Abstract
Visual field assessment is an important clinical evaluation for eye disease and neurological injury. We evaluated Octopus semi-automated kinetic peripheral perimetry (SKP) and Humphrey static automated central perimetry for detection of neurological visual field loss in patients with pituitary disease. We carried out a prospective cross-sectional diagnostic accuracy study comparing Humphrey central 30-2 SITA threshold programme with a screening protocol for SKP on Octopus perimetry. Humphrey 24-2 data were extracted from 30-2 results. Results were independently graded for presence/absence of field defect plus severity of defect. Fifty patients (100 eyes) were recruited (25 males and 25 females), with mean age of 52.4 years (SD = 15.7). Order of perimeter assessment (Humphrey/Octopus first) and order of eye tested (right/left first) were randomised. The 30-2 programme detected visual field loss in 85%, the 24-2 programme in 80%, and the Octopus combined kinetic/static strategy in 100% of eyes. Peripheral visual field loss was missed by central threshold assessment. Qualitative comparison of type of visual field defect demonstrated a match between Humphrey and Octopus results in 58%, with a match for severity of defect in 50%. Tests duration was 9.34 minutes (SD = 2.02) for Humphrey 30-2 versus 10.79 minutes (SD = 4.06) for Octopus perimetry. Octopus semi-automated kinetic perimetry was found to be superior to central static testing for detection of pituitary disease-related visual field loss. Where reliant on Humphrey central static perimetry, the 30-2 programme is recommended over the 24-2 programme. Where kinetic perimetry is available, this is preferable to central static programmes for increased detection of peripheral visual field loss.
Keywords: Humphrey, kinetic, octopus, perimetry, pituitary, static
INTRODUCTION
Perimetry is the systematic measurement of visual field function using different types and intensities of stimuli. Visual fields may be assessed by using moving (kinetic) targets, which outline the boundaries of visual field, or by using static (stationary on-off) targets, which map the sensitivity of the visual field.
Pituitary tumours account for 10–15% of clinically symptomatic intracranial neoplasms1 and contribute to a significant proportion of neurosurgical referrals to ophthalmology units. As well as visual dysfunction, complications include the effects of hormone hypersecretion, hypopituitarism, headaches, and epilepsy.2 The diagnosis of this type of lesion at an early stage is therefore of importance to the prognosis of the patient, particularly as early intervention is of known benefit.3–6
The management of patients with pituitary tumours includes surgical and medical treatments, and both have been shown to be beneficial to patients with pituitary tumour in terms of preservation of vision and amelioration of visual dysfunction.3–6 The prompt diagnosis of this disorder, with timely and appropriate intervention when vision is threatened, is an important clinical consideration.
Visual field defect is a common mode of presentation of these patients, and knowledge of the types of visual field abnormality in patients with pituitary tumour is therefore important. The cause of visual field loss may be due to direct compression of the tumour on the anterior visual pathways, and, although less direct, vascular or other mechanisms may also contribute.7 The typical field defects of bitemporal hemianopias and quadrantanopias are known to be associated with pituitary tumour, although other types of field defect have been described.8–13 Elkington2 reported visual field defects in 92.6% of his series, with the majority (70.7%) being varieties of bilateral temporal loss. Rowe and colleagues14 reported visual field defects in 56% of their cases, with bilateral field loss being most frequent but a mix of temporal and nasal loss. The variability of field defects can be explained by compression of the chiasm, optic nerves, and optic tracts or combinations of these structures.14 Compression of the chiasm may be symmetrical or asymmetrical relating to the tumour size and its degree of extension involving the chiasm, optic nerve, and optic tract.12 Symmetrical or asymmetrical compression is reflected by the presence of bilateral or unilateral visual field defects.14 This emphasises the importance of further investigation of patients presenting with field defects unexplained by ocular or other neurological disease.
