Abstract
Ocular ischaemic syndrome is a devastating eye disease caused by severe carotid artery stenosis. The purpose of the study was to develop a reliable rat model for this syndrome by means of common carotid artery occlusion and a controllable needle suture method. Adult Wistar rats were subjected to common carotid artery occlusion and sham surgery. The common carotid artery was ligated unilaterally or bilaterally with needles of different diameters, and ocular arterial filling time was examined by fluorescein fundus angiography at different time points. Haematoxylin-eosin staining of vessels and degree of stenosis were considered outcome measures. The ocular blood flow was monitored and measured by laser doppler flowmetry. Needles with a diameter of 0.4 mm were more effective in developing severe stenosis of the common carotid arteries compared with needles of other diameters. Bilateral common carotid artery occlusion was a more effective model than unilateral occlusion. The arterial filling time was significantly increased at 14 and 21 days after ligation (5.75 ± 0.45 and 6.27 ± 0.95 s, respectively) compared with arterial filling time before surgery (5.22 ± 0.64 s). The total blood flow in the sham surgery group was significantly higher than in the bilateral common carotid artery occlusion group. The fundus blood flow was statistically different between the two groups, whereas that of the anterior segment was not. In conclusion, the authors have established a rat model of ocular ischaemic syndrome via a controllable needle suture method, which was reliable up to 2–3 weeks after surgery.
Keywords: Animal model, controllable needle suture method, fluorescein fundus angiography, laser doppler flowmetry, retinal ischaemia
INTRODUCTION
Ocular ischaemic syndrome (OIS) occurs in the anterior and posterior segments of the eye and is caused by severe hypoperfusion of the eye, mostly secondary to common carotid artery stenosis. It is characterized by loss of visual acuity; inflammation of the conjunctiva, cornea, and anterior uvea; iris neovascularisation; mid-dilated poorly reactive pupil; cataract; neovascular glaucoma; and midperipheral retinopathy with dot and blot haemorrhages, microaneurysms, retinal arteriolar narrowing, capillary nonperfusion, and optic disc neovascularisation.1 Since OIS is usually underdiagnosed, has a poor prognosis and these patients usually suffer from severe ischaemic cardiovascular and cerebrovascular diseases, its 5-year survival rate after final diagnosis is only 60%.2 Only early detection, diagnosis, and timely treatment would allow increased survival rates and quality of life of these patients.
Monkeys, rabbits, rats, mice, guinea pigs, cats, dogs, and pigs have been used as animal models of retinal ischaemia.3–8 Rats are most widely used in laboratories because their vascular and retinal structures are closer to those of humans, and they are less expensive and are easier to obtain and handle. The most common rat models of retinal ischaemia include pressure elevation, optic nerve ligation, vessel ligation, photodynamic ablation, and endothelin administration. Slakter et al.9 were amongst the first to develop an animal model for carotid artery occlusive disease. Experimental data demonstrated that bilateral common carotid artery occlusion (BCCAO) in the rat results in an incomplete retinal ischaemia, leading to chronic ganglion cell loss, optic nerve degeneration, and other cerebral degeneration.10,11 The reduction of blood flow induced by BCCAO for 90 days in rats induces retinal degeneration and cellular death. Presently, no available model has provided direct evidence of retinal ischaemia. Our study establishes a rat model of OIS by BCCAO and a controllable needle suture method. This protocol, which uses fluorescein fundus angiography (FFA) and laser doppler flowmetry, provides an important tool to explore the pathogenesis and treatment of OIS.
MATERIALS AND METHODS
Animals
The protocol used in this study was approved by the institutional review board of Beijing Friendship Hospital affiliated to Capital Medicine University. The study adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Clean-grade Wister rats (6 months old; 270–300 g; Laboratory Animal Centre of Peking University) were used. Animals with cataracts or retinal diseases were excluded after slit lamp (Topcon SL-50; Suzhou Medical Appliance Factory) examination. They were assigned to two groups using a random number table: 24 were assigned to the sham surgery group and 68 to the stenosis group. Of these, 16 rats underwent bilateral ligation of the common carotid artery (BCCAO; 4 rats per group) with needles 0.3, 0.4, 0.6, and 0.9 mm in diameter; 4 rats underwent unilateral ligation of the common carotid artery with 0.4-mm-diameter needles; and 48 rats underwent BCCAO with 0.4-mm-diameter needles. All rats were subjected to FFA examination at different time points. Physiological indices such as weight, vital signs, behaviour, motion, and feeding were monitored constantly.
