Abstract
BACKGROUND: The prognostic value of tumor-infiltrating lymphocytes (TILs) in head and neck squamous cell carcinoma (HNSCC) remains controversial. Additionally, there is no standardized approach or cutoff value for evaluating TIL levels. The aim of this study was to establish a feasible method and criterion to assess TIL levels for future clinical practice and research use and to explore the relationship between TIL levels and prognosis. PATIENTS AND METHODS: This retrospective cohort study reviewed the records and pathological sections of 202 patients with HNSCC who were surgically treated at Beijing Stomatological Hospital, Capital Medical University, from January 1998 to January 2011. The predictor variable was the TIL level. The main outcome assessment parameters were disease-free survival (DFS) and disease-specific survival (DSS). RESULT: The T stage (P = .008), smoking history (P = .042), alcohol history (P = .048), need for radiotherapy (P = .012) and microscopic extracapsular spread (ECS) (P = .012) were associated with the TIL level. A cutoff value equal to 70% could be taken as a threshold for TIL assessment, with a TIL level higher than 70% associated with a better prognosis (DFS rate: 51.9%, P = .018; DSS rate: 59.3%, P = .049). The Cox regression model showed that the TIL level was an independent prognostic factor for DFS (hazard ratio (HR): 0.786, 95% CI: 0.618-0.999, P = .049). CONCLUSION: The TIL level is closely related to the prognosis of patients with HNSCC. A threshold value of 70% is appropriate for TIL assessment, as patients with a TIL level higher than 70% show a better prognosis. Thus, the TIL level might serve as an independent predictor for HNSCC recurrence.
Introduction
Approximately 635,000 new cases of head and neck cancer are diagnosed each year worldwide, and over 90% of cases are squamous cell carcinoma [1]. The treatments for head and neck squamous cell carcinoma (HNSCC) are improving; however, despite surgery with or without combined chemo-radiotherapy, the 5-year survival rate and the recurrence rate remain poor [2]. Thus, novel variables that can provide valuable information about prognosis are urgently needed for HNSCC.
Tumor-infiltrating lymphocytes (TILs) have been used to predict the prognosis of solid tumors for many years, as these cells serve as a readout of the interaction between the immune system and cancer cells [3]. There is emerging evidence to support the positive role of TILs in HNSCC, but there remain some controversial issues, and no standard process has been established for the evaluation of TILs. The immunoediting theory distinguishes the relationship between tumor cells and immune cells based on three phases: elimination, equilibrium and escape. This relationship persists for the entire duration of cancer progression [4]. Based on this theory, the immune system can have a positive effect by eliminating tumor cells, although the immune microenvironment may promote malignant cells to become more aggressive and capable of escaping immunological surveillance [5], [6]. Although current studies have presented arguments about the function of TILs, cumulative data suggest that these mononuclear cells are associated with favorable outcomes in squamous cell carcinoma and the response to chemo-radiotherapy [7], [8].
To explore the relationship between TILs and malignant tumors, formal approaches to evaluate the level of TILs exist in many fields, with the exception of HNSCC, and the TIL level has been applied in the clinic and in research trials [9], [10]. Some pathologists even believe that TILs possess more predictive value than conventional TNM staging.
In regards to HNSCC, the literature on TILs provides limited evidence of their prognostic role, and many findings have been based on small sample sizes [11]. Moreover, the different evaluation criteria used make the results of different studies incomparable. Due to these issues, an acceptable method to assess the level of TILs in HNSCC is urgently needed. Based on the methods used to evaluate TILs in other solid tumors (e.g., breast cancer, colorectal cancer, etc.) [9], [10], [12], in this study, we sought to establish a standardized procedure for measuring the TIL level and confirm the prognostic role of TIL levels in HNSCC.
In this study, we used hematoxylin and eosin (H&E) staining to observe and assess the infiltration of lymphocytes in the tumor stromal area and then analyze the relationship between TIL level and recurrence or survival rate. We focused on establishing a feasible approach and criterion to evaluate TILs, with the hope that the devised method can be used in common clinical practice and research for pathological diagnosis and prognostic adjudication.
Methods
Patient Samples
This research was conducted in full accordance with ethical principles, including the World Medical Association's Declaration of Helsinki (2002 version) and with approval of the Institutional Review Board of the Beijing Stomatological Hospital of Capital Medical University. Patient inclusion criteria included the following: (1) patients with a pathological diagnosis of squamous cell carcinoma; (2) patients with a primary tumor without evidence of distant metastasis; (3) patients who were treated primarily with surgery; (4) patients with no previous treatment; (5) patients with complete clinicopathological data and available tissue specimens; and (6) patients with a tumor located in the tongue, lower gingiva, upper gingiva, buccal mucosa, floor of the mouth, oropharynx, or hard palate. The exclusion criteria included preoperative chemotherapy or radiotherapy, failure to undergo surgery and the inability to obtain pathological slices. The medical records of patients who met the inclusion criteria between January 1998 and January 2011 for HNSCC at the Department of Oral and Maxillofacial-Head and Neck Oncology, Beijing Stomatological Hospital, Capital Medical University were retrospectively retrieved. All of the slides from the primary tumor sites for the selected patients were obtained.
