Abstract
The aim of this study was to assess the effect of idiopathic Optic perineuritis on the retinal nerve fiber layer, and determine the ability of optical coherence tomography to evaluate retinal nerve fiber loss after idiopathic Optic perineuritis. Four patients were assessed in this study. In all cases, average retinal nerve fiber layer was significantly thinner in the affected eye in comparison with the normal reference value and with the value for the contralateral normal eye at 12 months after the onset of optic perineuritis. Our study revealed that retinal nerve fiber layer loss occurs in idiopathic optic nerve sheath inflammation.
Keywords: OCT, optical coherence tomography, optic perineuritis, retinal nerve fiber layer, retinal nerve fiber layer loss
Introduction
Optic perineuritis (OPN) is an uncommon variant of orbital inflammatory disease, and the inflammatory process mainly involves the optic nerve sheath.1,2 In recent, OPN has generally been considered to represent a form of idiopathic inflammatory disease,2 while OPN occasionally occurs as a manifestation of specific infectious or inflammatory disorders, such as Wegener’s granulomatosis, sarcoidosis or syphilis.3–6
The prognosis for visual outcome in OPN patients is generally good, but optic nerve atrophy after optic nerve sheath inflammation can occur and worsen the prognosis in some cases of OPN patients.2
With the increasing use of optical coherence tomography (OCT), many researchers have observed that retinal nerve fiber layer thickness measured by OCT could be a good monitoring technique for quantifying axonal loss in optic neuropathy7,8 Therefore, OCT findings might be useful diagnostic technique and prognostic indicator in various optic neuropathy.
Although, OPN can be associated with optic nerve axonal damage, RNFL thickness changes in cases of OPN have not been previously described. Herein, we investigate a small case series of idiopathic unilateral, central vision affecting OPN patients who underwent OCT evaluations for RNFL thickness changes.
Materials and Methods
Case 1 (Table 1)
TABLE 1. Data of idiopathic optic perineuritis (OPN). Final best-corrected visual acuity (BCVA) and retinal nerve fiber layer thickness (RNFL), which was measured by spectral domain optical coherence tomography (SD-OCT, Carl Zeiss Meditec, Inc., Dublin, CA) at 12 months after optic perineuritis.
| Age/Sex/Eye | Initial BCVA | Visual field defect | Initial average RNFL thickness (μm) | Final BCVA | Final average RNFL thickness (μm) | Remark |
|---|---|---|---|---|---|---|
| 1/54/M/OD | HM | Diffuse | 123 | 20/40 | 75 | |
| 2/68/F/OD | CF | Diffuse | 86 | 20/32 | 68 | Recur |
| 3/51/M/OD | 20/32 | Central scotoma | 102 | 20/20 | 84 | Scleritis |
| 4/72/M/OD | 20/200 | Constriction of visual filed | 95 | 20/40 | 82 |
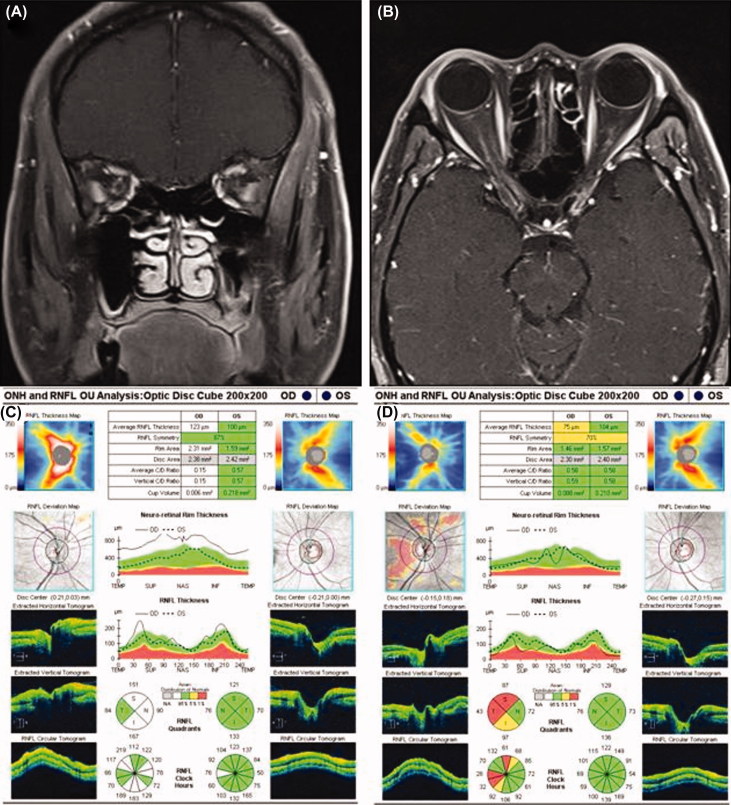
A 54-year-old man presented with a 3-day history of vision loss and pain associated with eye movements in his right eye. On examination, the visual acuity was 20/20 in the left eye and hand motion in the right eye. There was relative afferent pupil defect (RAPD) in the right eye. A visual field test showed normal results in the left eye and diffuse visual field defect in the right eye. The patient’s anterior segment showed a normal appearance and fundus examination showed right optic disc edema. Intraorbital optic nerve sheath enhancement without enhancement of the intrinsic optic nerve in the right eye was observed using contrast-enhanced orbital magnetic resonance image (MRI) (Figure 1A and B).