Surgical Procedure: Induction of Stenosis by a Controllable Needle Suture Method
The procedure was performed under anaesthesia induced by an intraperitoneal injection of 0.2% pentobarbital (30 mg/kg). Following a ventral midline incision (approximately 2–3 cm), the right common carotid artery (CCA), internal carotid artery (ICA), external carotid artery (ECA), and the vagus nerve were separated from the carotid sheath. Branches of the ECA were subjected to electric coagulation; the distal ECA was ligated and sheared (preliminary experiment) at about 1 cm from the origin of the CCA. Pinheads (needles) of different diameters were positioned beside the CCA and tied together by 5-0 silk sutures. The pinhead was then removed (Figure 1). This procedure resulted in different degrees of stenotic CCA, since the suture was left in place after reperfusion. The appearance (colour and pulsation condition) of the vessel wall was observed under the microscope. Animals assigned to sham surgery were submitted to the same procedure without ligation of the CCA. In order to reduce the variability of the procedure, one single experienced technician carried out all of the surgeries.
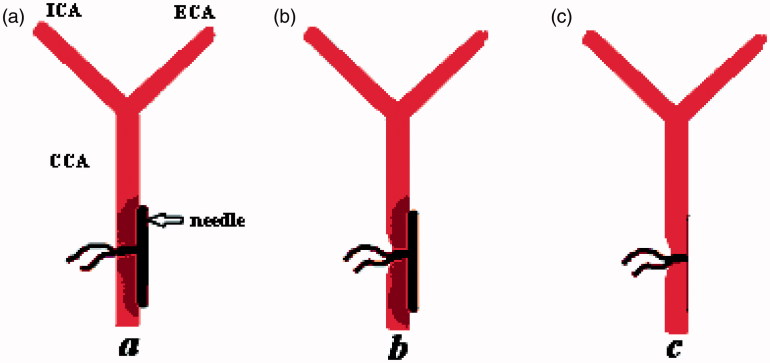
FIGURE 1.
The process of producing carotid stenosis. (a) A needle of 0.4 mm diameter was positioned beside the common carotid artery (CCA) at about 1.5 cm from the bifurcation; (b) the needle and CCA were tied together by 5-0 silk sutures; (c) the pinhead was then removed, and this procedure resulted in a certain degree of stenotic CCA, since the suture was left in place after reperfusion.
Pupillary Light Reflex
Each animal was examined for direct and consensual pupillary light reflex (PLR) with a light beam using a direct ophthalmoscope before and after surgery, everyday for the first week and then weekly up to 21 days. The examiner was blinded to whether the animals had been submitted to CCAO or sham surgery. Each animal was adapted to darkness for 5 min and then a beam of light was first directed to the right eye for evaluation of direct PLR. Subsequently, the beam was quickly moved to the left eye to assess the consensual reflex. The rat was left in the dark for 1 min for recovery and then the same procedure was repeated for the left eye. Loss of the pupillary reflex was defined as failure of pupil constriction after exposure to the light beam.
Fluorescein Fundus Angiography
FFA was investigated at different time points after surgery on the left eyes of rats from the two groups (fundus camera: TRC50IX retinal camera; Topcon, Itabashi, Tokyo). The procedure was performed under anaesthesia induced by an intraperitoneal injection of 0.3 mL/100 g of 10% chloral hydrate (Sinopharm Chemcial Reagent Co. Ltd, China). Cyclopentolate hydrochloride eye drops (Alcon Laboratories) were used as mydriatics. Rats were placed on the operating table for retinal observation. For this purpose, 0.5 mL/kg of 100 g/L sodium fluorescein (Guangzhou Baiyun Shan Ming Xing Pharmaceutical) was rapidly injected into the caudal vein. Dextran and hypromellose eye drops (Alcon Laboratories) were instilled approximately every 1–2 min in the eyes to prevent corneas from drying. The venous filling time was recorded.
Histology
All rats were euthanized with 10% chloral hydrate (5 mL) at given time points. The ligated carotid artery was rapidly dissected, soaked in 40 g/L formaldehyde for 24 h for fixation, and embedded in paraffin wax. Four-micron-thick serial sections were prepared for haematoxylin-eosin staining and vessel lumen evaluation.
Monitoring of Ocular Blood Flow
The ocular blood flow was monitored using laser doppler flowmeter (Moor Instruments, UK) before and 21 days after surgery.12 This approach allowed us to determine changes in blood volume per unit area. Blood flow was assessed in the iris (anterior segment) and fundus (posterior segment) and the values in sham and BCCAO groups were compared. In the laser room, starlight conditions induced mydriasis in rats.
The procedure was performed under anaesthesia by an intraperitoneal injection of 0.3 mL/100 g of 10% chloral hydrate. The animals were laterally recumbent to allow monitoring of the ocular blood flow. The blood flow was measured in the left eye. Data were recorded in high-resolution real-time imaging mode. Two equal areas were taken in the pupil and iris regions, where the decrease in blood flow was monitored and measured. The blood flow in the pupil area was considered as fundus and that in the iris area as anterior segment blood flow. Data are expressed as perfusion units (PU).
Statistical Analysis
Data are expressed as mean ± standard error. Student’s t test and analysis of variance were used (SPSS 17.0). Differences were considered significant when p < 0.05.