Management
All patients underwent surgery for resection of the primary tumor. The surgical procedure was selected by the surgeon according to the tumor site and local practice. Standard surgery, including radical tumor resection, neck dissection and the reconstruction of tissue defects (as necessary), was performed. Local excision of the primary site was performed with a minimum margin of 15 mm. Patients who had UICC stages pT3 and 4, pN+, perineural invasion and/or vascular emboli were recommended to receive radiation treatment, whereas patients who presented with extracapsular spread and/or positive margins were recommended to receive chemo-radiotherapy.
All tissue samples were obtained during the surgery and stored in paraffin. Tissues embedded in paraffin were sectioned into 4 μm sections and placed on a slide. After applying a standardized process for H&E staining, the slides were observed under a microscope (Olympus BX61) and scored using the approach described below. Samples from patients with oropharyngeal squamous cell carcinoma were also stained to assess the p16 status.
Approach and Threshold
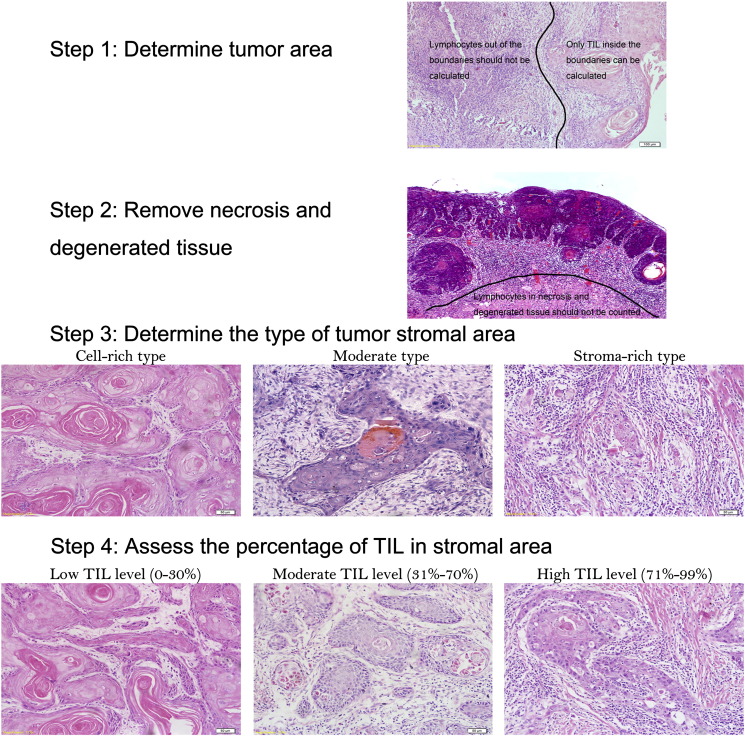
For this study, we analyzed a significant amount of literature on TILs, and the approach and criterion for evaluating the TIL level in the stromal area were as follows (Figure 1):
-
a.
Determine tumor area: We scanned the slide at low magnification and determined the boundaries of the tumor area. Only TILs inside the tumor boundaries were calculated.
-
b.
Remove necrotic and degenerated tissue: After sketching the boundaries, we removed necrotic and degenerated tissue inside the tumor area.
-
c.
Determine the type of tumor stromal area: The tumor stromal area can be divided into three categories: cell-rich, moderate and stroma-rich types. The cell-rich type is defined as more than 70% of the tumor area composed of carcinoma cells or tumor nest; when the stromal area is greater than 70%, it is classified as the stroma-rich type. The moderate type is defined as the intermediate situation.
-
d.
Assess TILs by quantifying only mononuclear cells (e.g., lymphocytes and plasmocytes); neutrophils in necrotic areas, dendritic cells and macrophages should be ruled out.
-
e.
Assess the percentage of TILs in the stromal area: We selected five typical views in each slide and assessed TILs score as the total stroma area divided by the lymphocyte-occupied area. The average value was considered the final score of the slide.
Figure 1.
Standardized approach of TIL evaluation for HNSCC patients.
The thresholds for evaluation of the TIL levels were established as follows: (1) 0% < low level ≤ 30%; (2) 30% < moderate level ≤ 70%; and (3) 70% < high level ≤ 99%.
The slides were first observed under a magnification of ×200 (ocular ×10, objective ×20). Then, the typical regions were used to represent the common situation inside the tumor and were evaluated under a magnification of ×400 (ocular ×10, objective ×40). Each slide was evaluated by two pathologists, and the results were considered only when the difference between observers was less than 10%. When the difference was more than 10% or divided into different TIL subgroups, the two pathologists determined the final score by consensus.
Statistical Analysis
The descriptive statistics used were frequencies, percentages, and means ± standard deviations. The outcome assessment parameters were disease-free survival (DFS) and disease-specific survival (DSS). DFS was defined as the time from the first day after treatment to disease progression or death from any cause. DSS was calculated from the time of the first operation to the time of death or last follow-up; patients who died from causes other than HNSCC were censored at the time of death. The Kaplan–Meier method was used to provide estimates of the DFS and DSS rates. Statistical significance was determined with the log-rank test. A Cox proportional hazards model (forward method) was used to adjust for the effects of other potential confounders. All of the tests were two-sided, and P values less than 0.05 were considered statistically significant. All of the statistical analyses were performed using SPSS software, version 17.0 for Windows (SPSS, Chicago, IL, USA).
Results
Patient Demographics
Between January 1998 and January 2011, a total of 202 successive patients with primary HNSCC were scheduled for radical surgery in our hospital. There were 134 (66.3%) males and 68 (33.7%) females. The mean age of the patients was 60.7 ± 13.5. The primary sites were the oral cavity in 191 patients (94.6%) and the oropharynx in 11 patients (5.4%). There was no statistically significant correlation between the natural history of tumors in HNSCC patients and the TIL level.