The assessment of loss of visual field is difficult and depends both upon the patient reporting their visual experience during testing, as well as the interpretation of these reports by a clinician. Visual fields are usually measured with perimeters such as the Humphrey field analyser, Octopus perimeter, and Goldmann perimeter. Although such methods do reduce the errors of interpretation and improve standardisation, the significant variability of such testing is still a well-recognised and studied phenomenon.15,16
Static automated perimetry has been shown to be adequate in neuro-ophthalmology practice, whereas kinetic perimetry is useful for patients with severe visual and neurological deficits and patients with peripheral visual field defects.17,18 In the context of early detection of visual involvement in pituitary tumours, it is important to be able to detect subtle visual field defects, particularly those that arise in the peripheral visual field, which is typically the area of visual field first compromised by pituitary compression. Given the advances in perimetry over recent years, with the current availability of faster thresholding programmes and semi-automated kinetic programmes, the purpose of this study is to compare these methods for diagnostic accuracy in detecting visual field defects due to pituitary disease. Our primary aim was to determine whether visual field results using the Humphrey perimeter (static) or Octopus perimeter (kinetic and static) are equally effective in detecting subtle visual field loss due to neurological impairment in pituitary disease.
Materials and Methods
Design
A prospective cohort study was undertaken in accordance with the Tenets of the Declaration of Helsinki. Regional ethics committee and institutional research and development unit approvals were obtained. We undertook a comparative study of the diagnostic performance (agreement between two diagnostic tests) in two hospital outpatient ophthalmology units.
Population
The target population was patients with pituitary disease attending National Health Service (NHS) eye clinic appointments for visual field assessment between April and July 2013.
Inclusion Criteria
We included adult patients aged 18 years or older with pituitary disease requiring visual field assessment, sufficient motor ability to sit at the perimeter unaided, able to press the response button, sufficient cognitive ability to understand and follow instructions for performing the test, and willingness to undergo standard assessment on both perimeters on the same day.
Exclusion Criteria
We excluded patients with poor reliability, determined by cumulative fixation loss and false-positive and/or false-negative catch trials of greater than 25%. For kinetic perimetry, visual field results were deemed unreliable if patient fixation was considered poor by the examiner (by observation on the Octopus eye monitor) or if the blind spot could not be mapped. For static perimetry, visual field results were deemed unreliable if a score of greater than 25% was recorded for fixation losses, false positives, and/or false negatives. We also excluded those undertaking visual field assessments other than 30-2 programmes and patients who were unable to sit for the duration of perimetry assessment, were unable to follow instructions for performing the test, or were too ill to complete the full assessment.
Recruitment
Patients with pituitary disease were recruited to assess the level of agreement for detection of visual field loss between the Octopus semi-automated kinetic peripheral perimetry and Humphrey static automated central perimetry techniques.
We recruited patients with pituitary disease attending visual field clinics. Patients were required to only undergo one additional visual field assessment at one clinic visit. No follow-up was required. Participants were pre-selected for the study by identifying patients consecutively from the waiting list for visual field assessment during the period April–July 2014. Thus, the selection procedure was not completely random. A selection bias existed in that the patients recruited to this study were booked to an outpatient visual field clinic for static perimetry. Therefore, there was an assumption that these patients had sufficient ability and cognition to undertake standard automated perimetry.
Patients attending the visual field clinic were approached and provided with a participant information sheet. Once the patient had time to read the sheet, they were asked whether they were interested in taking part. For those willing to participate, they were assessed against the inclusion criteria, after which informed, written consent was obtained. Nine patients declined to take part in the study. Reasons for declining to take part included a lack of time to undertake the additional test during the appointment. A further 10 patients failed to meet the inclusion criteria.
Visual Field Assessment Measures
The Humphrey 30-2 programme and Octopus semi-automated kinetic perimetry option were used for this study. The 30-2 programme was utilised on the Humphrey perimeter. This programme consists of 76 stimulus locations offset from the vertical and horizontal meridia and interspaced by 6-degree intervals. The programme assesses the visual field out to 30 degrees, and background illumination is set at 31.5 asp. We wished to consider the 24-2 programme, which consists of 54 stimulus locations. This was not assessed with our target population in this study. However, the target locations for the 24-2 were included in the 30-2 programme. Therefore, we extracted the data for these target locations.
A standardised kinetic strategy17 was programmed into the Octopus 900 perimeters used in this study such that the same programme was used across the two recruitment sites. Two stimuli of the same size (0.25 mm2) were used but of different intensity (I4e, 1000 apostilbs and I2e, 100 apostilbs). The peripheral visual field boundary and blind spot were assessed using a size I4e target. Central visual field boundary was assessed using a size I2e target. A minimum of 12 vectors were assessed for the peripheral visual field and 8 for the central visual field inclusive of vectors on and offset from the vertical and horizontal meridia moving centripetally, similar to previously reported testing strategies.17,18 Where a visual field defect was found, this was further evaluated by examiner intervention using additional vectors, with direction of target movement perpendicular to the boundary of the field defect. Following assessment, the response points along each vector were joined to form the isoptre for I4e and I2e targets, respectively. In addition, static points were assessed within the central 30 degrees of the visual field using the I4e target.