RESULTS
Rat Physiological Indices
In all rats, vital signs were stable. Animal behaviour was observed during the experiments. No differences were observed in motion, feeding, or weight between groups.
Slit-Lamp Examination
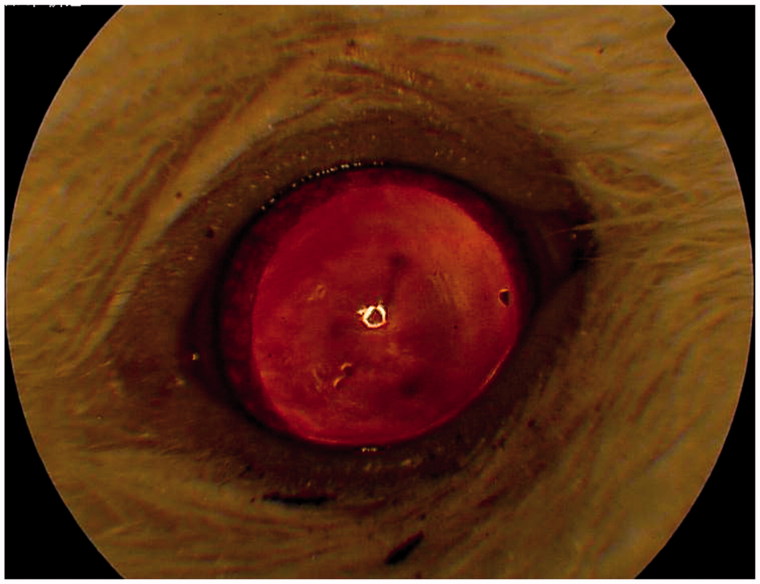
A cataract was found only in one eye of a rat belonging to the BCCAO group (ligated 0.4 mm needle) using slit-lamp examination (Figure 2). No cataracts were found in the sham surgery group.
FIGURE 2.
One rat in the bilateral carotid artery occlusion (BCCAO) group had a cataract in one eye.
PLR Examination
We observed PLR before and after surgery, every day for the first week and weekly up to 21 days. Before surgery, PLR of all animals was positive. Three rats from the BCCAO group lost direct PLR in both eyes (6.25%), whereas 45 maintained it in both eyes (93.75%). Loss of the PLR occurred during the first 3 days after surgery and its status did not change during the subsequent days and weeks after surgery. The consensual PLR was preserved in those rats with unilateral direct PLR loss. No animal from the sham group lost the PLR. No PLR delay was observed after surgery.
FFA Studies
Comparison of the Effects of Ligation with Different-Diameter Needles
Sixteen rats were subjected to BCCAO. They were divided into four groups and received ligation with 0.3-, 0.4-, 0.6-, and 0.9-mm-diameter needles by a controllable needle suture method. Ocular arterial filling time was observed on the left eyes by FFA before and 7 days after surgery. In the first group (0.3-mm needle), one rat died after ligation, whereas no filling was observed in the other three, suggesting that the CCA of this group was occluded due to the inadequate space left in the artery. This was confirmed by the histology of carotid artery carried out after FFA. The filling time of the other three groups (0.4-, 0.6-, and 0.9-mm needles; four rats in each group) were 4.93 ± 0.90, 5.03 ± 0.31, and 5.03 ± 0.34 s, respectively, before surgery and 5.67 ± 0.83, 5.23 ± 0.29, and 5.45 ± 0.31 s, respectively, after surgery. There was a significant difference before and after surgery in the 0.4-mm needle group (p = 0.045), but not in 0.6-mm and 0.9-mm needle groups (p = 0.066 and p = 0.224, respectively). These results indicate that a 0.4-mm-diameter needle was better for developing the severe stenosis of the carotid artery and was well suited as an OIS model (Table 1).
TABLE 1.
Comparison of arterial filling times (in seconds) before and after surgery when artery ligation was obtained with different diameter needles.
| Diameters of needles |
|||
|---|---|---|---|
| 0.4 mm | 0.6 mm | 0.9 mm | |
| Before surgery (s) | 4.93 ± 0.90 | 5.03 ± 0.31 | 5.03 ± 0.34 |
| After surgery (s) | 5.67 ± 0.83 | 5.23 ± 0.29 | 5.45 ± 0.31 |
| T | −3.326 | −2.828 | −1.529 |
| p | 0.045 | 0.066 | 0.224 |
Comparison between Bilateral and Unilateral Ligation
Four rats were subjected to BCCAO with a 0.4-mm needle by a controllable needle suture method, whereas four others received unilateral CCAO. The ocular arterial filling time was observed on the left eyes by FFA before and 7 days after surgery. The filling times in the BCCAO group were 4.93 ± 0.90 s before and 5.67 ± 0.83 s after surgery, whereas those of the unilateral ligation group were 5.05 ± 0.35 s before surgery and 5.40 ± 0.34 s after surgery. The statistical analysis showed a significant difference before and after surgery in the bilateral ligation group (p = 0.045), but no significant difference in the unilateral ligation group (p = 0.218). These results indicate that bilateral common carotid artery ligation is a better approach for an OIS model (Table 2).