Of the patients, 93 (46.0%) were smokers, 104 (51.5%) were non-smokers, and five (2.5%) had missing information. Smoking history was associated with the TIL level (P = .042), as patients with a history of smoking had a lower TIL level. There were 58 drinkers (28.7%) and 139 non-drinkers (68.8%), and non-drinkers showed a higher TIL level (P = .048). The numbers of patients at each clinical stage were as follows: T1 (n = 32, 15.8%), T2 (n = 53, 26.2%), T3 (n = 38, 18.8%), and T4 (n = 79, 39.2%). The clinical stage was associated with the TIL level (P = .008). One hundred forty-two (70.3%) of 202 patients underwent radiotherapy after surgery, and 60 patients (29.7%) did not undergo radiotherapy. The history of radiation was associated with the TIL level (P = .012). An analysis of p16 expression in all 11 patients with oropharyngeal squamous cell carcinoma revealed that two cases were positive, eight cases were negative, and one case was missing. The p16 status was not associated with the TIL level (P = .406).
The pathological nodal status of the patients was as follows: pN0 (n = 120, 59.4%), pN1 (n = 30, 14.9%), and pN2 (n = 52, 25.7%). No statistical correlation was found between the pathological nodal status and the TIL level (P = .130). A total of 169 successive cases were available for the analysis of histologic signs of severity (perineural invasion, vascular emboli, diffuse infiltration) and extracapsular spread (ECS) status. Specifically, perineural invasion was present in 23 cases (11.4%) and absent in 146 cases (72.3%); vascular emboli were present in five cases (2.5%) and absent in 164 cases (81.2%); and diffuse infiltration was present in 32 cases (15.8%) and absent in 137 cases (67.8%). There were no significant differences between the TIL level and perineural invasion (P = .150), vascular emboli (P = .286) and diffuse infiltration (P = .261). Notably, the ECS status was related to the TIL level (P = .012). There were nine ECS-positive cases (4.5%) and 160 ECS-negative cases (79.2%), and the patients who presented with ECS had a significantly lower level of TILs than those without ECS. Detailed data are available in Table 1.
Table 1.
Baseline Demographics of the 202 Patients in this Study
| Variable | No. (%) | Low TILs |
Moderate TILs |
High TILs |
P |
|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |||
| Age, yrs.: mean ± SD | 60.74 ± 13.48 | 0.906 | |||
| ≤60 | 97 (48.0%) | 32 (46.4%) | 36 (48.0%) | 29 (50.0%) | |
| >60 | 105 (52.0%) | 37 (53.6%) | 39 (52.0%) | 29 (50.0%) | |
| Sex | 0.097 | ||||
| Male | 134 (66.3%) | 51 (73.9%) | 43 (57.3%) | 40 (69.0%) | |