Full (normal) visual fields by kinetic assessment were defined as visual field results with isoptres for I4e and I2e falling within age-matched ranges (from the Octopus normative data set) and no focal defects within the isoptre area (apart from the blind spot in the temporal field). Visual field loss was defined as isoptre boundaries to either I4e or I2e targets constricted within the age-matched ranges, which could be global constriction or a defect type. The criteria for abnormality on Humphrey perimetry included mean deviation (MD) >2 dB and/or pattern standard deviation (PSD) value of >6 dB, ≥3 contiguous points at p < 5% forming a focal defect, glaucoma hemifield test outside normal limits, or a combination of any two of the above.
Classification of visual field types was according to nerve fibre layer and non-nerve fibre layer types.17,18 Visual field results were also graded according to the degree of visual field loss, if any, and ranging from 0 (normal visual field) to 5 (blinding visual field loss).19
The study protocol consisted of the 30-2 strategy visual field assessment and semi-automated kinetic visual field assessment with Humphrey perimetry and Octopus perimetry on the same day. The order of testing was randomised as to which of the two assessment types plus which eye (right/left) was undertaken first in order to take fatigue effect and learning effect into consideration. A short break of 5–10 minutes was allowed between testing on either perimeter. Age- and instrument-appropriate reading correction was used during the assessment. The assessments were undertaken by the same observer in each recruitment site using standardised computer automated programmes; therefore, variability between observers was not assessed.
Statistical Analysis
Our primary outcome measure was presence/absence of visual field loss using the Octopus and Humphrey perimeters. Visual field results from Humphrey and Octopus perimetry were assessed for presence or absence of visual field defects and for type and location of visual field defect as per classification options. Results of the evaluation of the Humphrey and Octopus methods to identify visual field loss were reported for the right and left eyes separately and for the eyes combined.
Level of agreement was measured using the Cohen’s kappa statistic when applicable (varying from 0 = no agreement to 1 = perfect agreement). Bearing in mind that the data of the right and left eyes cannot be strictly regarded as independent, level of agreement was assessed at patient level (i.e. three categories were considered, mainly no visual loss, visual loss in one eye, visual loss in both eyes).
Comparisons in grading between Octopus and Humphrey visual field scores were made.
RESULTS
Equal numbers of males and females were recruited (25 males and 25 females). Mean age at assessment was 52.4 years (SD = 15.7, range: 18–83 years). Following randomisation, 48% were tested with the Humphrey perimeter first and 52% were tested with the Octopus perimeter first. The right eye was tested first in 56% and the left eye tested first in 44%. Every patient had their right and left eyes tested (100 eyes).
Humphrey Perimetry
We classified visual fields for our 30-2 assessments using a binary response (yes/no) for presence of visual field loss. Eighty-five percent were classified as having visual field loss present. We reclassified the visual field by excluding the peripheral test locations that are tested in the 30-2 programme but not in the 24-2 programme (Figure 1) to determine whether visual field loss still remained even if the 24-2 programme had been run instead of the 30-2 programme. Eighty percent were classified as having visual field loss. Thus, the 24-2 programme would have missed five abnormal visual fields that were detected by the 30-2 programme (Table 1A). The classification by the two programmes at patient level is shown in Table 1(B). The non-weighted Kappa agreement coefficient between the two programmes is 0.72, 95% confidence interval (CI) (0.50, 0.94). Despite this value being considered as a good level of agreement between methods in some studies, the wide confidence interval indicates that the level of precision is low. In this case, note that since the 24-2 programme is encapsulated in the 30-2 programme, the outcomes from each programme are not independent from each other.
FIGURE 1.
