ABSTRACT
Ten patients with craniopharyngioma treated for the first time when younger than 18 were included. This study reviews the visual outcomes and provides information on visual field (VF) and optical coherence tomography (OCT) examination of craniopharyngioma. The best kappa concordance coefficients between VF and OCT parameters of atrophy were obtained for the ganglion cell (GC) thickness and the mean retinal nerve fibre layer (RNFL) thickness. The agreement between GC colour maps and VF defects was good. Optic nerve compression may be detected by RNFL measurement and GC analysis, and this may be valuable to predict visual recovery and in uncooperative patients to evaluate visual damage.
Keywords: Craniopharyngioma, ganglion cell colour map, optical coherence tomography, optic neuropathy children, visual field
INTRODUCTION
Craniopharyngioma is a tumour that develops from squamous cell nests derived from the primitive Rathke pouch.1–5 It is the most common suprasellar tumour in childhood and the second most common, after pituitary adenoma, in adults.1 Its incidence has been estimated to be of 1.4 cases per million children per year.6 Despite its histological classification as a benign tumour, it may be locally aggressive, with frequent extension and damage to adjacent structures and a high recurrence rate.4,5
Patients with craniopharyngioma are often diagnosed relatively late after the onset of the first symptoms.1 Children present with systemic symptoms more often than adults; these symptoms may include headache and nausea due to raised intracranial pressure, as well as diabetes insipidus, growth retardation, and delayed sexual development due to the disruption of the hypothalamic-pituitary pathway.1,5,7 Some patients present with visual loss as a result of compression of the visual pathways, with blurred vision being the most common visual complaint.1–7
Computed tomography (CT) usually shows an heterogeneous suprasellar mass with calcification and extension into the chiasm or third ventricle.1 Magnetic resonance imaging (MRI) can better determine tumour extension1 and is recommended for follow-up, although ophthalmic examinations are also considered useful for early detection of anterior visual pathway compression by recurrent tumour growth.1 However, children affected may find it hard to perform visual fields (VF). Furthermore, VF damage reflects impairment in optic nerve function that may or may not improve after treatment. In other compressive neuropathies, optical coherence tomography (OCT) has been shown to detect anatomical damage to the optic pathway and provide prognostic information.8 It requires only limited cooperation on the part of the patient in order to provide reliable measurements and therefore may be useful in patients unable to perform reliable VF.
Temporal retinal nerve fibre layer (RNFL) loss reflects damage to the papillomacular bundle, and it has been reported to be related to visual acuity loss.9,10 Mean RNFL loss is more often associated with visual field damage. Several studies have found that the ganglion cell layer (GC) is more strongly correlated with visual field defects than with the RNFL thickness.11
The purpose of this paper is to review visual outcomes in children with craniopharyngioma and to evaluate the correlation between visual field testing and OCT, as well as to evaluate the role of OCT for the prediction of visual outcomes after surgery.
MATERIALS AND METHODS
The charts of patients with craniopharyngioma who had been treated for the first time when younger than 18 were reviewed and considered for inclusion in the study. This first therapeutic procedure was not always performed in our hospital; therefore, symptoms and signs at presentation were not always available. This study was started when the Cirrus-HD model 4000 (Carl Zeiss Meditec) became available in our centre in 2010. Therefore, the first time the patients could be explored with this device will be considered the baseline visit.
These patients are followed in the neuro-ophthalmology department. At every visit, an extensive ophthalmic examination is performed, including at least best-corrected visual acuity (BCVA), pupillary responses, external ocular motor examinations, and anterior and posterior pole biomicroscopy and funduscopy. When patients are 7 or older, automated perimetry (Humphrey Field Analyser 750, Zeiss/Humphrey Systems, Dublin, CA) is performed, usually 24-2 tests, SITA (Swedish interactive threshold algorithm) standard strategy. Visual fields are considered reliable when false positives and fixation losses are lower than 10%. Type of visual field defect and mean deviation (MD) were recorded. When BCVA is less or equal to counting fingers, perimetry is not performed. In these cases, MD was considered −30 dB for statistical analysis. BCVA was considered normal if ≥0.9 (decimal scale).