| Female | 68 (33.7%) | 18 (26.1%) | 32 (42.7%) | 18 (31.0%) | |
| cT stage | 0.008 | ||||
| T1 | 32 (15.8%) | 8 (11.6%) | 12 (16.0%) | 12 (20.7%) | |
| T2 | 53 (26.2%) | 12 (17.4%) | 23 (30.7%) | 18 (31.0%) | |
| T3 | 38 (18.8%) | 9 (13.0%) | 15 (20.0%) | 14 (24.1%) | |
| T4 | 79 (39.2%) | 40 (58.0%) | 25 (33.3%) | 14 (24.1%) | |
| pN stage | 0.130 | ||||
| N0 | 120 (59.4%) | 36 (52.2%) | 44 (58.7%) | 40 (69.0%) | |
| N1 | 30 (14.9%) | 9 (13.0%) | 15 (20.0%) | 6 (10.3%) | |
| N2 | 52 (25.7%) | 24 (34.8%) | 16 (21.3%) | 12 (20.7%) | |
| N3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Site | 0.112 | ||||
| Oral cavity | 191 (94.6%) | 62 (89.9%) | 72 (96.0%) | 57 (98.3%) | |
| Oropharynx | 11 (5.4%) | 7 (10.1%) | 3 (4.0%) | 1 (1.7%) | |
| Smoking history | 0.042 | ||||
| Smoker | 93 (46.0%) | 39 (56.5%) | 25 (33.3%) | 29 (50.0%) | |
| Nonsmoker | 104 (51.5%) | 28 (40.6%) | 48 (64.0%) | 28 (48.3%) | |
| Missing | 5 (2.5%) | 2 (2.9%) | 2 (2.7%) | 1 (1.7%) | |
| Alcohol history | 0.048 | ||||
| Drinker | 58 (28.7%) | 26 (37.7%) | 13 (17.3%) | 19 (32.8%) | |
| Nondrinker | 139 (68.8%) | 41 (59.4%) | 60 (80.0%) | 38 (65.5%) | |
| Missing | 5 (2.5%) | 2 (2.9%) | 2 (2.7%) | 1 (1.7%) | |
| Perineural invasion | 0.150 | ||||
| Absence | 146 (72.3%) | 47 (68.1%) | 50 (66.7%) | 49 (84.5%) | |
| Presence | 23 (11.4%) | 10 (14.5%) | 9 (12.0%) | 4 (6.9%) | |
| Missing | 33 (16.3%) | 12 (17.4%) | 16 (21.3%) | 5 (8.6%) | |
| Vascular emboli | 0.286 | ||||
| Absence | 164 (81.2%) | 55 (79.7%) | 58 (77.3%) | 51 (87.9%) | |
| Presence | 5 (2.5%) | 2 (2.9%) | 1 (1.3%) | 2 (3.4%) | |
| Missing | 33 (16.3%) | 12 (17.4%) | 16 (21.3%) | 5 (8.6%) | |
| Diffuse infiltration | 0.261 | ||||
| Absence | 137 (67.8%) | 48 (69.6%) | 45 (60.0%) | 44 (75.9%) | |
| Presence | 32 (15.8%) | 9 (13.0%) | 14 (18.7%) | 9 (15.5%) | |
| Missing | 33 (16.3%) | 12 (17.4%) | 16 (21.3%) | 5 (8.6%) | |
| Microscopic ECS | 0.012 | ||||
| Absence | 160 (79.2%) | 50 (72.5%) | 57 (76.0%) | 53 (91.4%) | |
| Presence | 9 (4.5%) | 7 (10.1%) | 2 (2.7%) | 0 (0.0%) | |
| Missing | 33 (16.3%) | 12 (17.4%) | 16 (21.3%) | 5 (8.6%) | |
| Adjuvant radiotherapy | 0.012 | ||||
| Yes | 142 (70.3%) | 56 (81.2%) | 53 (70.7%) | 55 (68.8%) | |
| No | 60 (29.7%) | 13 (18.8%) | 22 (29.3%) | 25 (31.2%) | |
| p16 status* | 0.406 | ||||
| p16+ | 2 (18.2%) | 1 (14.3%) | 1 (33.3%) | 0 (0.0%) | |
| p16− | 8 (72.7%) | 6 (85.7%) | 1 (33.3%) | 1 (100.0%) | |
| Missing | 1 (9.1%) | 0 (0.0%) | 1 (33.3%) | (0.0%) |
Note: * p16 status was evaluated only in patients with oropharyngeal squamous cell carcinoma.
Patients With High TIL Level Showed a Better Prognosis