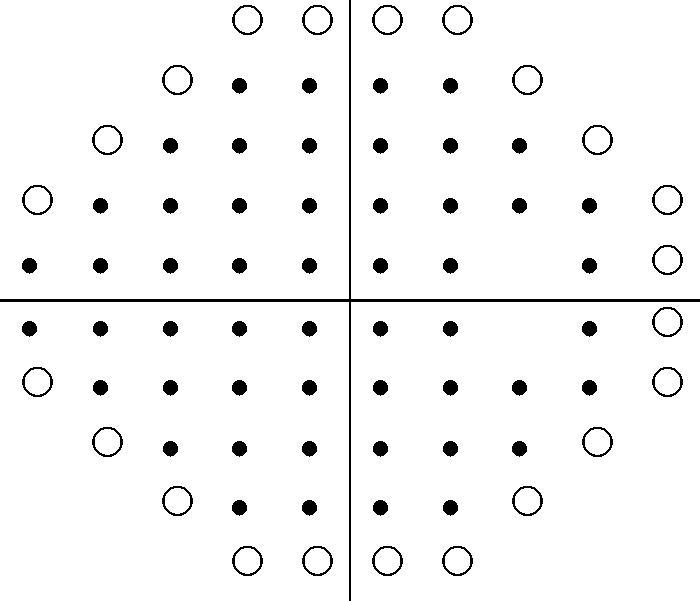
30-2 minus 24-2 peripheral points. This is an example for the right eye showing the stimulus locations points for the 30-2 and 24-2 threshold programmes. • indicates points seen on both 30-2 and 24-2 programmes. ○ indicates points seen only on the 30-2 programme but omitted from testing on the 24-2 programme.
TABLE 1. Humphrey 30-2 and 24-2 strategy responses.
| (A) Detection of visual loss | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Humphrey 30-2 |
|||||||||
| Left eye |
Right eye |
Both eyes |
|||||||
| Visual loss | No visual loss | Total | Visual loss | No visual loss | Total | Visual loss | No visual loss | Total | |
| Humphrey 24-2 | |||||||||
| Visual loss | 39 | 0 | 39 | 41 | 0 | 41 | 80 | 0 | 80 |
| No visual loss | 3 | 8 | 11 | 2 | 7 | 9 | 5 | 15 | 20 |
| Total | 42 | 8 | 50 | 43 | 7 | 50 | 85 | 15 | 100 |
(B) Strategy responses at patient level for the four programmes.
| Humphrey |
Octopus |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24-2 |
30-2 |
I2e |
I4e |
Overall |
|||||||||||||
| Method | Sub-method | Visual loss (eyes) | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 |
| Humphrey | 24-2 | 0 | – | – | – | 7 | 0 | 0 | 0 | 1 | 6 | 2 | 0 | 5 | 0 | 0 | 7 |
| 1 | – | – | – | 0 | 1 | 5 | 0 | 0 | 6 | 0 | 1* | 5 | 0 | 0 | 6 | ||
| 2 | – | – | – | 0 | 0 | 37 | 0 | 0 | 37 | 0 | 1 | 36 | 0 | 0 | 37 | ||
| 30-2 | 0 | 7 | 0 | 0 | – | – | – | 0 | 1 | 6 | 2 | 0 | 5 | 0 | 0 | 7 | |
| 1 | 0 | 1 | 0 | – | – | – | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | ||
| 2 | 0 | 5 | 37 | – | – | – | 0 | 0 | 42 | 0 | 2 | 40 | 0 | 0 | 42 | ||
| Octopus | I2e | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 1 | 0 | 0 | 1 | 0 | 0 | – | – | – | 0 | 0 | 1 | 0 | 0 | 1 | ||
| 2 | 6 | 6 | 37 | 6 | 1 | 42 | – | – | – | 2 | 2 | 45 | 0 | 0 | 49 | ||
| I4e | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | – | – | – | 0 | 0 | 2 | |
| 1 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | – | – | – | 0 | 0 | 2 | ||
| 2 | 5 | 5 | 36 | 5 | 1 | 40 | 0 | 1 | 45 | – | – | – | 0 | 0 | 46 | ||
| Overall | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | ||
| 2 | 7 | 6 | 37 | 7 | 1 | 42 | 0 | 1 | 49 | 2 | 2 | 46 | – | – | – | ||
Visual loss (eyes): 0 = no visual loss; 1 = visual loss in one eye; 2 = visual loss in both eyes.
*Humphrey 24-2 and 4e Octopus found visual loss in different eyes.