OCT scanning was performed in patients at least 4 years old, after mydriasis with 1% cyclopentolate, using Cirrus-HD model 4000 (Carl Zeiss Meditec). An internal fixation target was used whenever possible. To evaluate the RNFL thickness, macular thickness, and the GC, the optic disc cube protocol and the macular cube 512 × 128 acquisition protocols were performed. Mean average and temporal quadrant RNLF thickness, central foveal thickness, the total macular volume in the 6-mm ETDRS (Early Treatment Diabetic Retinopathy Study) ring, and the average GC thickness were recorded.
The Cirrus OCT compares the values for each exploration with the values of a built-in normative database; however, this database does not include children. Thus, OCT in children is harder to interpret; in daily practice, most physicians change the date of birth in order to have something with which to compare the values obtained. However, several groups have published OCT values for normal children. We compared the values for each patient with those of one such study designed to compile a normative database for healthy children in our country.12,13 As in adults, we considered values under 5th percentile (P5) and 1st percentile (P1) to be abnormally reduced.
The study was approved by our institutional review board and adhered to the tenets of the Declaration of Helsinki. Statistical analysis was performed using the Statistical Package for Social Sciences software (version 20.0; IBM, Armonk, NY). Agreement between affected visual fields and decreased OCT parameters was evaluated with the kappa coefficient.
RESULTS
Ten patients with craniopharyngioma were included in the study. There were four girls and six boys, with a median age at the time of the initial therapeutic procedure of 5 years (range from 3 to 16 years). All children underwent surgery for the removal of the tumour; case 8 also received adjuvant radiotherapy. So far, they have required between one and four procedures to control the tumour (mean of two procedures per child). Three cases needed additional surgical interventions to treat complications derived from the tumour (intracranial hypertension) or from surgery (cerebrospinal fluid fistula) (Table 1).
TABLE 1. Baseline characteristics of study patients.
| Child | Sex | Age at time of first surgery (years) | Time from surgery to the last visit (years) | Total number of surgical procedures | Systemic complications |
|---|---|---|---|---|---|
| 1 | Female | 3 | 4 | 3 | Obesity Panhypopituitarism |
| 2 | Male | 3 | 12 | 2 (plus a third one due to IH) | Obesity Diabetes insipidus ACTH deficit |
| 3 | Male | 4 | 3 | 2 | Short stature (GH) Diabetes insipidus |
| 4 | Female | 4 | 15 | 1 (plus a second one due to IH) | Panhypopituitarism Obesity |
| 5 | Female | 5 | 9 | 4 | Panhypopituitarism |
| 6 | Male | 5 | 0.5 | 1 | |
| 7 | Male | 5 | 0.5 | 2 | Short stature |
| 8 | Female | 10 | 8 | 1 (plus radiotherapy) | Panhypopituitarism |
| 9 | Male | 10 | 22 | 1 | Obesity Panhypopituitarism |
| 10 | Male | 16 | 1 | 2 (plus a third one due to CF fistula) | Panhypopituitarism |
IH = intracranial hypertension; CF = cerebrospinal fluid; ACTH = adrenocorticotropic hormone; GH = growth hormone.
Although two cases have been diagnosed and treated in the last year, the median time from the first surgical procedure to the last visit was 6 years. Tables 2 and 3 show the ophthalmic assessment at the last follow-up visit. There were no visual fields available for two children because at the last follow-up visit they were still younger than 7. Five eyes could not be tested due to amaurosis. MD ranged from −3.04 to −30 dB, with a mean MD for the 14 tested eyes of −16.53 dB (SD = 11.58 dB). Table 3 shows OCT parameters in the last visit. Only 3 out of 16 eyes had a mean RNFL thickness above the 1st percentile (P1 = 78.6 µm; P5 = 82.4 µm). Thus, most eyes had severe optic nerve damage according to the Cirrus OCT. However, only 8 out of 16 had a temporal quadrant RNFL thickness below P1 (P1 = 48.4 µm; P5 = 51.8 µm).12 The 18 eyes in which macular examination was performed showed a foveal thickness above P1 and only 3 eyes had values below P5 (P1 = 206 µm; P5 = 220.1 µm). The macular volume was below P1 in 6 eyes and below P5 in 11 eyes (P1 = 9.1 mm3; P5 = 9.4 mm3).12 Macular GC thickness was below P5 and P1 in 12 out of 16 explored eyes (P1 = 72 µm; P5 = 75 µm).13 Kappa concordance coefficients between visual fields (affected or not) and OCT parameters below normal values were statistically significant for the GC thickness (kappa = 1; p < 0.001 for P1 and P5) and the mean RNFL thickness (kappa = 0.632; p = 0.011 for P1 and P5).