The cut-off date for follow-up was December 1, 2013. Among the 202 patients, 27 (13.4%) were excluded because of loss to follow-up, and 175 patients provided follow-up data. The median follow-up time was 52 months (range, 5-153 months) for the survivors. No patients died during the perioperative period. During the follow-up period, 103 patients (58.9%) died. Eight of these patients died of causes other than cancer, including cardiovascular disease in two cases, lung infection in three patients and uncertain causes in two patients. A total of 109 patients (62.3%) developed recurrence. In addition, 16 patients developed a second primary carcinoma. The DFS rate was 37.7% (66/175), and the DSS rate was 45.7% (80/175).
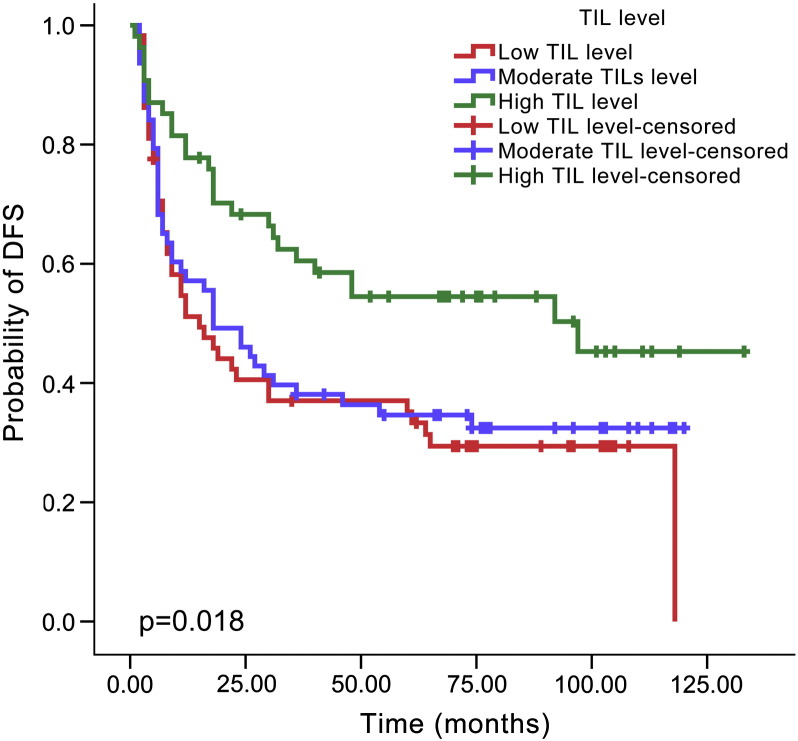
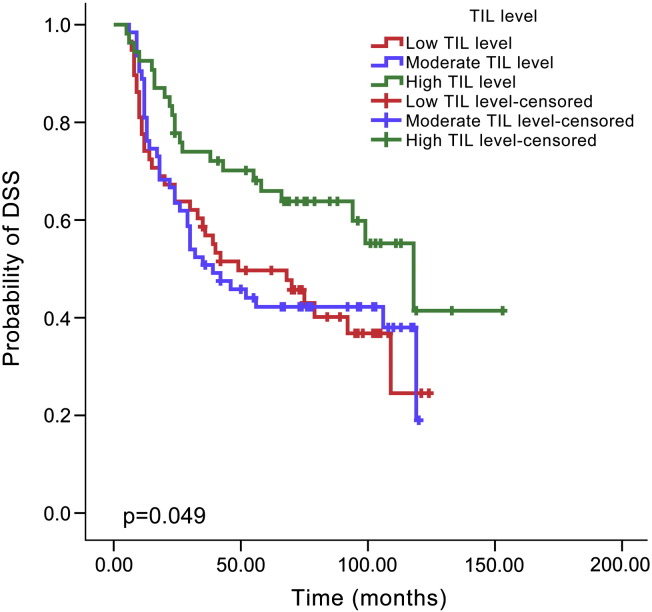
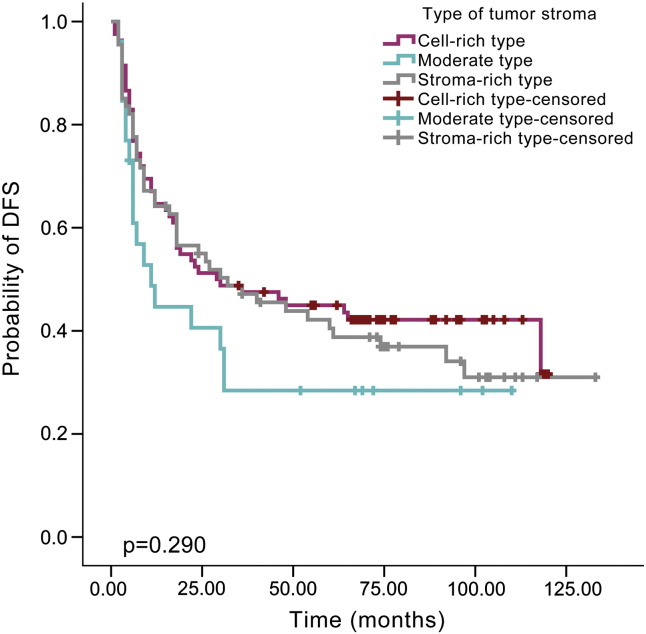
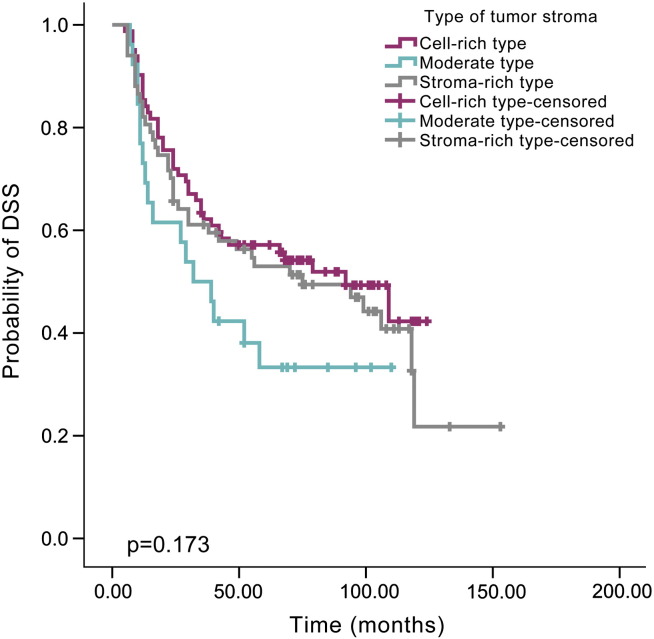
By Kaplan-Meier analysis, patients with a high TIL level showed a lower recurrence rate than those with a moderate TIL level (48.1% versus 66.7%, P = .024, Figure 2) or low TIL level (48.1% versus 70.7%, P = .006, Figure 2), but there was no difference between the low and moderate levels (70.7% versus 66.7%, P = .596, Figure 2). Similarly, improved DSS was noted for HNSCC patients with a high TIL level compared with moderate TIL level (40.7% versus 60.3%, P = .027, Figure 3) or low TIL level (40.7% versus 60.3%, P = .024, Figure 3). There was no significant difference in DSS between patients with low and moderate TIL levels (60.3% versus 60.3%, P = .915, Figure 3). The type of tumor stroma area was not related to patient prognosis, and there was no significant difference regarding recurrence between the cell-rich type and moderate type (58.5% versus 69.2%, P = .126), cell-rich type and stroma-rich type (58.5% versus 64.2%, P = .577) or moderate type and stroma-rich type (69.2% versus 64.2%, P = .252, Figure 4). The survival rate was also not significantly different between patients with the cell-rich type and moderate type (48.8% versus 65.4%, P = .074), cell-rich type and stroma-rich type (48.8% versus 56.7%, P = .478) or moderate type and stroma-rich type (65.4% versus 56.7%, P = .180, Figure 5).
Figure 2.
Kaplan–Meier survival curve for TIL level and DFS time according to different TIL levels.
Figure 3.
Kaplan–Meier survival curve for TIL level and DSS time according to different TIL levels.
Figure 4.
Kaplan–Meier survival curve for types of tumor stroma and DFS time according to different types of tumor stroma.
Figure 5.
Kaplan–Meier survival curve for types of tumor stroma and DSS time according to different types of tumor stroma.