Octopus Perimetry
In order to assess whether visual field loss in pituitary damage shows a different level of detection when comparing peripheral stimulus and a dimmer (so potentially more sensitive) central stimulus, we assessed the presence/absence of visual field loss to either stimulus. With the I4e target, 94% were classified as having visual field loss present and 6% were classed as normal visual fields (Table 2). With the I2e target results, 99% were classified as having visual field loss present and 1% had a normal visual field result. Overall, 100% were classified as having visual field loss present when I4e and I2e targets were combined.
TABLE 2. Octopus I4e and I2e strategy responses.
| I2e target |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Left eye |
Right eye |
Both eyes |
|||||||
| Visual loss | No visual loss | Total | Visual loss | No visual loss | Total | Visual loss | No visual loss | Total | |
| I4e target | |||||||||
| Visual loss | 46 | 1 | 47 | 47 | 0 | 47 | 93 | 1 | 94 |
| No visual loss | 3 | 0 | 3 | 3 | 0 | 3 | 6 | 0 | 6 |
| Total | 49 | 1 | 50 | 50 | 0 | 50 | 99 | 1 | 100 |
Humphrey and Octopus Perimetry
Eighty-five percent of Humphrey 30-2 results were classified as having visual field loss present compared with 100% of Octopus results (Table 3A). All results of visual field loss on Octopus perimeter were considered clinically valid based on the definition of visual field loss outlined in Materials and Methods and were therefore regarded as the gold standard. In comparison with Octopus perimeter results, the sensitivity of the Humphrey 30-2 method is 85%. Consequently, 15% of the cases with visual field loss would be missed by the Humphrey 30-2 method. When the Octopus results were compared with the Humphrey 24-2 results, the sensitivity of the 24-2 method was 80%; therefore, 20% of the cases with visual field loss were missed by this method (Table 3B).
TABLE 3. Humphrey and Octopus perimetry responses.
| (A) 30-2 versus Octopus | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Humphrey 30-2 |
|||||||||
| Left eye |
Right eye |
Both eyes |
|||||||
| Visual loss | No visual loss | Total | Visual loss | No visual loss | Total | Visual loss | No visual loss | Total | |
| Octopus | |||||||||
| Visual loss | 42 | 8 | 50 | 43 | 7 | 50 | 85 | 15 | 100 |
| No visual loss | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total |
42 | 8 | 50 | 43 | 7 | 50 | 85 | 15 | 100 |
| (B) 24-2 versus Octopus | |||||||||
| Humphrey 24-2 | |||||||||
| Octopus | |||||||||
| Visual loss | 39 | 11 | 50 | 41 | 9 | 41 | 80 | 20 | 100 |
| No visual loss | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 39 | 11 | 50 | 41 | 9 | 41 | 80 | 20 | 100 |
A qualitative comparison of Humphrey and Octopus results was undertaken in which one observer (F.R.) expressed an opinion of whether the visual field defect (if present) was depicted more clearly on either Humphrey or Octopus result or whether there was no difference in how clearly visual field defect was identified. The field defect was deemed easier to identify on Octopus in 48 results, easier to identify on Humphrey in 21 results, and with no difference in representation on either perimeter in 31 results.
We undertook a further qualitative comparison of whether the results from Octopus and Humphrey perimetry were a “match” for type of visual field defect: recorded as a binary result of match/mismatch. Fifty-eight percent were deemed a match and 42% were not, e.g. superior defect detected by both methods (Table 4A).
TABLE 4. Grading of visual field defect.
| (A) Type of visual field defect | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Octopus field defect type |
|||||||||||
| Normal | Arcuate | Functional | Homonymous hemianopia | Bitemporal hemianopia | Inferior defect | Inferior quadrantanopia | Superior defect | Increased blind spot | Vertical step | Total | |
| Humphrey field defect type | |||||||||||
| Normal | 1 | 0 | 7 | 0 | 0 | 0 | 0 | 9 | 0 | 4 | 21 |
| Arcuate | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Functional | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 10 |
| Homonymous hemianopia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Bitemporal hemianopia | 0 | 0 | 1 | 0 | 14 | 0 | 0 | 4 | 0 | 1 | 20 |
| Inferior defect | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 4 |
| Inferior quadrantanopia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| Superior defect | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 31 | 0 | 0 | 34 |
| Increased blind spot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 5 |
| Vertical step | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 1 | 0 | 24 | 1 | 15 | 2 | 0 | 50 | 0 | 7 | 100 |
Following a qualitative comparison between Octopus and Humphrey perimetry in severity of visual loss, only 50% of the cases were deemed a “match” (Table 4B).