TABLE 2. Ophthalmic examination at the last visit, including visual field results.
| BCVA |
Pallor disc |
VF RE |
VF LE |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Child | RE | LE | RAPD | Strabismus | RE | LE | MD | Defect | MD | Defect |
| 1 | 0.6 | 0.025 | LE | Exotropia | Diffuse | Diffuse | −18.23 | Temporal hemianopia | NP | |
| 2 | 0.6 | 0.5 | — | Orthotropia | No pallor | No pallor | −3.71 | Normal | −3.75 | Normal |
| 3 | 1 | 0.13 | LE | Exotropia | Temporal | Diffuse | −15.71 | Temporal hemianopia | NP | |
| 4 | 0.7 | 0.7 | — | Orthotropia | Temporal | Band atrophy | −7.12 | Left hemianopia | −6.17 | Left hemianopia |
| 5 | 0.3 | 0 | LE | Esotropia | Temporal | Diffuse | −20.42 | Temporal | NP | |
| 6 | 1 | 1 | — | Orthotropia | No | No | NP | NP | ||
| 7 | 0.9 | 0.4 | — | Orthotropia | No | Diffuse | NP | NP | ||
| 8 | 1 | 0.025 | LE | Orthotropia | Temporal | Diffuse | −3.04 | Temporal | −26.71 | Diffuse |
| 9 | 0.006 | 0.0125 | RE | Exotropia | Diffuse | Diffuse | NP | NP | ||
| 10 | 1 | 1 | — | Orthotropia | Temporal | Temporal | −5.40 | Left hemianopia | −4.15 | Left hemianopia |
BCVA = best-corrected visual acuity; RE = right eye; LE = left eye; RAPD = relative afferent pupillary defect; MD = mean deviation; NP = not performed.
TABLE 3. OCT parameters in the last visit.
| RNFL thickness (μurn) |
Macular parameters |
GC thickness |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RE |
LE |
RE |
LE |
(μm) |
||||||
| Child | Mean | Temporal | Mean | Temporal | MV (mm3) | FT (μm) | MV (mm3) | FT (μm) | RE | LE |
| 1 | 54 | 36 | 57 | 49 | 8.6 | 225 | 8.3 | 223 | 54 | 48 |
| 2 | 78 | 61 | 86 | 70 | 9.9 | 289 | 10.1 | 285 | 78 | 85 |
| 3 | 67 | 42 | 55 | 53 | 9.8 | 264 | 8.7 | 259 | 67 | 53 |
| 4 | 51 | 50 | 60 | 37 | 9.2 | 231 | 9.3 | 237 | 70 | 67 |
| 5 | 76 | 39 | 53 | 54 | 9.2 | 243 | 7.6 | 216 | 64 | 42 |
| 6 | 9.9 | 297 | 10.1 | 279 | 79 | 88 | ||||
| 7 | 97 | 56 | 85 | 77 | 9 | 273 | 9 | 239 | ||
| 8 | 76 | 36 | 72 | 35 | 9.4 | 243 | 9.3 | 235 | 69 | 67 |
| 9 | ||||||||||
| 10 | 64 | 29 | 77 | 44 | 8.7 | 215 | 9.5 | 220 | 55 | 63 |
RNFL = retinal nerve fibre layer; RE = right eye; LE = left eye; FT = foveal thickness; GC = ganglion cell.
