemporal | 7 | UD | UD | 22 | 42 | 32 | 56 |
| 10 | 49/M | Lt temporal | 10 | UD | UD | 15 | 34 | 27 | 47 |
| Mean | 48.1± 8.9 | 10.5 | 21.4 | 46.2 | 34.1 | 61 | |||
PLIC = Posterior limb of internal capsule; IC = internal capsule; Rt = right; Lt = left; NA = not available.
Comparing the two groups, no statistically significant difference was observed in the activities of daily living scale mBI (95% CI 14.3-4.5, p = 0.31) and FM score at 2 months (95% CI 15.2-5.35, p = 0.25) (fig. 1). The Ashworth tone scale and the MRC grade for hand muscles remained statistically nonsignificant at 2 months between groups 1 and 2 (p > 0.05).
Fig. 1.
Mean FM score and mBI in groups 1 and 2 at 2 months (p > 0.05).
Serum VEGF and BDNF Expression Levels
Twenty healthy age-matched controls had a mean VEGF level of 257 ± 102.3 pg/ml and a BDNF level of 16.6 ± 3.4 ng/ml. The baseline VEGF and BDNF levels were similar (p = 0.42 and p = 0.56, respectively) and hence were compared at 8 weeks. Mean VEGF at baseline was 336 ± 78.6 pg/ml in group 1 and 370 ± 91.3 pg/ml in group 2. All patients showed improvement within the groups at 8 weeks (p < 0.05), but no statistically significant difference was observed between the two groups at 8 weeks (mean 453.5 ± 89.1 vs. 408.4 ± 93.3 pg/ml, 95% CI 13.3-6.7, p = 0.96). The median values for VEGF between the groups 1 and 2 were also nonsignificant at 8 weeks (fig. 2) (442.1 vs. 400.3 pg/ml, p = 0.67).
Fig. 2.
Box plots showing VEGF in groups 1 and 2 at 8 weeks. No significant difference in the median values of VEGF was found in both groups (442.1 vs. 400.3 pg/ml; p = 0.67).
In group 1, mean BDNF at baseline was 18.7± 6.6 and 16.1± 7.6 ng/ml in group 2. There was no difference observed within the groups at 8 weeks (p = 0.45 and p = 0.68 for group 1 and group 2, respectively). No statistically significant improvement was observed between the groups (mean 32.8 ± 9.2 vs. 27.3 ± 9.1 ng/ml; 95% CI 5.7-1.2, p = 0.78) after 8 weeks of cell transplantation.
Correlation of Growth Factors with Stroke Subtype and Motor Recovery
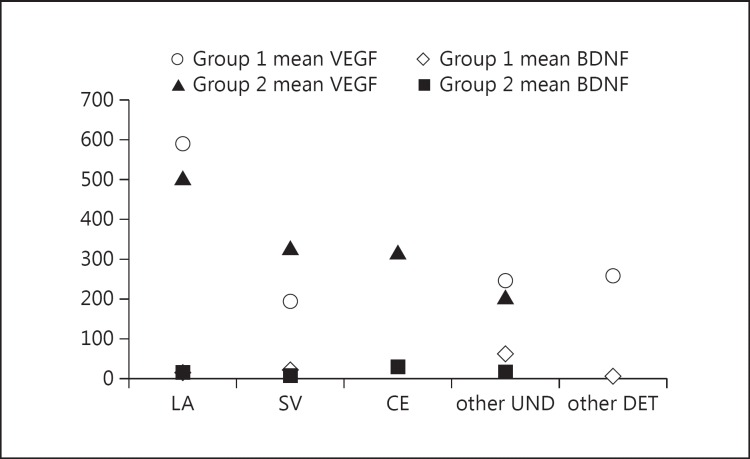
Out of 20 patients, all patients with LA disease were found to have a mean baseline VEGF of 500.9 pg/ml followed by small-vessel (SV) (p = 0.01, Mann-Whitney U test, one-tailed) and other stroke types, whereas 1 patient with CE stroke had a VEGF level of 316.4 pg/ml. BDNF showed the opposite, i.e, a high BDNF level was observed in stroke patients with other undetermined etiology followed by LA and SA strokes, with 28.2 ng/ml in 1 CE stroke (fig. 3). There was no causal relationship observed with risk factors, infarct size, or other laboratory parameters (platelets) for both BDNF and VEGF (Spearman coefficient r = 0.34, p = 0.56). We divided patients according to severity: severely affected (MRC grade = 1 with an FM score ≤25) and moderately affected (MRC grade > 2 ≤ 3 with an FM score of ∼25-45) and correlated them with the serum factors. Eleven of 20 patients were severely affected, with baseline median values of VEGF and BDNF concentrations of 316.4 (interquartile range 471.8-166.1) and 12.5 ng/ml (interquartile range 23.7-4.05), respectively. On the other hand, mean concentrations of VEGF in moderately affected patients at 2 months were 314.8 versus 289.8 pg/ml (95% CI ∼116.4-66.5, p = 0.45) and in the severely affected group 592.1 versus 487.6 pg/ml (p = 0.76) without any statistically significant improvement. BDNF also showed statistically insignificant results, with the severely affected group having a mean BDNF level of 28.1 ng/ml in group 1 and 23.5 ng/ml in group 2, and the same was observed in the moderately affected group (38.1 vs. 30.4 ng/ml, p = 0.32) (fig. 4a, b).