(B) Severity of visual field defect.
| Octopus field grade |
|||||||
|---|---|---|---|---|---|---|---|
| Normal | Minimal | Mild | Moderate | Marked | Blinding | Total | |
| Humphrey field grade | |||||||
| Normal | 1 | 13 | 7 | 0 | 2 | 0 | 23 |
| Minimal | 0 | 24 | 2 | 2 | 0 | 0 | 28 |
| Mild | 0 | 9 | 11 | 1 | 0 | 0 | 21 |
| Moderate | 0 | 3 | 4 | 7 | 0 | 0 | 14 |
| Marked | 0 | 0 | 3 | 3 | 5 | 1 | 12 |
| Blinding | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Total | 1 | 49 | 27 | 13 | 7 | 3 | 100 |
Test Duration
Duration of test per eye was recorded for both Octopus and Humphrey perimetry. Due to the possible effect of correlation between the left and right eye measurements, we performed a paired t test on the total duration per individual for the Humphrey and Octopus programmes. Duration of test for Humphrey 30-2 assessment was 9.34 minutes (SD = 2.02) and for Octopus kinetic assessment was 10.79 minutes (SD = 4.06). The Octopus assessment was slightly longer because of the combined kinetic and static assessments, and this was statistically significant in comparison with Humphrey perimetry, with a difference between means of 1.44 minutes (SD = 3.43; p = 0.004, paired t test).
DISCUSSION
All patients recruited to this pilot study had static threshold and kinetic perimetry within the same assessment visit. Types of visual field loss in pituitary disease typically include temporal defects (bitemporal hemianopia, bitemporal quadrantanopia) but also nasal visual field loss, scotomas, and wedge defects.14 The location and extent of visual field loss is dependent on the site of visual pathway compression.
When using central threshold perimetry, commonly utilised programmes in UK clinics are the 30-2 and 24-2 options on the Humphrey visual field analyser and G programme on the Octopus perimeter. Both the 30-2 and G programmes test the visual field out to 30 degrees in all directions from central fixation. The 24-2 programme tests the visual field out to 30 degrees in the nasal visual field but out to 24 degrees only in the superior, inferior, and temporal visual fields. Our first objective was to assess whether a difference in detection of visual field loss might occur with use of the 24-2 rather than the 30-2 programme where fewer points are tested in the temporal visual field. Although overall there was a good level of agreement between the two programmes, the 30-2 detected visual field loss in five more eyes (5%) than the 24-2 option.
Kinetic perimetry uses targets of different size and intensity to measure the field of vision. Our second objective was to assess whether a difference in detection of visual field loss might occur with use of different target intensities (size I4e versus size I2e). We observed a sensitivity for detection of visual field loss of 99% by the I2e target and of 94% by the I4e target. When both targets were used together, the combination detected all visual field defects. Combined use of both targets has been recommended in a previous study comparing peripheral static and kinetic programmes.17
Our third objective considered whether a difference in detection of visual field loss might occur with use of kinetic rather than threshold static perimetry. Visual field results on Octopus kinetic perimetry were compared with age-matched boundaries from the Octopus normative data set. Visual field results on Humphrey static perimetry were compared with the age-matched thresholds from the Humphrey normative statpac data set. These normative data sets are based on different populations, so it is theoretically possible, although unlikely, that visual field defects may show up more clearly on comparison with one normative data set than another. It is more likely that the differences relate to measurement of peripheral than purely central visual fields.