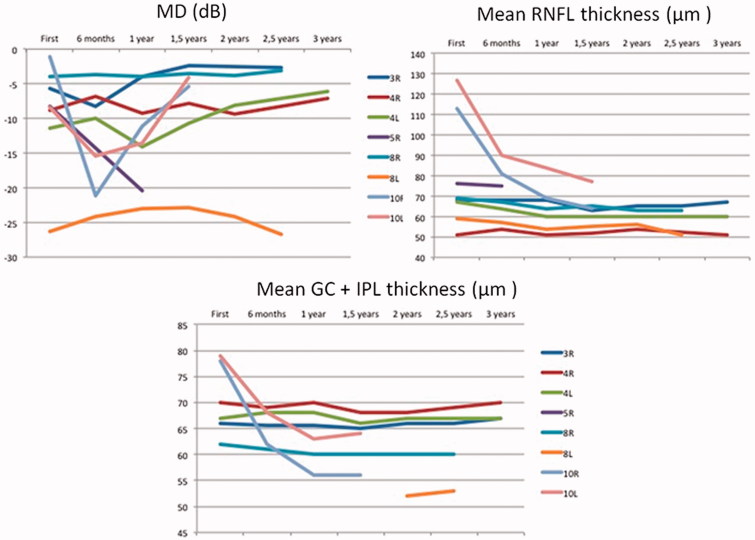
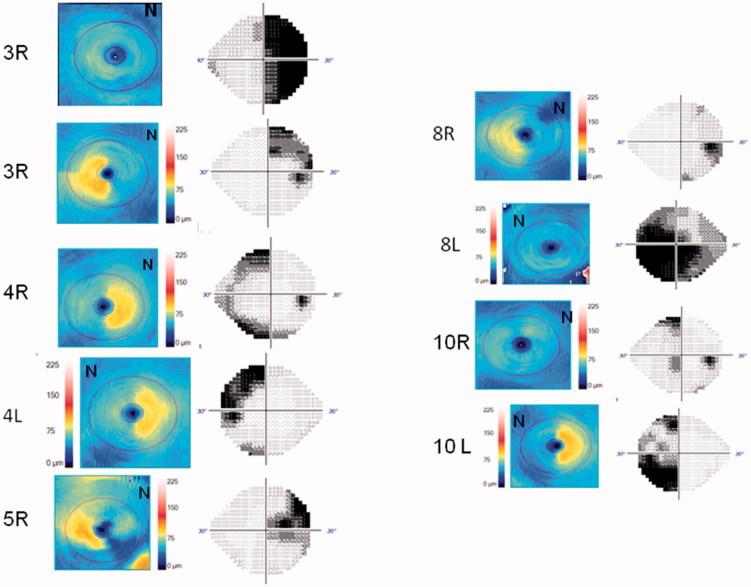
At least three reliable sequential visual fields could be performed in eight eyes of five children. Visual fields are difficult to perform and the MD values varied greatly, even in patients in whom there was no tumour recurrence. The variance of the VF MD of each eye during follow-up ranged from 0.124 to 7.441 in those without recurrence and from 26.184 to 75.381 in those with recurrences (Figure 1). OCT values fluctuated less. In patient 10, who presented with papilloedema, RNFL thickness decreased 49 and 50 µm in each eye, respectively. The variance ranged from 0.5 to 7.9 µm in all other cases. GC thickness values also showed less variation between visits. In case 10, GC thickness decreased 22 and 16 µm in each eye, respectively. The variance ranged from 0 to 0.81 in all other cases. Figure 1 shows the evolution of the MD of the visual fields and of the mean RNFL and GC thicknesses in those affected eyes with both tests available. Figure 2 shows the correspondence between VF and GC colour map. The agreement between GC colour maps and VF for the detection of diffuse or hemianopic defects was good. The kappa concordance coefficient was 0.667 (p = 0.014).
FIGURE 1.
Visual field and RNFL and GC thickness changes during the follow-up period of three years. Case 5 and 10 showed significant worsening of their visual fields due to recurrent tumoral growth. RNFL and GC thickness remained stable save for case 10 who initially showed papilledema that disappeared after surgery.
FIGURE 2.
Correspondance of VF with its GC color-map to identify diffuse or hemianopic defects.