TABLE 2.
Comparison of arterial filling times (in seconds) before and after surgery following bilateral or unilateral ligation.
| Bilateral ligation | Unilateral ligation | |
|---|---|---|
| Before surgery (s) | 4.93 ± 0.90 | 5.05 ± 0.35 |
| After surgery (s) | 5.67 ± 0.83 | 5.40 ± 0.34 |
| t | −3.326 | −1.552 |
| p | 0.045 | 0.218 |
Time Course of Ligation Effects
Sixteen rats received BCCAO with 0.4-mm needles by a controllable needle suture method. One rat that developed a cataract was excluded (Figure 2). The arterial filling time was observed on the left eyes by FFA before and at different time points after surgery. The filling time was 5.22 ± 0.64 s before surgery, and 5.49 ± 0.30, 5.09 ± 0.27, 5.63 ± 0.48, 5.75 ± 0.45, and 6.54 ± 0.67 s on the 1st, 3rd, 7th, 14th, and 21st days after surgery, respectively. No significant difference was found before and 1 week after surgery (p > 0.05). Instead, a statistically significant difference was found before surgery and 2 and 3 weeks after surgery (p = 0.012 and p < 0.001, respectively). These results indicate that 2–3 weeks after surgery was an appropriate period for the establishment of OIS (Table 3).
TABLE 3.
Comparison of arterial filling times (in seconds) before and up to 3 weeks after ligation.
| Before | Day 1 | Day 3 | Day 7 | Day 14 | Day 21 | |
|---|---|---|---|---|---|---|
| Filling time (s) | 5.22 ± 0.64 | 5.49 ± 0.30 | 5.09 ± 0.27 | 5.63 ± 0.48 | 5.75 ± 0.45 | 6.27 ± 0.95 |
| Mean difference | NA | −0.27333 | 0.13333 | −0.40667 | −0.52667 | −1.05333 |
| p | NA | 0.188 | 0.519 | 0.052 | 0.012 | 0.000 |
Long-Term FFA Results
The artery filling time assessed on the left eyes of 24 rats belonging to the sham group was 5.10 ± 0.67 s before and 5.23 ± 0.48 s 21 days after surgery with no statistical difference (p = 0.120). At the same time point, the artery filling time of the 48 left eyes from the BCCAO group (0.4-mm needle) was 5.44 ± 1.26 s before (Figure 3) and 6.50 ± 1.13 s after surgery (Figure 4). This difference was statistically significant (p < 0.001; Table 3). FFA showed venous dilatation and tortuosity, blurring of the optic disc, straightening of retinal arteries, and delay in the rate of retinal arterial and venous filling.
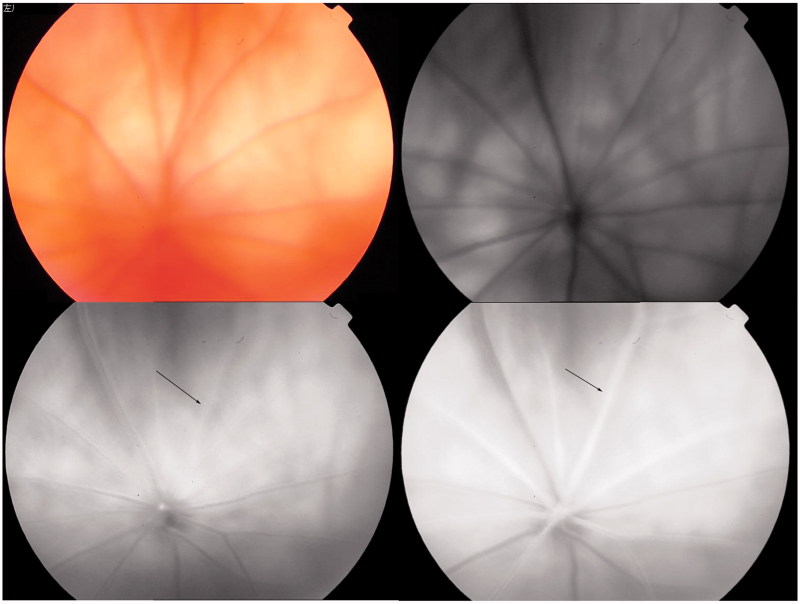
FIGURE 3.
Typical fluoroscein fundus angiography (FFA) performed on BCCAO rats before surgery. Upper left: fundus image; upper right: blue reflectance (red-free) image. The retina was observed after fluorescein sodium was injected into the caudal vein. Arterial filling appeared at 5.7 s (lower left) and complete engorgement at 6.7 s (lower right).