Fig. 3.
Graph showing mean VEGF in ischemic stroke. All patients with LA disease were found to have a high mean baseline VEGF in groups 1 and 2 followed by SV. One patient with CE stroke had a VEGF level of 316.4 pg/ml, patients with undetermined (UND) or determined (DET) stroke each had a mean VEGF level of 245.5 or 256.7 pg/ml.
Fig. 4.
a Association of serum VEGF according to stroke severity (severely and moderately affected patients) at baseline and at 2 months in groups 1 and 2. b Association of serum BDNF according to stroke severity (severely and moderately affected patients) at baseline and at 2 months in both groups.
Discussion
The hypothesis that stem cells work through paracrine signaling is being widely studied. It is defined as a communication between two different cells where one releases chemical mediators to its immediate environment, which leads to a change in behavior of a cell in its adjacent territory [2,14]. Autologous intravenous stem cell therapy is safe and feasible in chronic stroke as evidenced by this report and has been established by us in our previous trials as well [22,23] in which we used both naive, i.e., mononuclear, and culture-expanded, i.e., mesenchymal, stem cells. The endogeneous or intrinsic recovery mechanisms start just after an acute insult and are completed by 3 months. The exogenous recovery continues over a longer period, through cell-based therapies, gene therapy, neuromotor rehabilitation and others [3]. We recruited patients between 3 months and 1.5 years after the index event, so that the spontaneous recovery is over. As gliosis and scarring of the brain tissue appears beyond 2 years, we had screened patients within this range.
A mean of 59.9 × 106 mononuclear stem cells (each) were easily procured from 10 bone marrow samples in 1-2 h of culture procedure. Earlier studies received 50 million cells twice [24], 200-400 million cells [25], 34.6 million cells and 5-10 million cells [26]. Compared to our last trial, this study is a randomized placebo-controlled study with the same cell dosage. Savitz et al. [27], in a recent study, conducted autologous mononuclear stem cell transplantation in 10 subjects. Eight received 10 million cells/kg body weight, 1 received 7 million cells/kg body weight, and 1 received 8 million/kg body weight. There was no difference observed in functional outcomes with the three kinds of dosage. STARTING-2 collaborators have suggested autologous culture-expanded mesenchymal stem cells to a dose of 1 × 106 cells/kg, the equivalent human dose found to be effective in a rat stroke model (1 × 105 to 3 × 106 cells/rat) based on mean body weight [28]. Cell-enhanced recovery has been reported with chronic delivery of cells even at 1 month after ischemia [29]. The best route of transplantation still needs to be established although many clinical trials are opting intravenous as the mode of transplantation being safe and easily tolerable [30].
A very recent study published by Prasad et al. [31] investigated the safety and efficacy of intravenous mononuclear stem cell transplantation in subacute ischemic stroke in which 58 patients received a mean of 280.75 million bone marrow stem cells at a median of 18.5 days after stroke onset. There was no significant difference between the bone marrow stem cell arm and the control arm in the Barthel Index score (63.1 vs. 63.6, p = 0.92), modified Rankin scale shift analysis (p = 0.53) or score >3 (47.5 vs. 49.2%, p = 0.85), and NIHSS score (6.3 vs. 7.0, p = 0.53), which is similar to our study, as we did not observe any statistically significant improvement between both groups on Ashworth tone scale, MRC grade, FM scale and mBI (95% CI 15.2-5.35, p = 0.25; 95% CI 14.3-4.5, p = 0.31, respectively) with a short follow-up until 2 months.