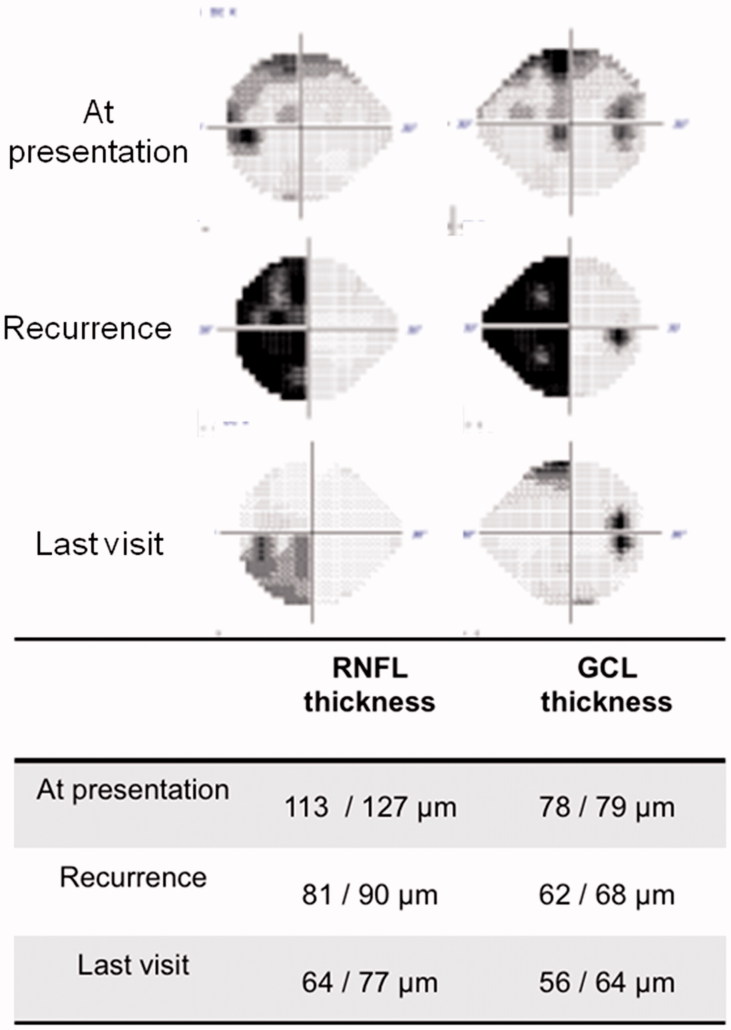
In case 10, Cirrus OCT was available before and after surgical treatment of a tumour recurrence. RNFL thickness was 81 µm in the right eye and 90 µm in the left eye, and the patient recovered most of the VF defect, as shown in Figure 3. Case 3 also had a recurrence before Cirrus OCT was available. The left eye was already blind at that time with a Stratus-OCT RNFL thickness of 47 µm; vision did not improve after surgery. The right eye showed a temporal hemianopia (−15.71 dB) with a Stratus-OCT RNFL thickness before surgery of 79 µm. After surgery, the VF only showed a mild superior temporal defect (−2.68 dB).
FIGURE 3.
Case 10 presented with severe left homonimous hemianopia that recovered after surgery as predicted by his OCT study.
DISCUSSION
Optic neuropathy is a frequent result of craniopharyngioma in children despite the low rate of cases presenting with visual complaints.7,14,15 Optic nerve damage may be due to chronic papilloedema, radiotherapy, direct compression, or manipulation during surgery. Direct compression is considered the main cause of visual loss.16
OCT in sellar tumours may predict visual recovery after surgery. In patients with visual field defects, if the RNFL thickness is not under normal values, visual recovery is frequent. However, if the RNFL thickness is already decreased before surgery, the chances of visual recovery are lower. The explanation for this may be that compression initially leads to impaired function, which is reflected as visual field damage. Retinal ganglion cell death occurs after prolonged compression, and there is a time delay before RNFL thinning is detected on OCT. Thus, RNFL thinning reflects irreversible ganglion cell damage. This predictive role has been established in adults with pituitary adenoma.8 Although OCT may also have a similar predictive role in children, craniopharyngioma may behave differently than adenomas because complete resection is difficult and the risk of recurrence is high (34–75%).17–21 In this case series, pre-treatment OCT was available in four subjects. Two were children too young to perform visual fields. In the two patients who had reliable presurgical VF and OCT, visual fields improved in both eyes in patient 10 and in only one eye of patient 3. This eye was the only one that had severe RNFL damage on OCT.
After surgery, OCT reflects the damage sustained by the optic nerve and is correlated with visual acuity and visual fields. Bialer et al. evaluated 20 children with craniopharyngioma with OCT.14 The RNFL thickness was evaluated in 16 cases, with follow-up examinations available in 10 cases.22–24 They found a correlation between RNFL thickness and BCVA; RNFL thickness was also significantly thinner in those eyes with visual field defects.14 In Bialer et al.'s study, among the 15 children who were able to perform visual fields, 4 showed bilateral temporal defects, 3 unilateral temporal defects, 1 inferior homonimous quadrantopia, and 1 a severe unilateral defect.14 These were the visual defects that children showed at presentation; therefore, they were less severe than our results after a mean of 5 years of follow-up. In our series, 60% of eyes had a BCVA below 0.5, although only in 20% of the children were both eyes similarly affected. This rate is similar to that reported previously.1,14,25 More frequent than decreased acuity was the presence of a visual field defect, which is consistent with the higher prevalence of decreased mean RNFL thickness as compared with temporal RNFL damage that we observed. In our case series, only one out of eight tested cases showed normal visual fields. The most common defect was a temporal hemianopia in one eye and a diffuse and severe loss in the fellow eye (50%), which would be consistent with previous studies that report bitemporal hemianopia to be the most common initial visual field defect.1,2 The second-most frequent defect was homonymous hemianopia (25%).