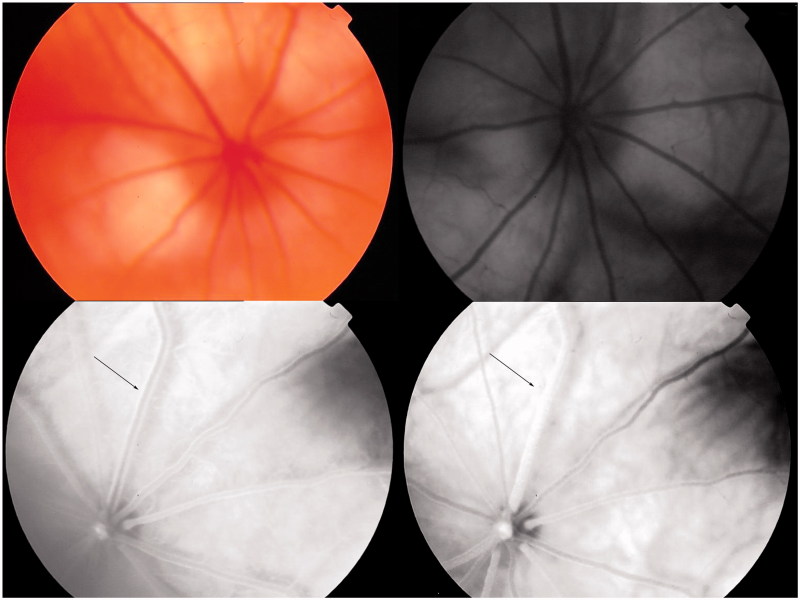
FIGURE 4.
Typical FFA performed on BCCAO rats 21 days after surgery. Upper left: fundus image; upper right: blue reflectance (red-free) image. The retina was observed after fluorescein sodium was injected into caudal vein. Arterial filling appeared at 6.7 s (lower left) and complete engorgement at 8.2 s (lower right), a longer filling time compared with that before surgery.
Histology
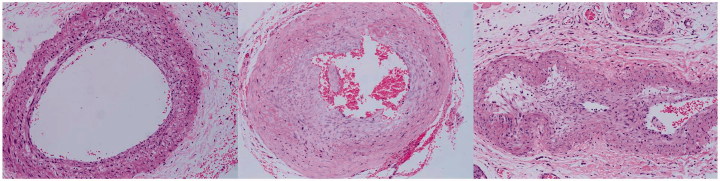
Seven days after surgery, an incision was made to check the carotid arteries after FFA. The common carotid arteries of all rats of the sham group (n = 5) remained unobstructed and pulsating under microscope inspection. The common carotid arteries of the BCCAO group were stenotic but with blood flowing through and pulsating normally (n = 5). The common carotid arteries of rats were pink and pulsating well with the coil on (Figure 5).
FIGURE 5.
Micrographs of haematoxylin and eosin–stained CCA after FFA. No stenosis was apparent in CCA of the sham group (left); an obvious stenosis was present in CCA of the BCCAO group (middle; CCA ligated by a 0.6-mm needle; right: CCA ligated by a 0.4-mm needle) with red blood cells in the lumen.
Ocular Blood Flow Monitoring
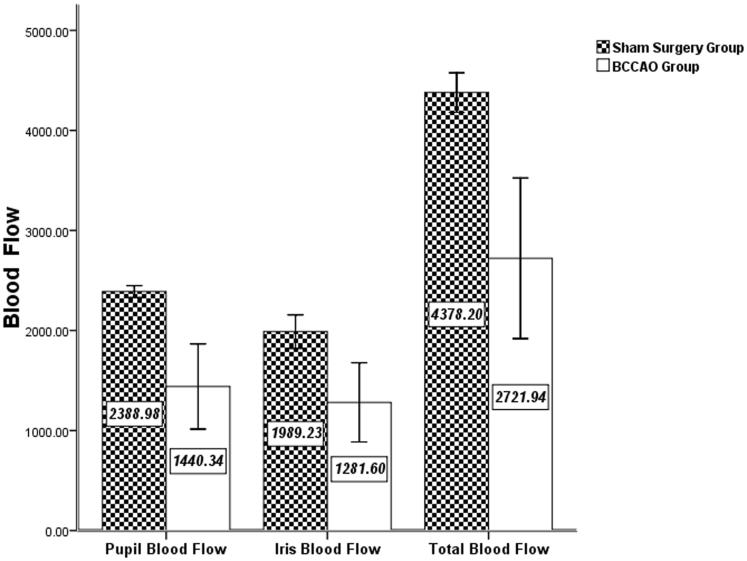
The blood flow was measured in the iris (anterior segment) and fundus (posterior segment) by laser doppler flowmetry (Figure 6). There were 4 rats in the sham surgery and 22 in the BCCAO group. The total blood flow of the sham surgery group (4378.20 ± 197.36 PU) was significantly higher than that of BCCAO group (2721.94 ± 802.55 PU; p = 0.038). The pupil area blood flow of the sham surgery group (2388.98 ± 59.12 PU) was significantly higher than that of the BCCAO group (1440.34 ± 426.15 PU; p = 0.032). The iris area blood flow of the sham surgery group (1989.23 ± 167.54 PU) was higher than that of the BCCAO group (1281.60 ± 395.93 PU), but the difference was not statistically significant (p = 0.091).
