Abstract
Coexisting myasthenia gravis and neuromyelitis optica spectrum disorder was reported as a rare association, with only 26 reported cases in the literature. The authors report the case of a middle-aged Chinese woman with bilateral recurrent optic neuritis and seropositive ocular myasthenia gravis who was subsequently diagnosed with neuromyelitis optica spectrum. She was tested seropositive for the neuromyelitis optica immunoglobulin G (NMO-IgG) and had elevated antinuclear antibody titres, but workup for other autoimmune disorders were negative. She was subsequently prescribed with azathioprine and pyridostigmine, and showed good control of both autoimmune disorders. To the best of the authors’ knowledge, this is the first reported case in the literature of a Chinese patient with seropositivity for both anti-acetylcholine receptor and NMO-IgG without a thymic disorder. Testing of NMO-IgG may be considered in patients with optic neuritis with underlying autoimmune disorders even in the absence of transverse myelitis for the detection of associated neuromyelitis optica spectrum disorders.
Keywords: Neuromyelitis optica, NMO-IgG seropositivity, ocular myasthenia
Case Report
Neuromyelitis optica (NMO) is a rare disorder with reported prevalence ranging from 0.44 to 2.50 per 100,000.1–4 NMO is a B-cell-mediated inflammatory and demyelinating disorder of the central nervous system, often presenting with severe and bilateral recurrent optic neuritis and transverse myelitis that are radiological distinguished from multiple sclerosis (MS).5,6 Early detection of NMO is facilitated by the testing for the neuromyelitis optica immunoglobulin G (NMO-IgG) antibody. An early diagnosis is important because its treatment differs from that of MS and the early use of systemic immunosuppressants may reduce relapse and improve clinical outcome.7,8 NMO spectrum disorder is a limited form of NMO with seropositivity for NMO-IgG but without the complete clinical features of NMO.5 Seropositive NMO spectrum disorder has been reported to be associated with other autoimmune diseases, most commonly systemic lupus erythematosus (SLE), Sjögren syndrome, antiphospholipid syndrome, and sarcoidosis.9 The coexistence of NMO spectrum disorder and ocular MG is rare, with only 26 reported cases in the literature worldwide and no reported cases for Chinese ethnicity. Of these 26 reported cases, only 17 had confirmatory seropositivity for both MG and NMO spectrum disorder, but none belonging to Chinese ethnicity.10 We report the first case in the Chinese population of a middle-aged woman with bilateral optic neuritis and seropositivity for both ocular MG and NMO spectrum disorder and subsequently treated with azathioprine and pyridostigmine for the NMO spectrum disorder and ocular MG, respectively. Informed patient consent was sought and granted prior to publication and no patient personal information was revealed in this case report.
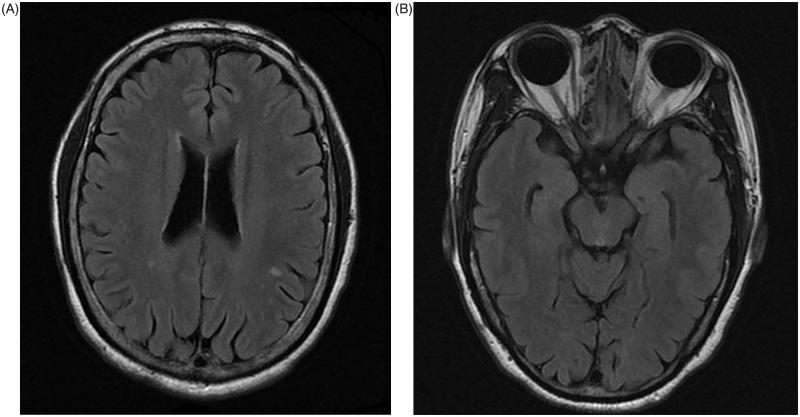
A 59-year-old Chinese woman without family history of autoimmune disease presented with a first episode of right eye retrobulbar optic neuritis at the age of 51, resulting in visual impairment in her right eye (Snellen visual acuity was 0.1 on presnetation). She then developed recurrent left eye retrobulbar neuritis, with three attacks until the age of 55. There were no systemic neurological features and no transverse myelitis. She was treated with 3 days of intravenous pulse methyleprednisolone followed by 11 days of oral prednisolone as per the Optic Neuritis Treatment Trial during each acute episode of optic neuritis.11 Magnetic resonance imaging (MRI) of the brain and orbit performed at around 2 months after treatment revealed non-specific T2 fluid-attenuated inversion recovery (FLAIR) hyperintense foci over the white matter of the bilateral corona radiate and centrum semiovale. There was right optic atrophy, compatible with the history of recurrent optic neuritis, but no residual FLAIR hyperintensities were noted at the optic nerves at the time of the MRI orbit (Figure 1A, B).