Recently, several studies have focused on GC analysis in sellar tumours. Moon et al. studied the relationship between visual field recovery and changes in the retinal GC layer in 19 adults with sellar tumours, including two patients with craniopharyngioma. They found that the GC layer was more strongly correlated with visual field defects than mean RNFL thickness, and they concluded that the selective measurement of the GC layer can be a useful parameter for evaluating the damage induced by chiasmal compression.11 Although our case series is small and therefore our data should be interpreted with caution, in our study visual field defects were correlated with mean RNFL thickness and even more strongly with GC layer thickness.
Trans-synaptic retrograde degeneration of retinal ganglion cells following retrogeniculate visual pathway lesions has been reported.26,27 Initially, progressive thinning of the RNFL following occipital lobe/optic radiation damage due to stroke was described.27 Thinning of the RNFL was asymmetric and topographically congruent with lesions of the visual pathway located between the chiasm and the occipital lobe.26,28–31 There also is also evidence supporting the correlation between the pattern of both RNFL loss and macular GC thinning and the type of visual field defect in other optic neuropathies.32–36 Kanamori et al. showed how colour-coded maps could help to visualize the pattern of GC thinning in four cases of optic tract syndrome.31 Our colour maps (Figure 2), also reflected a good topographic concordance between visual field damage and the areas represented in cold colours (blue). Only in two eyes was the visual field defect milder and more limited than would be expected in the presence of diffuse GC atrophy (1R and 10R). Since OCT can be performed in younger children than visual fields, and macular protocols are easier to perform than optic nerve protocols, these maps would allow us to estimate visual field damage in young children.
Figure 1 shows some examples of visual field and RNFL and ganglion cell thickness changes during the follow-up period of 3 years. During follow-up, cases 5 and 10 showed significant worsening of their visual fields, which prompted us to repeat MRI. In both cases, recurrent tumour growth was detected. Case 10 was operated quickly and, as predicted by the analysis of the RNFL thickness, recovered most of the visual field (Figure 3). Case 5 has not received further treatment yet. It must be borne in mind that as mentioned before, there is a time delay between retinal GC death and detection of RNFL or ganglion cell layer thinning on OCT. Thus, correlations between visual damage and OCT parameters in acute phases of the disease are not as clear as in stable nerves.
In summary, young children who may not perform reliable visual fields can be scanned with OCT. Optic nerve damage may be detected both by RNFL measurement and GC analysis, and this information may be valuable in patients still untreated to predict visual recovery and in uncooperative patients after surgery to estimate visual damage. Although we consider that visual fields are irreplaceable to detect compression of the visual pathway in time to avoid irreversible damage, OCT may be a valid substitute when patients are unable to perform perimetry. Therefore, both visual fields and OCT should be performed in patients with craniopharyngioma whenever possible. Although the absence of a built-in normative database for children in commercially available OCT scanners can represent a problem for interpreting OCT examinations, normative data are now available in the literature.
ACKNOWLEDGMENTS
The following departments were involved in performance of the study: Department of Ophthalmology, La Paz University Hospital, and Department of Ophthalmology, Ramón y Cajal University Hospital.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Note: Figures 1 and 2 of this article are available in colour online at www.informahealthcare.com/oph.