FIGURE 6.
Comparison of ocular blood flow between sham surgery and BCCAO groups. Data are expressed in PU; n = 4 in sham and n = 22 in BCCAO groups. The total blood flow of the sham surgery group was significantly greater than that of the BCCAO group (p = 0.038). The pupil area blood flow of the sham surgery group was significantly greater than that of the BCCAO group (p = 0.032). The iris area blood flow of the sham surgery group was greater than that of the BCCAO group, but the difference was not statistically significant (p = 0.091).
DISCUSSION
The current study demonstrated significant histopathological changes in the ischaemic retina following controllable needle suture BCCAO. Several experimental methods are presently available to induce ischaemic retina: (1) increasing the intraocular pressure by continuous perfusion of the anterior chamber in the rabbit,13 (2) surgical occlusion and reperfusion of the common carotid artery in the Mongolian gerbil,14,15 and (3) surgical permanent occlusion of the bilateral common carotid arteries in the rat.16–19 These models are all extremely complicated to establish because the vessel walls are easily damaged, the stenosis is not constant, and the degree of stenosis is difficult to control. Since the current study was aimed at establishing a new animal model of OIS and investigating its possible underlying mechanism, it was essential to cause chronic cerebral hypoperfusion. To achieve this condition, a high intraocular pressure in the anterior chamber perfusion was not enough. In addition, because of the vascular anatomical structure in the central nervous system (CNS) of the Mongolian gerbil, permanent surgical occlusion of the bilateral common carotid arteries causes complete termination of the blood supply to the forebrain and 40–60% of the gerbils die shortly after the surgery. Therefore, the Mongolian gerbil model can be used as a transient ischaemia and reperfusion model.14,20 Available data demonstrated that the blood flow after BCCAO reached 30–53% of the control in the brain and 16–27% of the preoperative condition in the eye.21 This indicated that, in rats, the blood supply is maintained long after BCCAO. Due to this level of blood supply to the eye and brain, rats subjected to this surgery maintained their vision (as judged by behavioural observations) and only 18.2% of the animals died during the experiments.21 Thus, the present experimental method was adequate to induce chronic hypoperfusion of the eye and ischaemia of the retina as a model of OIS.
A series of needles of different diameters (0.3, 0.4, 0.6, and 0.9 mm) were used to control the stenotic degree of CCA. In the first group (0.3-mm needle), one rat died after ligation, whereas no filling was observed in the other three, suggesting that the CCA of this group was occluded due to the inadequate space left in the artery. This was confirmed by the histology of carotid artery. The obstruction of CCA may have resulted in acute cerebral ischaemia and led to the death of the rat. For ethical reasons, we abandoned this group. However, histopathological examinations showed severe CCA stenosis in rats that received surgery with 0.4-mm needles. FFA of the 0.4-mm group showed retinal ischaemia and delay of arterial filling time. Mild to moderate CCA stenosis was observed during histopathological examination of the 0.6-mm and 0.9-mm needle groups. Based on FFA, there was no significant difference between the delays of arterial filling times in these two groups. Transient ischaemia could not be established due to collateral circulation. Therefore, we believe that needles with diameters of 0.4 mm represent the best solution to induce severe stenosis of CCA. This procedure is simple, highly reproducible, and the degree of stenosis is controllable. The model simulates hypoperfusion of the brain caused by CCA stenosis and has been used in research of angioplasty, including stent placement and balloon dilatation, in severe CCA stenosis.22 In our animal model, occlusion of both CCAs cut off blood supply to the retina; however, some retinal perfusion was maintained by retrograde blood flow to the ophthalmic artery through the circle of Willis.
One rat of the BCCAO group had a cataract in the left eye, which was possibly the result of anterior ischaemia, an important component of OIS. Blockage of major proximal arteries such as the common carotid and internal carotid arteries, which branch to the ophthalmic artery, causes retinal ischaemia. The severity of the insult depends on the number of occluded vessels and their anastomoses. Unilateral occlusion of the CCA produces subtle retinal changes, whereas bilateral occlusion causes ophthalmoscopic evidence of retinal ischaemia, retinal ganglion cell (RGC) loss, optic atrophy, and cerebral damage in rats that have vertebral arteries.10 However, occlusion of both bilateral carotid and vertebral arteries may cause serious ocular and cerebral ischaemia or even death in animals after a long period of occlusion. In line with these findings, our study showed that the OIS model could be established only if both common carotid arteries were ligated.