This ongoing study was planned to examine the efficacy not only on clinical, functional and radiological outcome measures but on the release of growth factors (VEGF and BDNF) after 2 months. The growth factors such as bFGF, VEGF, and angiopoietin-1, and cardiac levels of angiogenic ligands are released as a consequence of neural repair after brain insult [32,33]. BDNF is involved in many facets of brain function, including neuroplastic changes that underlie motor learning. It exerts its effects on neuroplasticity by facilitating long-term potentiation, by promoting dendritic growth and remodeling [34,35]. This was observed in all the patients who had been administered a neuromotor regime for 2 months. Patients in group 2 showed a statistically significant increase (baseline to 8 weeks) in BDNF as compared to VEGF (p = 0.01 vs. p = 0.53) indicating that BDNF is more likely to be involved in neuroplastic changes, which is supported by the literature [36]. Several animal studies have demonstrated significantly increased BDNF mRNA levels in the rat hippocampus after a single bout of 6 h voluntary wheel running [37]. The percent gain in BDNF was high compared to VEGF (68 vs. 20%), suggesting that unlike other growth factors, BDNF is secreted in the central nervous system and blood stream through both repair initiated and an activity-dependent pathway. The activity-dependent secretion is crucial to the role of BDNF in promoting neuroplasticity in circuits activated in response to enrichened experience, i.e., stem cells along with exercise [38]. In a related work [39], it was found that a critical amount or ‘threshold’ of poststroke rehabilitation must be met to obtain functional recovery. Animals exceeding this threshold exhibited forelimb recovery and showed significant increases in motor cortex BDNF levels, while rats receiving less rehabilitation did not recover and BDNF levels remained at control levels. All groups received motor imagery and aerobic form of training for 8 weeks, which was 1 h in the hospital settings and rest exercises were advised at home. These findings indicate that BDNF also plays a key role in poststroke rehabilitation and consequently BDNF may be an important target to enhance recovery in stroke patients. Serum BDNF levels rise during aerobic exercise, and quickly return to baseline levels upon exercise cessation, approximately 10-15 min after exercise offset [40].
The mean concentration of both VEGF and BDNF in the serum of patients with stroke was significantly higher than that of the healthy controls. VEGF is a key mediator of angiogenesis, which is an important process leading to reperfusion of ischemic brain tissue after acute stroke [41]. An increase in VEGF was observed from baseline to 2 months but did not reach statistically significant levels in both groups. Mean VEGF expression was lowest in the serum of patients with SV, increasing in stroke with other origin and being the greatest in LA stroke in both groups, suggesting that VEGF could be a marker indicating the size of the infarct, which is supported by previous literature [42,43]. We did not find any correlation with platelets in any of our patients. It has been observed that transplantation of bone marrow mesenchymal stem cells into ischemic brain resulted in gain of coordinated function in rats due to bone marrow cell soluble factor release, resulting in inhibited scar formation, increased angiogenesis and neuronal commitment [44,45]. Matsuo et al. [46] posited that increased plasma VEGF values last for at least 90 days in all stroke subtypes, and the clinical significance varies for different stroke types. It was found that the severely affected group had a high mean VEGF of 592.1 versus 487.6 pg/ml (p = 0.76), without any statistically significant improvement between groups 1 and 2 (fig. 4a, b). Serum VEGF at baseline was higher in severely affected patients than in moderately affected patients (316.1 vs. 257.4 pg/ml), which remained high at 2 months predicting a good functional recovery, suggesting that in chronic strokes (without classification into stroke subtype and volume), VEGF might have been increased already at acute onset in severely affected patients and function as an angiogenic [47] and neuroprotective molecule. Thus, higher VEGF values would attenuate neuronal death and reduce infarct volume, although infarct volumes are not depicted in this analysis.