FIGURE 1.
(A) The coronal image represents the enhancement around, rather than within, the right optic nerve. (B) Orbital magnetic resonance imaging (MRI) with contrast enhanced and fat suppression shows, enhancement around the right intraorbital optic nerve in axial view. (C) A baseline spectral domain-optical coherence tomography (SD-OCT) image shows peripapillary retinal nerve fiber layer (RNFL) thickening in the right eye relative to the left eye, which is consistent with the observed optic disc edema in the right eye. (D) Twelve months after idiopathic optic perineuritis (OPN), there is RNFL thinning in the right eye relative to the left eye.
Laboratory findings revealed a CRP of 0.9 mg/dl (normal range: 0.0–0.3), while ESR, ACE, FTA, and ANCA were all within the normal range. The results of hematology test, renal and liver function tests, urine analysis, and chest radiography were normal. Based on the clinical presentation and radiologic findings, the patient was diagnosed as having right idiopathic optic perineuritis (OPN). An initial SD-OCT (Carl Zeiss Meditec, Inc., Dublin, CA) examination showed RNFL thickening (average RNFL thickness: 123 μm) in the right eye due to optic disc swelling (Figure 1C). Treatment with prednisolone (60 mg/day) was initiated, 10 days after symptom onset. Subsequently, the steroid dose was gradually tapered and the patient’s vision was improved to 20/40 after 12 months. Although recurrence of symptoms has not been detected, RNFL loss (average RNFL thickness: 75 μm) and optic disc atrophy in the right eye were observed 12 months after OPN (Figure 1D).
Case 2 (Table 1)
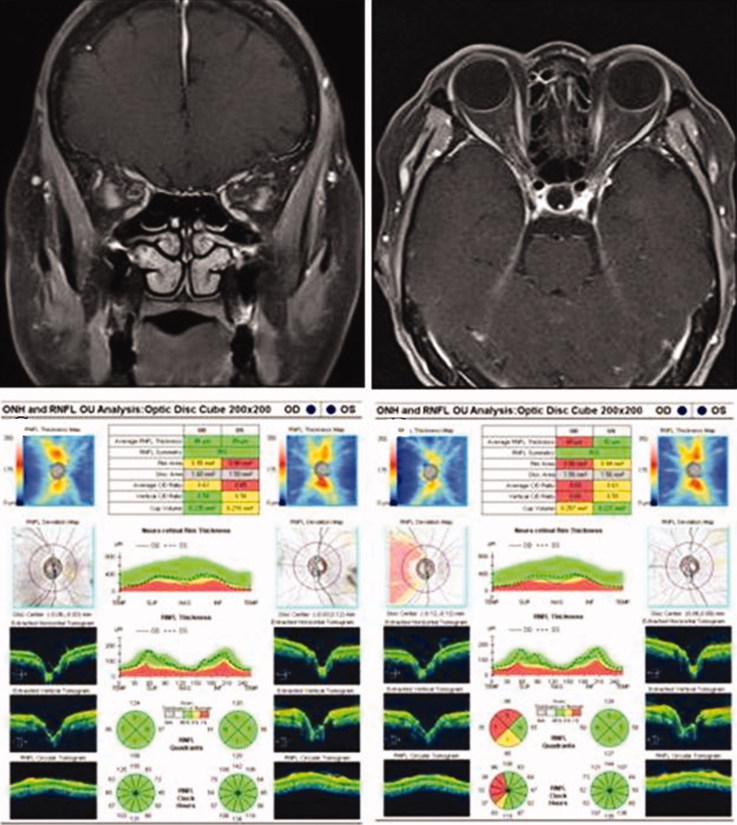
A 68-year-old woman was transferred from the local eye hospital with a diagnosis of retrobulbar acute optic neuritis. She complained of blurred vision in her right eye and ocular pain/headache associated with eye movement that had persisted for 1 week. Her visual acuity was counting fingers and disc edema was not observed on her right eye. Diffuse visual field defect and RAPD were detected in her right eye. Laboratory tests revealed a CRP of 0.4 mg/dl and an ESR of 30 mm/hour, while ACE, FTA, and ANCA were all within the normal range. The results of hematology test, renal and liver function tests, urine analysis, and chest radiography were all within normal limits. Fat suppressed T2-weighted orbital MRI showed high-intensity areas around her right optic nerve and perineural fat tissue (Figure 2A and B).