Suture ligation is commonly used, but lately intraluminal suture ligation has also been used for transient artery occlusion and reperfusion.23 Slakter et al.9 were one of the earliest groups to develop an animal model of carotid artery occlusive diseases. Their data indicate that retinal ischaemia could be induced by BCCAO in rats, resulting in chronic damage of RGCs, degeneration of the optic nerve, and other cerebral damage.10,11 Barnett and Osborne believed that flow reduction 7 days after BCCAO could lead to glial degeneration accompanied by increased glial fibrillary acidic protein, although it did not cause significant tissue damage.1,24,25 However, after only 90 days of BCCAO, direct PLR disappeared in most of the animals,10,26 the retina degenerated, cells died, and electroretinogram (ERG) showed a permanent reduction in amplitude of a and b waves.1,26,27 Lavinsky et al.28 studied retinal changes 30 days after BCCAO, including disappearance of the pupillary light reflex and reduction in thickness of the inner plexiform layer (IPL) and cell count of RGCs. Kim et al.29 used a similar method to induce BCCAO in two different rat strains. Before BCCAO, all Wistar and Sprague-Dawley (SD) rats exhibited an intact PLR. However, all Wistar rats that survived BCCAO eventually lost the PLR in both eyes, whereas all surviving SD rats showed intact PLR in both eyes. The PLR loss was detectable 1 day post BCCAO induction and remained unchanged throughout the experiment. Our results are in agreement with those of Kim et al.; however, their study did not mention how to control the stenotic degree of the CCA.
We found that there was no significant difference between FFA arterial filling time before BCCAO and that measured 1, 3, and 7 days after BCCAO, similar to what was reported by Barnett and Osborne.24 However, there was a significant difference between FFA arterial filling time before BCCAO and 14 and 21 days after BCCAO, which suggested the presence of hypoperfusion ischaemia of the retina at 14 days after BCCAO. Extending our observation for another week, we found that the retinal hypoperfusion ischaemia of the rats did not improve at all.
Retinal damage and dysfunction of BCCAO rats is very similar to human OIS. There are many similarities between humans and rats in most cerebral arteries, including general organisation, vascular structure observed by light and electron microscopy, and morphological changes related to cerebrovascular diseases. Therefore, the rat is most likely a suitable animal model of human cerebrovascular diseases. OIS is a serious ocular disease with potentially blinding risks. This disease is too rare to be appropriately studied in humans. We recommend rat BCCAO retinal ischaemia to model human OIS and explore new treatments.
Acknowledgement
The study was supported by the Li Huanying Funding (Basic and clinical cooperation issues) from Capital Medical University.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Note: Figures 1–5 of this article are available in colour online at www.informahealthcare.com/oph.
References
- 1.Bachman JA. Exacerbation of ocular ischemic syndrome following cataract surgery. Clin Eye Vis Care 1995;7:139–142 [Google Scholar]
- 2.Sivalingam A, Brown GC, Magargal LE, Menduke H. The ocular ischemic syndrome. II. Mortality and systemic morbidity. Int Ophthalmol 1989;13:187–191 [DOI] [PubMed] [Google Scholar]
- 3.Brooks DE, Källberg ME, Cannon RL, Komàromy AM, Ollivier FJ, Malakhova OE, Dawson WW, Sherwood MB, Kuekuerichkina EE, Lambrou GN. Functional and structural analysis of the visual system in the rhesus monkey model of optic nerve head ischemia. Invest Ophthalmol Vis Sci 2004;45:1830–1840 [DOI] [PubMed] [Google Scholar]
- 4.Uckermann O, Uhlmann S, Pannicke T, Francke M, Gamsalijew R, Makarov F, Ulbricht E, Wiedemann P, Reichenbach A, Osborne NN, Bringmann A. Ischemia-reperfusion causes exudative detachment of the rabbit retina. Invest Ophthalmol Vis Sci 2005;46:2592–2600 [DOI] [PubMed] [Google Scholar]
- 5.Yilmaz T, Kükner AS, Aydemir O, Ozercan HI, Naziroğlu M. Aprotinin reduces ischemia-reperfusion injury in the retina of guinea pigs. Eur J Ophthalmol 2003;13:642–647 [DOI] [PubMed] [Google Scholar]
- 6.Roth S. Post-ischemic hyperemia in the cat retina: the effects of adenosine receptor blockade. Curr Eye Res 1995;14:323–328 [DOI] [PubMed] [Google Scholar]
- 7.Madl JE, McIlnay TR, Powell CC, Gionfriddo JR. Depletion of taurine and glutamate from damaged photoreceptors in the retinas of dogs with primary glaucoma. Am J Vet Res 2005;66:791–799 [DOI] [PubMed] [Google Scholar]