FIGURE 1.
Axial MRI (A) brain and (B) orbit showing non-specific T2 FLAIR hyperintense foci over the white matter of the bilateral corona radiate and centrum semiovale.
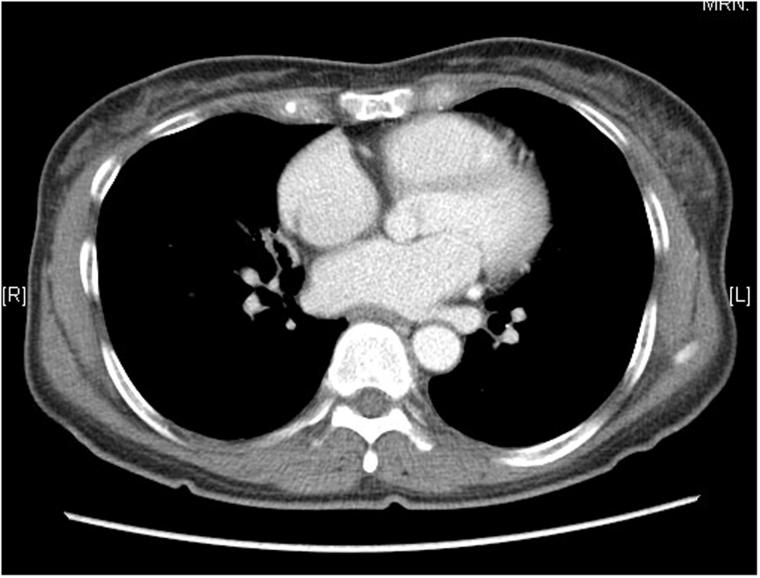
At the age of 56, she developed variable bilateral upper lid ptosis with fatigability. There was no systemic weakness. The diagnosis of ocular MG was confirmed by an elevated titre of anti-acetylcholine receptor antibodies in serum (8.83 nmol/L; normal: <0.45 nmol/L) as well as a positive ice test and Tensilon test. Computerised topography (CT) scan of thorax demonstrated no thymic mass nor medistenal mass (Figure 2). Her seropositive ocular MG was controlled with pyridostigmine 30 mg three times a day.
FIGURE 2.
CT thorax showing no thymic nor mediastinal mass.
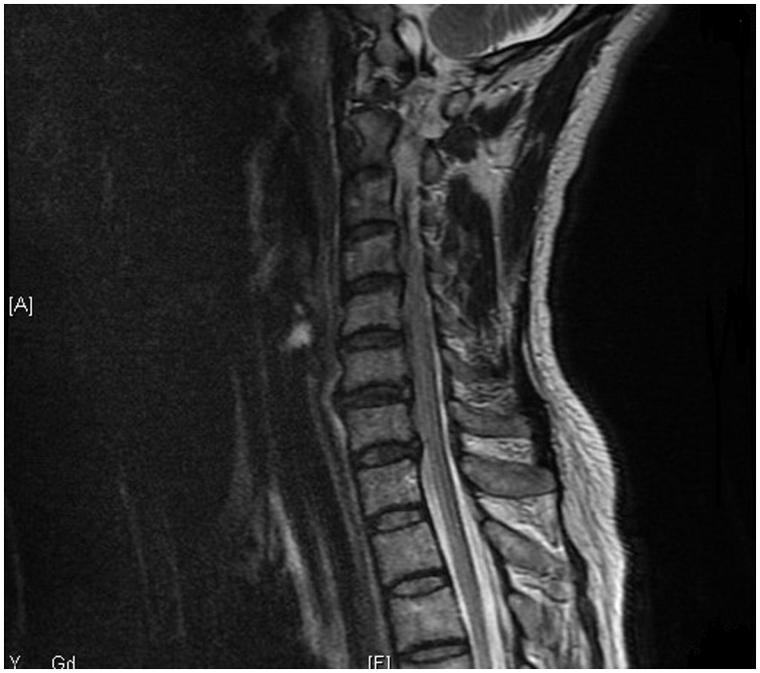
Six months later, she presented with a fourth attack of left retrobulbar optic neuritis and was referred to our neuro-ophthalmology clinic. Lumbar puncture revealed no oligoclonal bands. The anti-aquaporin-4 titre was 2+ positive based on the cell-based indirect immunofluorescence assay for the autoantibody using a 3-point scale score system as described by Chan et al.12 Complete rheumatological blood workup and systemic workup by rheumatologists only revealed elevated ANA titre (1:128) without elevated double-stranded DNA; however, there was no evidence of other autoimmune diseases. A serial MRI brain and orbit revealed no interval change as compared with the previous scan. Neurological examination revealed no typical features of myelitis. A whole-spine MRI showed no evidence of transverse myelitis and only some mild features of cervical degeneration was noted (Figure 3). She was started on azathioprine 50 mg daily in addition to her pyridostigmine regimen. Routine blood taking revealed no features of toxicities from the azathioprine.