FIGURE 2.
(A) Doughnut-like ring enhancement around the right intraorbital optic nerve in the coronal view. (B) Contrast enhanced orbital MRI shows tram track sign in right orbit. (C) Initial OCT demonstrates normal RNLF thickness in both eyes. (D) RNFL loss in the right eye revealed by SD-OCT at the 12-month follow-up examination.
Baseline OCT (average RNFL thickness of right eye: 86 μm) demonstrated normal RNFL thickness in both eyes (Figure 2C). Steroid pulse therapy was initiated 10days after symptom onset. After seven days, her visual acuity improved to 20/20 in the right eye. Subsequently, the steroid therapy was tapered off. However, after one month, there was a recurrence of symptoms in her right eye, so treatment with prednisolone (50 mg/day) was started. Subsequently, the steroid dose was tapered off more gradually. Her vision was 20/32 with relative RNFL loss (average RNFL thickness: 68 μm) in the right eye after 16 months (Figure 2D).
Discussion
Optical coherence tomography is an in vivo histological technique that can be used to detect retinal abnormalities, including those resulting from axonal loss in diseases that affect the anterior optic pathway which caused by inflammation, compression, and infiltration.9 During the past few years, several studies have suggested that RNFL thickness measured by OCT can be used to detect axonal loss in demyelinating diseases, and can be applied to monitor disease severity and treatment efficacy.7–9 In addition, it has been suggested that OCT can help differentiate demyelinating diseases, and OCT findings can be long-term prognostic factors on the basis of the severity of axonal loss.10,11
OPN has been reported as a type of idiopathic orbital inflammatory disease and the diagnosis of OPN is most commonly based on the magnetic resonance image findings, along with the clinical characteristics.2,12 Neuro imaging in OPN patient typically shows a characteristic pattern of enhancement around the optic nerve, like tram-track on axial views and doughnut on coronal views.2,12 In addition, hazy enhancement of orbital fat surround optic nerve sheath or extraocular muscle, are observed in some cases of OPN patient.2,12 Clinically, OPN patients show dramatic response to corticosteroid treatment, but recurrence after tapering or discontinuation of treatment is common.1,2
In OPN, the main focus of inflammatory process occurs in optic nerve sheath, and this finding is supported by pathologic study and radiologic findings; optic nerve sheath and around orbital fat enhancement in orbital MRI. However, optic atrophy has been observed and worsens the prognosis in some cases of OPN patients. Pathologic study revealed mechanism of optic nerve injury in OPN. Vasculitis and necrobiotic granulomas were observed in full thickness biopsy specimen.2,13 Visual loss was caused by in large part to secondary ischemic infarction of the optic nerve, apparently due to circumferential compression of the optic nerve periphery by the compartment effect of the thickened optic nerve sheath.13,14 In addition, the inflammation in optic nerve sheath may release cellular toxic materials which may inhibit optic nerve regeneration and cause the demyelination of optic nerve,15 like other inflammatory optic neuropathies.16 Therefore, we postulate that optic nerve sheath inflammation without optic nerve involvement can be the cause of optic nerve axonal damage, and subsequent RNFL loss. In this study, we found that peripapillary average RNFL was significantly thinner in the affected eye when compared to normal reference values and to the values for the unaffected contralateral normal eye. These findings are consistent with our hypothesis that optic nerve sheath inflammation can cause retrograde axonal degeneration.
Although corticosteroid treatment elicits a dramatic response in patients with OPN in literature,17 in some cases of OPN patient have been shown worsen the prognosis if initiation of treatment is delayed or recurrent attack.2 Our patients has shown good response to steroid treatment, and recovered visual acuity and visual field defect after steroid therapy, but RNFL loss was observed. Therefore, we propose that early and aggressive treatment to eliminate inflammatory injury in the optic nerve sheath may prevent RNFL loss and restore visual function in server cases of OPN patients who has recurrent disease or sever central visual loss.