Previous comparative studies have contrasted semi-automated kinetic perimetry using static perimetry within the central 30 degrees in ocular diseases such as advanced glaucoma, optic neuritis, and optic nerve head drusen. These studies have reported good comparisons and test-retest reliability.12,20–24 Similar comparisons for neuro-ophthalmic cases have been reported with equal reliability in 77% of eyes.25 In our study with the 30-2 programme assessment, 85% of results showed visual field loss and 15% were normal with full central visual fields. With kinetic assessment, 100% of results showed visual field loss. Thus, 15% of results with peripheral visual field loss were missed by central threshold assessment, which was statistically significant. This is clinically important, as pituitary disease can cause peripheral visual field loss and early diagnosis is essential to allow prompt intervention. In this study we did not undertake a peripheral static testing programme. A previous study compared the Humphrey peripheral static screening programme (full field 120) with an Octopus peripheral kinetic strategy.18 A match for normal or abnormal visual field results was reported for 87% of the cases. The authors concluded that although the FF120 was useful for detection of visual field defects, Octopus kinetic perimetry was preferable, as it provided added information of the defect depth and size plus a more representative view of the visual field defect.18
Visual field results are displayed quite differently between kinetic and threshold programmes, and given the potential for stato-kinetic dissociation (where static results appear worse than kinetic results26), we considered whether either method displayed the visual field result more clearly than the other. This was determined purely by a qualitative evaluation of each visual field result from either perimeter as described previously.19 In 42% of the assessments there was a difference in the grading of visual field defect observed between Octopus and Humphrey (30-2 programme). Mismatch of visual field results typically was related to differences in normal versus functional, superior defect, or vertical step results, and more extensive visual fields on one perimeter result such as hemianopia depicted on one result with inferior or superior defect on the other. This has been similarly reported previously.18 In relation to pituitary disease, 2.6% had normal Humphrey results but corresponding Octopus results showed peripheral superior defects. Octopus perimetry was twice as likely to show a clearer representation of the visual field than Humphrey perimetry. On comparing severity of field loss, 50% were graded equally as having the same severity of defect on either perimeter. A difference of one grade, e.g. minimal versus mild and mild versus moderate, was found for 33%; 13% in which the Humphrey result was normal but the Octopus result showed a mild visual field defect. A difference of two grades, e.g. minimal versus moderate and mild versus marked was found for 15%; 7% in which the Humphrey result was normal but the Octopus result showed a mild visual field defect. Such differences are clinically significant but mainly represent the presence of a peripheral visual field defect found on Octopus perimetry that was not/less evident on the central programme from Humphrey perimetry. We acknowledge that these clinical grades of mismatch of visual fields for visual field defect type and severity are purely based on qualitative evaluation where there is the potential for observer bias, similar to the reported literature.17,18,27,28
CONCLUSIONS
There is clinical significance to detection of visual field loss in pituitary disease, and capturing peripheral loss is important to the early diagnosis of chiasmal involvement. Based on our results, we recommend that, where central threshold perimetry is conducted on patients with pituitary disease, the full 30 degrees is tested and programmes such as the 24-2 option are avoided. Where it is possible to measure kinetic peripheral visual fields, this is preferable to static central visual fields for increased detection of peripheral visual field loss. When conducting kinetic perimetry, the combined use of peripheral (I4e) and central (I2e) targets increases sensitivity to detection of visual field loss.
Acknowledgements
We should like to thank the patients who took part in this study, and Nicola Beere, Royal Bolton Hospital, for permission to use their perimeter.