Senescence in stem cells is one phenomenon that is currently being studied and recently published data suggest that their efficacy is limited by natural aging [48]. The impact of aging on stem cell populations differs between tissues and depends on a number of intrinsic/extrinsic factors, including systemic changes associated with immune system alterations. The mean age of patients in our study was a relatively younger population (48.6 ± 9.6 years), so we assume that efficacy was not greatly compromised. Comorbid or risk factors like age, hypertension, or diabetes were observed in our group, but we did not find any correlation with the cell effects nor did we find a low yield in the correlation with these risk factors. The bone marrow harvest from each patient was not compromised in spite of all the risk factors. Owing to ethical concerns and rampant use of stem cells without regulation, the institute ethics committee gave approval for 10 patients initially. These patients will be followed up for up to 6 months to 2 years to gauge the efficacy in terms of serum growth factor, spectroscopic analysis and functional imaging. Both groups showed improvement with increased clinical and activity of daily living scores, which would urge the researchers to ponder over psychoimmunological/placebo effects of mononuclear stem cells.
Doll et al. [49], in a review article, reported that an increase in the production of pro-inflammatory cytokines and a decrease in the production of anti-inflammatory cytokines are correlated with a larger infarct size and worse clinical outcome. TNF-α, IL-1β, IL-6 and IL-10 are some cytokines that are known to get upregulated after acute stroke. IL-6 is elevated in plasma during the first week after stroke, while IL-1β is elevated in the cerebrospinal fluid of severe stroke patients with peak levels at 2-3 days. TNF-α-positive neurons peak between 2 and 3 days after stroke and TNF-α-positive astrocytes peak between 15 h and 14 days. Pro-inflammatory cytokines after mononuclear stem cell infusion has also been reported [50]. Since this trial studies mononuclear stem cell infusion in the chronic stage of stroke, where acute inflammatory pathways might have stabilized, cytokine measurement might not serve as a good marker. Cytokines released from mononuclear stem cells can be measured and may account as another outcome measure to study efficacy in future trials.
There is evidence suggesting that human neural stem cells, human umbilical cord blood and BM-MNCs secrete glial cell-derived neurotrophic factor and BDNF, IGF-1 and VEGF, which may protect dysfunctional motor neurons, thereby prolonging the lifespan of the stroke-induced animals [51]. They are known to secrete NT-3, which supports the survival, and differentiation of existing stroke-induced damage to the brain and central nervous system caused by focal or global ischemia [52,53].
The pathophysiology of stroke within the first few days includes infarct progression, active inflammation and edema formation and loss of autoregulatory control from the brain. The acute and subacute phase may not be conducive for cell transplantation. Stem cell research is in the nascent stage and is being studied in all stages of stroke. In chronic stroke, the rationale of cell infusion is upregulation of growth factors, prevention of ongoing cell death, enhancement of synaptic connectivity between the host and graft, cell differentiation and integration acting as scaffolds and chaperons. A very small sample size, dose of cells, site/mode of transplantation, no fMRI data analyzed and lack of in vivo monitoring of the intravenously transplanted cells are a few limitations which do not infer efficacy of BM-MNC in chronic stroke patients. The heterogeneity of stroke, infarct etiologies, topography, injury location and size are hurdles to prove the same. The interim analysis shows that the research may be continued as no risk was observed in both groups, which indicates that cell transplantation was safe in 10 of these patients.
Conclusion
Novel research directions aspire to use hematopoietic stem cells as biologically active drug delivery vehicles to facilitate tissue regeneration. Autologous intravenous mononuclear stem cell transplantation is safe and feasible. No conclusion on efficacy can be addressed with this report. It is likely that the paracrine mediators are expressed/released in a temporal and spatial manner after stem cell infusion and exercise training exerting different effects depending on the microenvironment of the host after injury.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgement
The study is funded by the Department of Science and Technology, under the Ministry of Science, New Delhi, India. All authors have contributed equally.