FIGURE 3.
MRI spine showing no evidence of transverse myelitis and only some mild features of cervical degeneration.
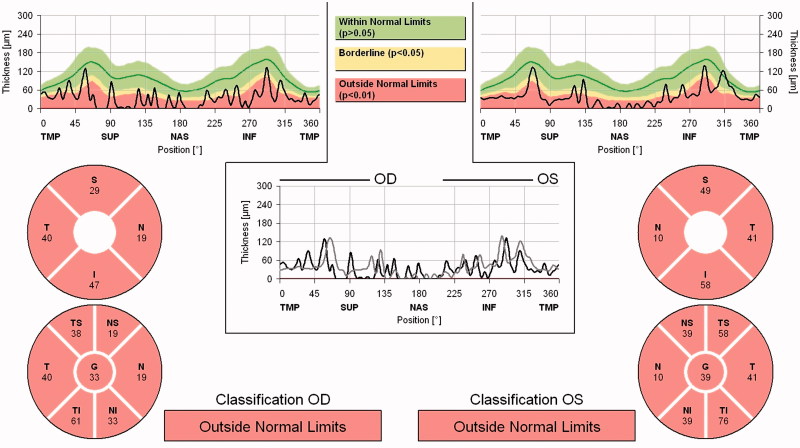
On last follow-up, more than 3 years after the start of azathioprine and pyridostigmine, ophthalmic examination revealed the absence of ptosis or diplopia. There was a relative afferent papillary defect over her right eye with bilateral optic disc pallor. Best-corrected Snellen visual acuity was 0.05 and 0.6 over her right and left eye, respectively. Spectralis optical coherence topography (Heidelberg Engineering, Carlsbad, CA, USA) demonstrated generalised thinning of the bilateral peripapillary retinal nerve fibre (RNFL) layers with an average RNFL thickness of 33 and 39 µm over the right and left eye, respectively (Figure 4). There were no relapses of optic neuritis for 3 years with the maintenance dose of azathioprine 50 mg twice daily for the NMO spectrum disorder and pyridostigmine 30 mg four times a day for the ocular MG.
FIGURE 4.
Spectralis OCT demonstrating generalised RNFL thinning over both eyes.
Discussion
NMO-IgG antibody is a specific test for NMO and NMO spectrum disorders, with a specificity of 99–100% and sensitivity of 58–91%; detection rates vary between the different immune assays.13–15
McKeon et al. demonstrated that 34% of NMO patients are harboured with autoimmune antibodies against neural tissues and muscles.16 Patients with MG may trigger the onset of NMO spectrum disorder after thymectomy. In Leite et al.’s series of 16 subjects, more than 70% of those with coexisting MG and NMO spectrum disorder had a previous thymectomy, although the exact pathophysiological explanation is yet to be determined due to the rarity of this association.17 The current evidence of the coexistence of MG and NMO spectrum disorder is summarised in Table 1.16,17
TABLE 1.