Limitations of this study include the retrospective nature of data collection and the potential selection bias caused by the small cases. However, we observed the possibility of significant RNFL loss in our case series, and suggest that OCT could be a useful examination technique to assess retrograde RNFL damage after optic nerve sheath inflammation.
Declaration of interest: The authors did not have any financial and proprietary interests during the conduct of the study. The authors did not receive any public or private support during the conduct of the study.
Note: Figures 1 and 2 of this article are available in colour online at http://informahealthcare.com/oph.
References
- 1.Yuen SJ, Rubin PA. Idiopathic orbital inflammation: ocular mechanisms and clinicopathology. Ophthalmol Clin North Am 2002;15:121–126 [DOI] [PubMed] [Google Scholar]
- 2.Purvin V, Kawasaki A, Jacobson DM. Optic perineuritis: clinical and radiographic features. Arch Ophthalmol 2001;119:1299–1306 [DOI] [PubMed] [Google Scholar]
- 3.Yu-Wai-Man P, Crompton DE, Graham JY, Black FM, Dayan MR. Optic perineuritis as a rare initial presentation of sarcoidosis. Clin Experiment Ophthalmol 2007;35:682–684 [DOI] [PubMed] [Google Scholar]
- 4.Morotti A, Liberini P, Padovani A. Bilateral optic perineuritis as the presenting feature of giant cell arteritis. BMJ Case Rep 2013;29:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takazawa T, Ikeda K, Nagaoka T, Hirayama T, Yamamoto T, Yanagihashi M, Tochikubo T, Iwasaki Y. Wegener granulomatosis-associated optic perineuritis. Orbit 2014;33:13–16 [DOI] [PubMed] [Google Scholar]
- 6.Parker SE, Pula JH. Neurosyphilis presenting as asymptomatic optic perineuritis. Case Rep Ophthalmol Med 2012;2012:621872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fjeldstad AS, Carlson NG, Rose JW. Optical coherence tomography as a biomarker in multiple sclerosis. Expert Opin Med Diagn 2012;6:593–604 [DOI] [PubMed] [Google Scholar]
- 8.Oliveira C, Cestari DM, Rizzo JF III. The use of fourth-generation optical coherence tomography in multiple sclerosis: a review. Semin Ophthalmol 2012;27:187–191 [DOI] [PubMed] [Google Scholar]
- 9.Mendoza-Santiesteban CE, Gonzalez-Garcia A, Hedges TR III Hernandez-Silva Y, Columbie-Garbey Y, Fernández-Cherkasova L, Santiesteban-Freixas R, Casali SV. Optical coherence tomography for neuro-ophthalmologic diagnoses. Semin Ophthalmol 2010;25:144–1454 [DOI] [PubMed] [Google Scholar]
- 10.Fernandes DB, Raza AS, Nogueira RG, Wang D, Callegaro D, Hood DC, Monteiro ML. Evaluation of inner retinal layers in patients with multiple sclerosis or neuromyelitis optica using optical coherence tomography. Ophthalmology 2013;120:387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monteiro ML, Fernandes DB, Apóstolos-Pereira SL, Callegaro D. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2012;53:3959–3966 [DOI] [PubMed] [Google Scholar]
- 12.Fay AM, Kane SA, Kazim M, Millar WS, Odel JG. Magnetic resonance imaging of optic perineuritis. J Neuroophthalmol 1997;17:247–249 [PubMed] [Google Scholar]
- 13.Margo CE, Levy MH, Beck RW. Bilateral idiopathic inflammation of the optic nerve sheaths. Light and electron microscopic findings. Ophthalmology 1989;96:200–206 [DOI] [PubMed] [Google Scholar]
- 14.Winterkorn JM, Odel JG, Behrens MM, Hilal S. Large optic nerve with central retinal artery and vein occlusions from optic neuritis/perineuritis rather than tumor. J Neuroophthalmol 1994;14:157–159 [PubMed] [Google Scholar]
- 15.Hykin PG, Spalton DJ. Bilateral perineuritis of the optic nerves. J Neurol Neurosurg Psychiatry 1991;54:375–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bessero AC, Clarke PG. Neuroprotection for optic nerve disorders. Curr Opin Neurol 2010;23:10–15 [DOI] [PubMed] [Google Scholar]
- 17.Tatsugawa M, Noma H, Mimura T, Funatsu H. High-dose steroid therapy for idiopathic optic perineuritis: a case series. J Med Case Rep 2010;4:404–407 [DOI] [PMC free article] [PubMed] [Google Scholar]