References
- 1.Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 2.Goldman SA, Windrem MS. Cell replacement therapy in neurological disease. Philos Trans R Soc Lond B Biol Sci. 2006;361:1463–1475. doi: 10.1098/rstb.2006.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scadden DT. Stem cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 4.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 5.Domen J, Weissman IL. Self-renewal, differentiation or death: regulation and manipulation of hematopoietic stem cell fate. Mol Med Today. 1999;5:201–208. doi: 10.1016/S1357-4310(99)01464-1. [DOI] [PubMed] [Google Scholar]
- 6.Brennemann M, Sharma S, Harting M, Strong R, Cox CS. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthony DF, Sheils PG. Exploiting paracrine mechanisms of tissue regeneration to repair damaged organs. Transplant Res. 2010;2:13–17. doi: 10.1186/2047-1440-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnaik PR, Devitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukahara T, Yonekawa Y, Tanaka K, Ohara O, Wantanabe S, Kimura T, et al. The role of brain-derived neurotrophic factor in transient forebrain ischemia in the rat brain. Neurosurgery. 1994;34:323–331. doi: 10.1227/00006123-199402000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, et al. Intravenous brain-derived neurotrophic factor enhances post stroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 11.Zachary I. Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. NeuroSignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- 12.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barkho BZ, Zhao X. Adult neural stem cells: response to stroke injury and potential for therapeutic applications. Curr Stem Cell Res Ther. 2011;6:327–338. doi: 10.2174/157488811797904362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loewen SC, Anderson BA. Reliability of motor assessment and Barthel Index. Phys Ther. 1999;8:1077–1081. doi: 10.1093/ptj/68.7.1077. [DOI] [PubMed] [Google Scholar]
- 16.Ismail S, Bilger Y, Evren Y, Rdvani A. Brunnstrom recovery stage and motricity index for the evaluation of upper extremity in stroke: analysis for correlation and responsiveness. Int J Rehabil Res. 2009;32:228–231. doi: 10.1097/MRR.0b013e32832a62ad. [DOI] [PubMed] [Google Scholar]
- 17.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1972;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin A, Srivastava MVP, Kumaran SS, Mohanty S, Bhatia R. Autologous intravenous mononuclear stem cell transplantation in chronic ischemic stroke. J Stem Cells Regen Med. 2012;8:181–189. doi: 10.46582/jsrm.0803011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergado JA, Monteagudo CS, Ramirez PH, Gonzalez RM. Autologous bone marrow stem cell transplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27:151–161. doi: 10.3233/RNN-2009-0483. [DOI] [PubMed] [Google Scholar]
- 20.Bhasin A, Srivastava MVP, Kumaran SS, Bhatia R, Mohanty S. Neural interface of mirror therapy in chronic stroke: a functional imaging study. Neurol India. 2012;60:570–576. doi: 10.4103/0028-3886.105188. [DOI] [PubMed] [Google Scholar]
- 21.Ploughman M, Austin MW, Glynn L, Corbett D. The effects of poststroke aerobic exercise on neuroplasticity: a systematic review of animal and clinical studies. Transl Stroke Res. 2015;6:13–28. doi: 10.1007/s12975-014-0357-7. [DOI] [PubMed] [Google Scholar]
- 22.Bhasin A, Srivastava MVP, Kumaran SS, Mohanty S, Bhatia R, Garg A, Airan B. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93–104. doi: 10.1159/000333381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhasin A, Srivastava MVP, Kumaran SS, Mohanty S, Bhatia R. Stem cell therapy: a clinical trial in stroke. Clin Neurol Neurosurg. 2013;115:1003–1008. doi: 10.1016/j.clineuro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Kondziolka D, Wechsler L, Meltzer C, Goldstein S. Transplantation of cultured neuronal cells for patients with stroke. Neurology. 2000;55:565–570. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- 25.Savitz SI, Dinsmore J, Wu S. Neural transplantation of fetal porcine cells in patients with basal ganglia infarcts; a preliminary safety and feasibility study. Cerebrovas Dis. 2005;2:101–112. doi: 10.1159/000086518. [DOI] [PubMed] [Google Scholar]
- 26.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 27.Savitz SI, Mishra V, Kasam M, Juneja H, Cox CS, Alderman S, Aisiku I, Kar S, Gee A, Grotta JC. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 28.Kim SJ, Moon GJ, Chang WH, Kim YH, Bang OY, STARTING-2 collaborator Intravenous transplantation of mesenchymal stem cells preconditioned with early phase stroke serum: current evidence and study protocol for a randomized trial. Trials. 2013;14:317. doi: 10.1186/1745-6215-14-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen SH, Li Y, Chen J, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 30.Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Alderman S, Aisiku I, Kar S, Gee A, Grotta JC. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 31.Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP, Hassan KM, Prabhakar S, Marwaha N, Khandelwal N, Misra UK, Kalita J, Nityanand S, InveST Study Group Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45:3618–3624. doi: 10.1161/STROKEAHA.114.007028. [DOI] [PubMed] [Google Scholar]