Previous reports on coexisting NMO spectrum disorder and MG.
| References | Sex M:F ratio | Ethnicity (%) | MG phenotype (%) | Thymectomy rate (%) | NMO-IgG positivity rate (%) | Anti-Ach receptor positivity rate (%) |
|---|---|---|---|---|---|---|
| Leite et al.17 | 1:15 | Caucasian (68.8%) Mixed African descent (18.9%) Mixed Arab and Caucasian (6.3%) Japanese/Asian (6.3%) | Generalized (81.3%) Ocular (18.8%) | 68.8% | 43.8% | 100.0% |
| Jarius et al.10 | 1:9 | Caucasian (100.0%) | Generalized (40.0%) Ocular (60.0%) | 90.0% | 100.0% | 90.0% (results not available in 1 case) |
| Yau et al. (this work) | 0:1 | Chinese (100%) | Ocular (100%) | 0% | 100% | 100.0% |
Our patient presented with bilateral recurrent optic neuritis prior to the onset of ocular MG and was later confirmed to have NMO spectrum disorder via seropositivity for NMO-IgG. It has been reported in the literature that in 90–98% of subjects, the diagnosis of MG preceded that of NMO spectrum disorder with a lapse time ranging from 4 to 16 years.10,17 Although thymectomy has been postulated as a triggering factor for NMO spectrum disorder,17 a low index of suspicious for NMO spectrum disorder may lead to its delayed diagnosis due to the absence of the typical systemic features of NMO.
Our case was unique in that she presented with bilateral recurrent optic neuritis, which may suggest that she may have had a NMO spectrum disorder prior to her onset of ocular MG. Our case reiterates the importance of the testing for NMO-IgG in those with ocular MG presenting with optic neuritis or other atypical systemic neurological signs.10,16
Typical MRI spine features in NMO consists of T2 hyperintensities over three or more vertebral segments.5 More than 50% of subjects with NMO have normal MRI brain scans at the initial phase, whereas 84.8% will develop abnormal brain lesions on serial scans.9 Most of the brain lesions are non-specific and concentrated in regions where the aquaporin-4 channels are expressed.18 The MRI brain features of our patient are in line with the current knowledge in the literature.
Patients with NMO-related optic neuritis often show more marked thinning of the RNFL when compared with MS.19 Our patient had an average RNFL thickness of only 33 and 39 µm over the right and left eye, respectively, which was much thinner than the mean average thickness of those with idiopathic optic neuritis at 3 months post attack (103.9 ± 6.0 µm).20
To the best of our knowledge, this is the first reported case in the literature of a Chinese patient presenting with coexisting seropositive NMO spectrum disorder and ocular MG. Clinicians should have a high index of suspicion for NMO or NMO spectrum disorders in those with recurrent optic neuritis and underlying systemic autoimmune diseases. The earlier detection and treatment with systemic immunosuppressant therapy can potentially reduce relapse and alter disease progression in NMO spectrum disorders.21
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Note: Figure 4 of this article is available in colour online at www.informahealthcare.com/oph.