TIL Level is an Independent Risk Factor for DFS and DSS
Using Cox proportional hazards regression models, we first estimated independent predictive factors for DFS. The univariate analyses in the entire group showed that the TIL level (hazard ratio (HR): 0.730, 95% CI: 0.578-0.924, P = .009), pN status (HR: 1.418, 95% CI: 1.149-1.750, P = .001), and site (HR: 2.642, 95% CI: 1.324-5.273, P = .006) were related to DFS. Multivariate analysis demonstrated that the TIL level (HR: 0.786, 95% CI: 0.618-0.999, P = .049) and pN status (HR: 1.351, 95% CI: 1.091-1.673, P = .006) were independent risk factors for DFS.
The univariate analyses of all patients evaluated for DSS revealed that the TIL level (HR: 0.758, 95% CI: 0.590-0.974, P = .031), T stage (HR: 1.225, 95% CI: 1.015-1.480, P = .035), pN status (HR: 1.692, 95% CI: 1.351-2.118, P < .001), site (HR: 3.173, 95% CI: 1.583-6.359, P = .001), and sex (HR: 0.615, 95% CI: 0.386-0.980, P = .041) were correlated with DSS. The multivariate analysis confirmed that pN status (HR: 1.600, 95% CI: 1.250-2.049, P < .001) and site (HR: 2.253, 95% CI: 1.093-4.642, P = .028) were independent predictive factors for DSS (Table 2).
Table 2.
Univariate Analysis and Multivariate Analysis for the Estimation of Risk Factors for the DFS and DSS in Patients with HNSCC
| Variable | Hazard Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Univariate analysis for DFS | |||
| TIL level (high, moderate, low) | 0.730 | 0.578-0.924 | 0.009 |
| Type of tumor stromal area (cell-rich, middle, stroma-rich) | 1.062 | 0.869-1.299 | 0.555 |
| T stage (T1, T2, T3, T4) | 1.055 | 0.888-1.255 | 0.542 |
| pN status (N0, N1, N2, N3) | 1.418 | 1.149-1.750 | 0.001 |
| Site (Oral versus Oropharynx) | 2.642 | 1.324-5.273 | 0.006 |
| Age (≤60 versus >60) | 1.030 | 0.706-1.504 | 0.877 |
| Sex (male versus female) | 0.873 | 0.577-1.320 | 0.519 |
| Tobacco use (absence versus presence) | 1.057 | 0.750-1.489 | 0.753 |
| Alcohol use (absence versus presence) | 1.050 | 0.725-1.520 | 0.796 |
| Radiotherapy (Yes versus No) | 1.072 | 0.720-1.597 | 0.731 |
| Multivariate analysis for DFS | |||
| TIL level (high, moderate, low) | 0.786 | 0.618-0.999 | 0.049 |
| pN status (N0, N1, N2, N3) | 1.351 | 1.091-1.673 | 0.006 |
| Site (oral versus oropharynx) | 1.935 | 0.947-3.952 | 0.070 |
| Univariate analysis for DSS | |||
| TIL level (High, moderate, low) | 0.758 | 0.590-0.974 | 0.031 |
| Type of tumor stromal area (cell-rich, middle, stroma-rich) | 1.089 | 0.878-1.350 | 0.439 |
| T stage (T1, T2, T3, T4) | 1.225 | 1.015-1.480 | 0.035 |
| pN status (N0, N1, N2, N3) | 1.692 | 1.351-2.118 | <0.001 |
| Site (oral versus oropharynx) | 3.173 | 1.583-6.359 | 0.001 |
| Age (≤60 versus >60) | 0.919 | 0.613-1.380 | 0.685 |
| Sex (male versus female) | 0.615 | 0.386-0.980 | 0.041 |
| Tobacco use (absence versus presence) | 1.220 | 0.836-1.780 | 0.303 |
| Alcohol use (absence versus presence) | 1.438 | 0.964-2.147 | 0.075 |
| Radiotherapy (Yes versus No) | 1.073 | 0.701-1.643 | 0.744 |
| Multivariate analysis for DSS (forward method) | |||
| Site (oral versus oropharynx) | 2.253 | 1.093-4.642 | 0.028 |
| pN status (N0, N1, N2, N3) | 1.600 | 1.250-2.049 | <0.001 |
| TILs level (high, moderate, low) | 0.843 | 0.647-1.098 | 0.206 |
| T stage (T1, T2, T3, T4) | 0.991 | 0.800-1.228 | 0.934 |
| Sex (male versus female) | 0.703 | 0.438-1.129 | 0.145 |
*Note: DFS, disease-free survival; DSS, disease-specific survival; TILs: Tumor infiltrating lymphocyte.
Discussion
As indicated previously, many studies are incomparable because of the inconsistency in assessment approach and threshold, making the role of TILs in HNSCC uncertain [13]. Based on approaches for evaluating TIL levels in other fields, we developed a method aimed at evaluating TIL levels in HNSCC, and the results proved feasible.
Many authors have suggested that the TIL level carries more predictive value than TNM stage, and others believe that the TIL level can provide detailed stratification on the basis of TNM stage. T stage is the foundation of the TNM stage, and it has been proven to be directly related to prognosis. Our study found that TILs were associated with T stage; specifically, more advanced tumors showed fewer infiltrating lymphocytes. Moreover, patients with a lower TIL level were found to require radiation treatment after surgery. These results indicated that patients with higher TIL levels may have a better prognosis.