- 32.Sobrino T, Arias S, Rodriguez-González R, Brea D, Silva Y, de la Ossa NP, Agulla J, Blanco M, Pumar JM, Serena J, Dávalos A, Castillo J. High serum levels of growth factors are associated with good outcome in intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29:1968–1974. doi: 10.1038/jcbfm.2009.182. [DOI] [PubMed] [Google Scholar]
- 33.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 34.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39:728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 35.Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem Res. 2003;28:1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- 36.Sun J, Ke Z, Ping Yip P, Hu XL, Zheng XX, KY Tong KY. Gradually increased training intensity benefits rehabilitation outcome after stroke by BDNF upregulation and stress suppression. Biomed Int. 2014;92:57–62. doi: 10.1155/2014/925762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen MJ, Russo-Neustadt AA. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus. 2009;19:962–972. doi: 10.1002/hipo.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ke Z, Yip SP, Li L, Zheng XX, Tong KY. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS One. 2011;8:6. doi: 10.1371/journal.pone.0016643. e16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mang CS, Campbell KL, Ross CJ, Boyd LA. Promoting neural plasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013;93:1707–1716. doi: 10.2522/ptj.20130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas Vega S, Strüder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121:59–65. doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- 41.Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J. Serial measurement of vascular endothelial growth factor and transforming growth factor beta1 in serum of patients with acute ischemic stroke. Stroke. 2000;31:1863–1870. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- 42.Cobbs CS, Chen J, Greenberg DA, Graham SH. Vascular endothelial growth factor expression in transient focal cerebral ischemia in the rat. Neurosci Lett. 1998;249:79–82. doi: 10.1016/s0304-3940(98)00377-2. [DOI] [PubMed] [Google Scholar]
- 43.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 44.Lee SC, Lee KY, Kim YJ, Kim SH, Koh SH, Lee YJ. Serum VEGF levels in acute ischaemic strokes are correlated with long-term prognosis. Eur J Neurol. 2010;17:45–51. doi: 10.1111/j.1468-1331.2009.02731.x. [DOI] [PubMed] [Google Scholar]
- 45.Lennmyr F, Ata KA, Funa K, Olsson Y, Terent A. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. J Neuropathol Exp Neurol. 1998;57:874–882. doi: 10.1097/00005072-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Matsuo R, Ago T, Kamouchi M, Kuroda J, Kuwashiro T, Hata J, Sugimori H, Fukuda K, et al. Clinical significance of plasma VEGF value in ischemic stroke - research for biomarkers in ischemic stroke (REBIOS) study. BMC Neurol. 2013;13:32–39. doi: 10.1186/1471-2377-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26:943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- 48.Nurkovic JS, Volarevic V, Lako M, Armstrong L, Arsenijevic N, Stojkovic M. Aging of stem and progenitor cells: mechanisms, impact on the therapeutic potential and rejuvenation. Rejuvenation Res. 2016;19:3–12. doi: 10.1089/rej.2015.1676. [DOI] [PubMed] [Google Scholar]
- 49.Doll DN, Barr TL, Simpkins JW. Cytokines: their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis. 2014;5:294–306. doi: 10.14336/AD.2014.0500294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma S, Yang B, Strong R, Xiao Xi XP, Brenneman M, Grotta JC, Aronowski J, Savitz SI. Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. J Neurosci Res. 2014;88:2869–2876. doi: 10.1002/jnr.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao L, Zou Z, Tian H, Zhang Y, Zhou H, Liu L. Stem cell-based therapies for ischemic stroke. Biomed Res Int. 2014;2014:468748. doi: 10.1155/2014/468748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalluri HS, Dempsey RJ. Growth factors, stem cells, and stroke. Neurosurg Focus. 2008;24:3–8. doi: 10.3171/FOC/2008/24/3-4/E13. [DOI] [PubMed] [Google Scholar]
- 53.Lindvall O, Kokaia Z. Stem cell research in stroke: how far from the clinic? Stroke. 2011;42:2369–2375. doi: 10.1161/STROKEAHA.110.599654. [DOI] [PubMed] [Google Scholar]