References
- 1.Bedi G, Usmani N, Delgado S, Sheremata W. Prevalence and ethnic origin of patients with neuromyelitis optica in South Florida. Mult Scler 2009;15:S34 [Google Scholar]
- 2.Cabrera-Gomez JA, Kurtzke JF, Gonzalez-Quevedo A, Lara-Rodríguez R. An epidemiological study of neuromyelitis optica in Cuba. J Neurol 2009;256:35–44 [DOI] [PubMed] [Google Scholar]
- 3.Rivera JF, Kurtzke JF, Booth VJ, Corona VT., 5th Characteristics of Devic’s disease (neuromyelitis optica) in Mexico. J Neurol 2008;255:710–715 [DOI] [PubMed] [Google Scholar]
- 4.Cabre P, Heinzlef O, Merle H, Buisson GG, Bera O, Bellance R, Vernant JC, Smadja D. MS and neuromyelitis optica in Martinique (French West Indies). Neurology 2001;56:507–514 [DOI] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489 [DOI] [PubMed] [Google Scholar]
- 6.Wingerchuk DM. Evidence for humoral autoimmunity in neuromyelitis optica [review]. Neurol Res 2006;28:348–353 [DOI] [PubMed] [Google Scholar]
- 7.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112 [DOI] [PubMed] [Google Scholar]
- 8.Wingerchuk DM, Weinshenker BG. Neuromyelitis optica. Curr Treat Options Neurol 2008;10:55–66 [DOI] [PubMed] [Google Scholar]
- 9.Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, Montalban X, Du Pasquier RA, Polman CH, Sorensen PS, Hemmer B. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol 2010;17:1019–1032 [DOI] [PubMed] [Google Scholar]
- 10.Jarius S, Paul F, Franciotta D, de Seze J, Münch C, Salvetti M, Ruprecht K, Liebetrau M, Wandinger KP, Akman-Demir G, Melms A, Kristoferitsch W, Wildemann B. Neuromyelitis optica spectrum disorders in patients with myasthenia gravis: ten new aquaporin-4 antibody positive cases and a review of the literature. Mult Scler 2012;18:1135–1143 [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Cleary PA, Anderson MM, Jr, Keltner JL, Shults WT, Kaufman DI, Buckley EG, Corbett JJ, Kupersmith MJ, Miller NR. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med 1992;326:581–588 [DOI] [PubMed] [Google Scholar]
- 12.Chan KH, Jason SC Kwan, Philip WL Ho, Jessica WM Ho, Andrew CY Chu, David B Ramsden. Aquaporin-4 autoantibodies in neuromyelitis optica spectrum disorders: comparison between tissue-based and cell-based indirect immunofluorescence assays. J Neuroinflamm 2010,7:50. doi: 10.1186/1742-2094-7-50 [DOI] [PMC free article] [PubMed]
- 13.Misu T, Fujihara K, Itoyama Y. Neuromyelitis optica and anti-aquaporin 4 antibody—an overview. Brain Nerve 2008;60:527–537 [PubMed] [Google Scholar]
- 14.Fazio R, Malosio ML, Lampasona V, De Feo D, Privitera D, Marnetto F, Centonze D, Ghezzi A, Comi G, Furlan R, Martino G. Antiaquaporin 4 antibodies detection by different techniques inneuromyelitis optica patients. Mult Scler 2009;15:1153–1163 [DOI] [PubMed] [Google Scholar]
- 15.Waters P, Vincent A. Detection of anti-aquaporin-4 antibodies in neuromyelitis optica: current status of the assays. Int MS J 2008;15:99–105 [PubMed] [Google Scholar]
- 16.McKeon A, Lennon VA, Jacob A, Matiello M, Lucchinetti CF, Kale N, Chan KH, Weinshenker BG, Apiwattinakul M, Wingerchuk DM, Pittock SJ. Coexistence of myasthenia gravis and serological markers of neurological autoimmunity in neuromyelitis optica. Muscle Nerve 2009;39:87–90 [DOI] [PubMed] [Google Scholar]
- 17.Leite MI, Coutinho E, Lana-Peixoto M, Apostolos S, Waters P, Sato D, Melamud L, Marta M, Graham A, Spillane J, Villa AM, Callegaro D, Santos E, da Silva AM, Jarius S, Howard R, Nakashima I, Giovanni G, Buckley C, Hilton-Jones D, Vincent A, Palace J. Myasthenia gravis and neuromyelitis optica spectrum disorder: a multicenter study of 16 patients. Neurology 2012;78:1601–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 2006;63:964–968 [DOI] [PubMed] [Google Scholar]
- 19.Ratchford JN, Quigg ME, Conger A, Frohman T, Frohman E, Balcer LJ, Calabresi PA, Kerr DA. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology 2009;73:302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yau GSK, Lee JWY, Lau PPK, Tam VTY, Wong WWY, Yuen CYF. Prospective study on retinal nerve fibre layer thickness changes in isolated unilateral retrobulbar optic neuritis. Sci World J Epub ahead of print 21 Oct 2013; Article ID 694613, doi:10.1155/2013/694613 [DOI] [PMC free article] [PubMed]
- 21.Papeix C, Vidal JS, de Seze J, Pierrot-Deseilligny C, Tourbah A, Stankoff B, Lebrun C, Moreau T, Vermersch P, Fontaine B, Lyon-Caen O, Gout O. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler 2007;13:256–259 [DOI] [PubMed] [Google Scholar]