By Kaplan–Meier analysis, we confirmed a significant difference in prognosis between the high TIL level group and the other two groups. Patients with high TIL levels showed a better outcome in both DFS and DSS. Using the Cox regression model, TIL level was identified as an independent predictor of DFS and was closely related to DSS. Therefore, the TIL level has great predictive value for HNSCC patients, and these results are in accordance with those of previous studies and our hypothesis.
A few previous studies have focused on the relationship between health behaviors and TILs, specifically smoking and drinking history [14]. In this study, smokers and drinkers tended to have lower TIL levels, which is consistent with the results of Wolf et al. [14]. Unhealthy behaviors may damage the liver, thereby weakening the immune response.
As an immune biomarker, the TIL level reflects the ability of the immune system to protect against malignant cells. The immune system targets cancer by lysing tumor cells and secreting cytokines [15], [16]. We predict that only when there are sufficient numbers of lymphocytes or antitumor factors can the immune microenvironment eliminate tumor tissue, which is why only high TIL levels were associated with better prognosis. Interestingly, ECS was also found to be related to the TIL levels, as patients with ECS showed lower TIL levels. Notably, no patients in the subgroup of high TIL levels developed ECS. The lack of TILs in the primary site reflected the immune deficiency. Therefore, we speculated a lack of TILs in the primary site could reflect the immune status of the corresponding metastatic lymph nodes. For these patients, we hypothesized that the immunity of metastatic nodes was not sufficient to eliminate the tumor and that this deficiency allowed the carcinoma cells to be able to break through the lymph node capsule. To test this hypothesis, a further study of the association between the TIL level of the primary site and the ECS of metastatic lymph nodes might be required and will be performed in the future.
From the microscopic perspective, the development of tumor tissues leads to hypoxia, which decreases the pH and significantly changes the tissue microenvironment. Previous studies have shown that the expression of signaling molecules such as VEGF, GPD1L and HIF-1 changes during cancer progression, and some researchers now believe that these factors act on lymphocytes and alter the proportion of T cell subsets. For instance, Yu et al. [17] reported that lymphocytes in the tumor microenvironment can affect the balance between the immune response and tolerance, leading to different outcomes. With tumor development, the total number of lymphocytes is decreased and the number of immunosuppressive cells is increased, thereby weakening the immune environment of the tumor. However, the TIL mechanism of action remains controversial, and future studies are required to address TIL function [17], [18], [19].
In recent years, the localization of TILs has received increasing attention [18], [20], [21], [22]. These studies have generally categorized TILs into two groups based on whether they are in contact with tumor cells: stromal lymphocytes (sTILs) and intratumoral lymphocytes (iTILs). Like much previous research, our study focused on sTILs. In practice, we found that there were also immune cells present outside of the tumor margins. Balermpas et al. [21] reported that the intratumoral localization of TILs influences their prognostic impact, and Taube et al. [20] and Tumeh et al. [22] reported that the localization of T cells was associated with PD-L1-expressing cells. However, because there is no official recommendation for assessing TILs outside of tumor boundaries, we did not count these lymphocytes using our method.
Considering our results in terms of daily clinical practice, we recommend 70% as a threshold for clinical practice and research trials to predict the prognosis of HNSCC patients. Although we found that 70% was the best cutoff point for the TIL level, for future TIL assessment, we recommend that the TIL level be divided into three groups. Currently, immunohistochemistry (IHC) is commonly used in research on TILs (e.g., to detect CD3+, CD8+, and CD45+ cells), but the effect of T lymphocyte subsets remains unclear [23], [24]. Based on the approach we established in this study, we will explore the clinical value of TIL subtyping using IHC, and the three-group approach might provide additional information. This study used H&E staining to confirm the relationship between TILs and prognosis, as H&E staining is convenient and easier to carry out in clinical practice.
This study was retrospective in nature and was restricted to patient subsets with available samples. Additionally, this study used only H&E staining as the method of pathological analysis. Thus, all results should be considered exploratory. This study may also be criticized for lack of data on some important baseline factors, including the depth of invasion and tumor thickness. These limitations will be given further consideration in future studies.
Conclusion
The assessment approach and criterion we established to evaluate TILs in HNSCC have been proven feasible. The TIL level was clearly related to patient prognosis, and 70% was identified as a valid threshold for TIL assessment, with TIL levels higher than 70% associated with a better prognosis. Thus, the TIL level may serve as an independent predictor for DFS in HNSCC patients.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China (81302350 and 81570957), the Beijing Science and Technology Committee (Z161100000516201), the Beijing Nova programme (Z151100000315045), China, and Discipline construction fund of Beijing Stomatological Hospital (15-09-12).
Conflicts of interest: None declared.
Contributor Information
Zhien Feng, Email: jyfzhen@126.com.
Zhengxue Han, Email: hanf1989@hotmail.com.