REFERENCES
- 1.Chen C, Okera S, Davies PE, Selva D, Crompton JL. Craniopharyngioma: a review of long-term visual outcome. Clin Experiment Ophthalmol 2003;31:220–228 [DOI] [PubMed] [Google Scholar]
- 2.Karavitaki N, Brufani C, Warner JT, Adams CBT, Richards P, Ansorge O, Shine B, Turner HE, Wass JA. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol 2005;62:397–409 [DOI] [PubMed] [Google Scholar]
- 3.Kennedy HB, Smith RJ. Eye signs in craniopharyngioma. Br J Ophthalmol 1975;59:689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poretti A, Grotzer MA, Ribi K, Schönle E, Boltshauser E. Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol 2004;46:220–229 [DOI] [PubMed] [Google Scholar]
- 5.Clark AJ, Cage TA, Aranda D, Parsa AT, Auguste KI, Gupta N. Treatment-related morbidity and the management of pediatric craniopharyngioma: a systematic review. J Neurosurg Pediatr 2012;10:293–301 [DOI] [PubMed] [Google Scholar]
- 6.Haupt R, Magnani C, Pavanello M, Caruso S, Dama E, Garrè ML. Epidemiological aspects of craniopharyngioma. J Pediatr Endocrinol Metab 2006;19:289–293 [PubMed] [Google Scholar]
- 7.Rath SR, Lee S, Kotecha RS, Taylor M, Junckerstorff RC, Choong CS. Childhood craniopharyngioma: 20-year institutional experience in Western Australia. J Paediatr Child Health 2013;49:403–408 [DOI] [PubMed] [Google Scholar]
- 8.Danesh-Meyer H V, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci 2008;49:1879–1885 [DOI] [PubMed] [Google Scholar]
- 9.Noval S, Visa J, Contreras I. Visual field defects due to optic disk drusen in children. Graefes Arch Clin Exp Ophthalmol 2013;251:2445–2450 [DOI] [PubMed] [Google Scholar]
- 10.Rajjoub RD, Trimboli-Heidler C, Packer RJ, Avery RA. Reproducibility of retinal nerve fiber layer thickness measures using eye tracking in children with nonglaucomatous optic neuropathy. Am J Ophthalmol 2015;159:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon CH, Hwang SC, Ohn Y-H, Park TK. The time course of visual field recovery and changes of retinal ganglion cells after optic chiasmal decompression. Invest Ophthalmol Vis Sci 2011;52:7966–7973 [DOI] [PubMed] [Google Scholar]
- 12.Barrio-Barrio J, Noval S, Galdós M, Ruiz-Canela M, Bonet E, Capote M, Lopez M. Multicenter Spanish study of spectral-domain optical coherence tomography in normal children. Acta Ophthalmol 2013;91:56–63 [DOI] [PubMed] [Google Scholar]
- 13.Galdos M, Barrio-Barrio J, Noval S, Ruiz-Canela M, Bonet E, Capote M, Garamendi E. Multicenter macular ganglion cell analysis: normative paediatric reference range. Acta Ophthalmol 2014;92:326–327 [DOI] [PubMed] [Google Scholar]
- 14.Bialer OY, Goldenberg-Cohen N, Toledano H, Snir M, Michowiz S. Retinal NFL thinning on OCT correlates with visual field loss in pediatric craniopharyngioma. Can J Ophthalmol 2013;48:494–499 [DOI] [PubMed] [Google Scholar]
- 15.Harbert MJ, Yeh-Nayre LA, O'Halloran HS, Levy ML, Crawford JR. Unrecognized visual field deficits in children with primary central nervous system brain tumors. J Neurooncol 2012;107:545–549 [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg-Cohen N, Ehrenberg M, Toledano H, Kornreich L, Snir M, Yassur I, Cohen IJ, Michowiz S. Preoperative visual loss is the main cause of irreversible poor vision in children with a brain tumor. Front Neurol 2011;2:62. doi: 10.3389/fneur.2011.00062. eCollection 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Güemes Hidalgo M, Muñoz Calvo MT, Fuente Blanco L, Villalba Castaño C, Martos Moreno GA, Argente J. Secuelas endocrinológicas en niños y adolescentes supervivientes de tumores del sistema nervioso central tras 5 años de seguimiento. An Pediatría 2014;80:357–364 [DOI] [PubMed] [Google Scholar]
- 18.Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Tyler-Kabara EC, Wang EW, Snyderman CH. Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg 2013;119:1194–1207 [DOI] [PubMed] [Google Scholar]