- 8.Morén H, Gesslein B, Undrén P, Andreasson S, Malmsjö M. Endovascular coiling of the ophthalmic artery in pigs to induce retinal ischemia. Invest Ophthalmol Vis Sci 2011;52:4880–4885 [DOI] [PubMed] [Google Scholar]
- 9.Slakter JS, Spertus AD, Weissman SS, Henkind P. An experimental model of carotid artery occlusive disease. Am J Ophthalmol 1984;97:168–172 [DOI] [PubMed] [Google Scholar]
- 10.Stevens WD, Fortin T, Pappas BA. Retinal and optic nerve degeneration after chronic carotid ligation: time course and role of light exposure. Stroke 2002;33:1107–1112 [DOI] [PubMed] [Google Scholar]
- 11.Wakita H, Tomimoto H, Akiguchi I, Lin JX, Ihara M, Ohtani R, Shibata M. Ibudilast, a phosphodiesterase inhibitor, protects against white matter damage under chronic cerebral hypoperfusion in the rat. Brain Res 2003;992:53–59 [DOI] [PubMed] [Google Scholar]
- 12.Chamot SR, Movaffaghy AM, Petrig BL, Riva CE. Blood flow in the human iris measured by laser Doppler flowmetry. Microvasc Res 1999;57:153–161 [DOI] [PubMed] [Google Scholar]
- 13.Veriac S, Tissie G, Bonne C. Oxygen free radicals adversely affect the regulation of vascular tone by nitric oxide in the rabbit retina under high intraocular pressure. Exp Eye Res 1993;56:85–88 [DOI] [PubMed] [Google Scholar]
- 14.Kuroiwa T, Bonnekoh P, Hossmann KA. Laser doppler flowmetry in CA1 sector of hippocampus and cortex after transient forebrain ischemia in gerbils. Stroke 1992;23:1349–1354 [DOI] [PubMed] [Google Scholar]
- 15.Tomimoto H, Wakita H, Akiguchi I, Nakamura S, Kimura J. Temporal profiles of accumulation of amyloid β/A4 protein precursor in the gerbil after graded ischemic stress. J Cereb Blood Flow Metab 1994;4:565–573 [DOI] [PubMed] [Google Scholar]
- 16.Hangai M, Yoshimura N, Hiroi K, Mandai M, Honda Y. Inducible nitric oxide synthase in retinal ischemia-reperfusion injury. Exp Eye Res 1996;63:501–506 [DOI] [PubMed] [Google Scholar]
- 17.Hart CT, Haworth S. Bilateral common carotid occlusion with hypoxic ocular sequelae. Br J Ophthalmol 1971;55:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujishima M, Ishitsuka T, Nakatomi Y, Tamaki K, Omae T. Change in local cerebral blood flow following bilateral carotid artery occlusion in spontaneously hypertensive and normotensive rats. Stroke 1981;12:874–876 [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya M, Sako K, Yura S, Yonemasu Y. Cerebral blood flow and histopathological changes following permanent bilateral carotid artery ligation in Wistar rats. Exp Brain Res 1992;89:87–92 [DOI] [PubMed] [Google Scholar]
- 20.Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol 1994;87:484–492 [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M, Kuroiwa T, Shimokawa R, Okeda R, Tokoro T. Nitric oxide synthase expression in ischemic rat retinas. Jpn J Ophthalmol 2000;44:235–244 [DOI] [PubMed] [Google Scholar]
- 22.Kleinedler JJ, Orchard EA, Foley JD, Rogers LK, Hebert VY, Dugas TR. A dietary approach to increase in-stent stenosis and face validity of a rat model for arterial angioplasty and stenting. Atherosclerosis 2011;219:484–491 [DOI] [PubMed] [Google Scholar]
- 23.Block F, Grommes C, Kosinski C, Schmidt W, Schwarz M. Retinal ischemia induced by the intraluminal suture method in rats. Neurosci Lett 1997;232:45–48 [DOI] [PubMed] [Google Scholar]
- 24.Barnett NL, Osborne NN. Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Exp Eye Res 1995;61:83–90 [DOI] [PubMed] [Google Scholar]
- 25.Osborne NN, Block F, Sontag KH. Reduction of ocular blood flow results in glial fibrillary acidic protein (GFAP) expression in rat retinal Muller cells. Vis Neurosci 1991;7:637–639 [DOI] [PubMed] [Google Scholar]
- 26.Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SA. Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res 2000;859:96–103 [DOI] [PubMed] [Google Scholar]
- 27.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 2004;23:91–147 [DOI] [PubMed] [Google Scholar]
- 28.Lavinsky D, Arterni NS, Achaval M, Netto CA. Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefes Arch Clin Exp Ophthalmol 2006;244:199–204 [DOI] [PubMed] [Google Scholar]
- 29.Kim SK, Cho KO, Kim SY. White matter damage and hippocampal neurodegeneration induced by permanent bilateral occlusion of common carotid artery in the rat: comparison between Wistar and Sprague-Dawley strain. Korean J Physiol Pharmacol 2008;12:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]