References
- 1.Feng Z., Xu Q.S., Niu Q.F., Qin L.Z., Li J.Z., Su M., Li H., Han Z. Risk factors for patients with multiple synchronous primary cancers involving oral and oropharyngeal subsites. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:360–366. doi: 10.1016/j.oooo.2015.10.031. http://dx.doi.org/10.1016/j.oooo.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 2.Wang W.L., Lee C.T., Lee Y.C., Hwang T.Z., Wang C.C., Hwang J.C., Tai C.M., Chang C.Y., Tsai S.S., Wang C.P. Risk factors for developing synchronous esophageal neoplasia in patients with head and neck cancer. Head Neck. 2011;33:77–81. doi: 10.1002/hed.21397. http://dx.doi.org/10.1002/hed.21397 [DOI] [PubMed] [Google Scholar]
- 3.Rosenbery S.A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 4.Mittal D., Gubin M.M., Schreiber R.D., Smyth M.J. New insights into cancer immunoediting and its three component phases — elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. http://dx.doi.org/10.1016/j.coi.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy S.A., Taylor J.M., Terrell J.E., Islam M., Li Y., Fowler K.E., Wolf G.T., Teknos T.N. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. http://dx.doi.org/10.1002/cncr.23615 [DOI] [PubMed] [Google Scholar]
- 6.Lee J.J., Chang Y.L., Lai W.L., Ko J.Y., Kuo M.Y., Chiang C.P., Azuma M., Chen C.W., Chia J.S. Increased prevalence of interleukin-17-producing CD4(+) tumor infiltrating lymphocytes in human oral squamous cell carcinoma. Head Neck. 2011;33:1301–1308. doi: 10.1002/hed.21607. http://dx.doi.org/10.1002/hed.21607 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen N., Bellile E., Thomas D., McHugh J., Rozek L., Virani S., Peterson L., Carey T.E., Walline H., Moyer J. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma (HNSCC) Head Neck. 2016;38:1074–1084. doi: 10.1002/hed.24406. http://dx.doi.org/10.1002/hed.24406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balermpas P., Michel Y., Wagenblast J., Seitz O., Weiss C., Rödel F., Rödel C., Fokas E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110:501–509. doi: 10.1038/bjc.2013.640. http://dx.doi.org/10.1038/bjc.2013.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salgado R., Denkert C., Demaria S., Sirtaine N., Klauschen F., Pruneri G., Wienert S., Van den Eynden G., Baehner F.L., Penault-Llorca F. The evaluation of tumor-infiltrating lymphocytes (TILs)in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. http://dx.doi.org/10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pagès C., Tosolini M., Camus M., Berger A., Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. http://dx.doi.org/10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 11.Rajjoub S., Basha S.R., Einhorn E., Cohen M.C., Marvel D.M., Sewell D.A. Prognostic significance of tumor-infiltrating lymphocytes in oropharyngeal cancer. Ear Nose Throat J. 2007;86:506–511. [PubMed] [Google Scholar]
- 12.Denkert C., Loibl S., Noske A., Roller M., Müller B.M., Komor M., Budczies J., Darb-Esfahani S., Kronenwett R., Hanusch C. Tumor-associated lymphocytes as an independent predictor of response to Neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. http://dx.doi.org/10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 13.Uppaluri R., Dunn G.P., Lewis J.S., Jr. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in head and neck cancers. Cancer Immun. 2008;8:16. [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf G.T., Chepeha D.B., Bellile E., Nguyen A., Thomas D., McHugh J., University of Michigan Head and Neck SPORE Program Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral Oncol. 2015;51:90–95. doi: 10.1016/j.oraloncology.2014.09.006. http://dx.doi.org/10.1016/j.oraloncology.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin Z., Blankenstein T. CD4+ T cell-mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFNg receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–686. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 16.Mumberg D., Monach P.A., Wanderling S., Philip M., Toledano A.Y., Schreiber R.D., Schreiber H. CD4(+) T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc Natl Acad Sci U S A. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu X., Zhang Z., Wang Z., Wu P., Qiu F., Huang J. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol. 2016;18:497–506. doi: 10.1007/s12094-015-1391-y. http://dx.doi.org/10.1007/s12094-015-1391-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassilakopoulou M., Avgeris M., Velcheti V., Kotoula V., Rampias T., Chatzopoulos K., Perisanidis C., Kontos C.K., Giotakis A.I., Scorilas A. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22:704–713. doi: 10.1158/1078-0432.CCR-15-1543. http://dx.doi.org/10.1158/1078-0432.CCR-15-1543 [DOI] [PubMed] [Google Scholar]
- 19.Pagès F., Kirilovsky A., Mlecnik B., Asslaber M., Tosolini M., Bindea G., Lagorce C., Wind P., Marliot F., Bruneval P. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. http://dx.doi.org/10.1200/JCO.2008.19.6147 [DOI] [PubMed] [Google Scholar]
- 20.Taube J.M., Klein A., Brahmer J.R., Xu H., Pan X., Kim J.H., Chen L., Pardoll D.M., Topalian S.L., Anders R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. http://dx.doi.org/10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balermpas P., Rödel F., Liberz R., Oppermann J., Wagenblast J., Ghanaati S., Harter P.N., Mittelbronn M., Weiss C., Rödel C. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b + myeloid cells in recurrences. Br J Cancer. 2014;111:1509–1518. doi: 10.1038/bjc.2014.446. http://dx.doi.org/10.1038/bjc.2014.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. http://dx.doi.org/10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogino T., Shigyo H., Ishii H., Katayama A., Miyokawa N., Harabuchi Y., Ferrone S. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66:9281–9289. doi: 10.1158/0008-5472.CAN-06-0488. http://dx.doi.org/10.1158/0008-5472.CAN-06-0488 [DOI] [PubMed] [Google Scholar]
- 24.Badoual C., Hans S., Rodriguez J., Peyrard S., Klein C., Agueznay Nel H., Mosseri V., Laccourreye O., Bruneval P., Fridman W.H. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. http://dx.doi.org/10.1158/1078-0432.CCR-05-1886 [DOI] [PubMed] [Google Scholar]