- 19.Clark AJ, Cage TA, Aranda D, Parsa AT, Sun PP, Auguste KI, Gupta N. A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv Syst 2013;29:231–238 [DOI] [PubMed] [Google Scholar]
- 20.Jo KW, Shin HJ, Kong DS, Seol H-J, Nam D-H, Lee J-I. Treatment outcomes of pediatric craniopharyngioma : a 15-year retrospective review of 35 cases. J Korean Neurosurg Soc 2012;52:37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalapurakal JA, Goldman S, Hsieh YC, Tomita T, Marymont MH. Clinical outcome in children with craniopharyngioma treated with primary surgery and radiotherapy deferred until relapse. Med Pediatr Oncol 2003;40:214–218 [DOI] [PubMed] [Google Scholar]
- 22.Rebolleda G, González-López JJ, Muñoz-Negrete FJ, Oblanca N, Costa-Frossard L, Álvarez-Cermeño JC. Color-code agreement among stratus, cirrus, and spectralis optical coherence tomography in relapsing-remitting multiple sclerosis with and without prior optic neuritis. Am J Ophthalmol 2013;155:890–897 [DOI] [PubMed] [Google Scholar]
- 23.Pierro L, Gagliardi M, Iuliano L, Ambrosi A, Bandello F. Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Invest Ophthalmol Vis Sci 2012;53:5912–5920 [DOI] [PubMed] [Google Scholar]
- 24.Costa-Cunha LVF, Cunha LP, Malta RFS, Monteiro MLR. Comparison of Fourier-domain and time-domain optical coherence tomography in the detection of band atrophy of the optic nerve. Am J Ophthalmol 2009;147:56–63 [DOI] [PubMed] [Google Scholar]
- 25.Drimtzias E, Falzon K, Picton S, Jeeva I, Guy D, Nelson O, Simmons I. The ophthalmic natural history of paediatric craniopharyngioma: a long-term review. J Neurooncol 2014;120:651–656 [DOI] [PubMed] [Google Scholar]
- 26.Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 2009;132:628–634 [DOI] [PubMed] [Google Scholar]
- 27.Jindahra P, Petrie A, Plant GT. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain 2012;135:534–541 [DOI] [PubMed] [Google Scholar]
- 28.Nakamura M, Ishikawa-Tabuchi K, Kanamori A, Yamada Y, Negi A. Better performance of RTVue than Cirrus spectral-domain optical coherence tomography in detecting band atrophy of the optic nerve. Graefes Arch Clin Exp Ophthalmol 2012;250:1499–1507 [DOI] [PubMed] [Google Scholar]
- 29.Tatsumi Y, Kanamori A, Kusuhara A, Nakanishi Y, Kusuhara S, Nakamura M. Retinal nerve fiber layer thickness in optic tract syndrome. Jpn J Ophthalmol 2005;49:294–296 [DOI] [PubMed] [Google Scholar]
- 30.Ostri C, Damgaard B, Hamann S. Optical coherence tomography documenting retinal nerve fiber loss in traumatic optic chiasmal syndrome. Acta Ophthalmol 2012;90:792–794 [DOI] [PubMed] [Google Scholar]
- 31.Kanamori A, Nakamura M, Yamada Y, Negi A. Spectral-domain optical coherence tomography detects optic atrophy due to optic tract syndrome. Graefes Arch Clin Exp Ophthalmol 2013;251:591–595 [DOI] [PubMed] [Google Scholar]
- 32.Danesh-Meyer H V, Carroll SC, Foroozan R, Savino PJ, Fan J, Jiang Y, Vander Hoorn S. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Invest Ophthalmol Vis Sci 2006;47:4827–4835 [DOI] [PubMed] [Google Scholar]
- 33.Weinreb RN, Shakiba S, Sample PA, Shahrokni S, van Horn S, Garden VS, Asawaphureekorn S, Zangwill L. Association between quantitative nerve fiber layer measurement and visual field loss in glaucoma. Am J Ophthalmol 1995;120:732–738 [DOI] [PubMed] [Google Scholar]
- 34.Iester M, Swindale N V, Mikelberg FS. Sector-based analysis of optic nerve head shape parameters and visual field indices in healthy and glaucomatous eyes. J Glaucoma 1997;6:370–376 [PubMed] [Google Scholar]
- 35.Bosworth CF, Sample PA, Williams JM, Zangwill L, Lee B, Weinreb RN. Spatial relationship of motion automated perimetry and optic disc topography in patients with glaucomatous optic neuropathy. J Glaucoma 1999;8:281–289 [PubMed] [Google Scholar]
- 36.Yamashita T, Miki A, Iguchi Y, Kimura K, Maeda F, Kiryu J. Reduced retinal ganglion cell complex thickness in patients with posterior cerebral artery infarction detected using spectral-domain optical coherence tomography. Jpn J Ophthalmol 2012;56:502–510 [DOI] [PubMed] [Google Scholar]