Declaration of interest: The authors do not have any commercial or proprietary interest in the Octopus 900 perimeter and Humphrey automated perimeter or Haag Streit International and Carl Zeiss UK. Haag Streit has provided the loan of the Octopus 900 perimeter for the conduct of this research study. Haag Streit and Carl Zeiss UK had no role in the design or conduct of this research.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Levy A, Lightman SL. Diagnosis and management of pituitary tumors. BMJ 1994;308:1087–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elkington SG. Pituitary adenoma—preoperative symptomatology in a series of 260 patients. Br J Ophthalmol 1968;52:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peter M, De Tribolet N. Visual outcome after transphenoidal surgery for pituitary adenomas. Br J Neurosurg 1995;9:151–157 [DOI] [PubMed] [Google Scholar]
- 4.Mbanya JC, Mendelow AD, Crawford PJ, Hall K, Dewar JH, Kendall-Taylor P. Rapid resolution of visual abnormalities with medical therapy alone in patients with large prolactinomas. Br J Neurosurg 1993;37:519–527 [DOI] [PubMed] [Google Scholar]
- 5.Sullivan LJ, McNeill P. Visual outcomes of pituitary adenoma surgery. St. Vincent's Hospital 1968–1987. J Clin Neuro-Ophthalmol 1991;11:262–267 [PubMed] [Google Scholar]
- 6.Findlay G, McFadzean RM, Teasdale G. Recovery of vision following treatment of pituitary tumors: application of a new system of visual assessment. Trans Ophthalmol Soc U K 1983;103:212–216 [PubMed] [Google Scholar]
- 7.Wybar K. Chiasmal compression. Proc R Soc Med 1977;70:307–317 [PMC free article] [PubMed] [Google Scholar]
- 8.Trobe JD. Chromophobe adenoma presenting with a hemianopic temporal arcuate scotoma. Am J Ophthalmol 1974;77:388–392 [DOI] [PubMed] [Google Scholar]
- 9.Katz J, Sommer A. A longitudinal study of the age-adjusted variability of automated visual fields. Arch Ophthalmol 1987;105:1083–1086 [DOI] [PubMed] [Google Scholar]
- 10.Heijl A, Lindgren A, Lindgren G. Test-retest variability in glaucomatous eyes. Am J Ophthalmol 1989;108:130–135 [DOI] [PubMed] [Google Scholar]
- 11.Kearns TP, Rucker CW. Arcuate defects in the visual fields due to chromophobe adenoma of the pituitary gland. Am J Ophthalmol 1958;45:505--507 [DOI] [PubMed] [Google Scholar]
- 12.Keltner JL, Johnson CA, Cello KE, Edwards MA, Bandermann SE, Kass MA, Gordon MO for the OHTS Group Classification of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol 2003;121:643–650 [DOI] [PubMed] [Google Scholar]
- 13.Heijl A, Patella VM, Bengtsson B. Effective Perimetry (Zeiss Visual Field Primer). 4th ed. Dublin, California/Jena, Germany: Carl Zeiss Meditec, Inc.; 2012 [Google Scholar]
- 14.Rowe FJ, Thompson C, Webster AR. Incidence of bitemporal hemianopic visual field defects in pituitary tumours. In: Louly M, Doyle M, Hirai T, Tomlinson E, editors. Transactions of the 9th International Orthoptic Congress, Kyoto, Japan, 8--11 October. International Orthoptic Association; 1995:279–283
- 15.Hershenfeld SA, Sharpe JA. Monocular temporal hemianopia. Br J Ophthalmol 1993;77:424–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bird AC. Field loss due to lesions at the anterior angle of the chiasm. Proc R Soc Med 1972;65:519–520 [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe FJ, Rowlands A. Comparison of diagnostic accuracy between Octopus 900 and Goldmann kinetic visual fields. BioMed Res Int 2014;214829. doi: 10.1155/2014/214829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe FJ, Noonan CP, Manuel M. Comparison of Octopus semi-automated kinetic perimetry and Humphrey peripheral static perimetry in neuro-ophthalmic cases. ISRN Ophthalmol 2013;753202. doi: 10.1155/2013/753202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wall M, George DN. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain 1991;114:155–180 [PubMed] [Google Scholar]
- 20.Keltner JL, Johnson CA, Spurr JO, Beck RW. Comparison of central and peripheral visual field properties in the optic neuritis treatment trial. Am J Ophthalmol 1999;128:543–553 [DOI] [PubMed] [Google Scholar]
- 21.Nowomiejska K, Vonthein R, Paetzold J, Zagorski Z, Kardon R, Schiefer U. Comparison between semiautomated kinetic perimetry and conventional Goldmann manual kinetic perimetry in advanced visual field loss. Ophthalmology 2005;112:1343–1354 [DOI] [PubMed] [Google Scholar]
- 22.Nowomiejska K, Rejdak R, Zagorski Z, Zarnowski T. Comparison of static automated perimetry and semi-automated kinetic perimetry in patients with bilateral visible optic nerve head drusen. Acta Ophthalmol 2009;87:801–805 [DOI] [PubMed] [Google Scholar]
- 23.Nowomiejska K, Vonthein R, Paetzold J, Zagorski Z, Kardon R, Schiefer U. Reaction time during semi-automated kinetic perimetry (SKP) in patients with advanced visual field loss. Acta Ophthalmol 2010;88:65–69 [DOI] [PubMed] [Google Scholar]
- 24.Nevalainen J, Paetzold J, Krapp E, Vonthein R, Johnson CA, Schiefer U. The use of semi-automated kinetic perimetry (SKP) to monitor advanced glaucomatous visual field loss. Graefes Arch Clin Exp Ophthalmol 2008;246:1331–1339 [DOI] [PubMed] [Google Scholar]
- 25.Szatmáry G, Biousse V, Newman NJ. Can Swedish interactive thresholding algorithm fast perimetry be used as an alternative to Goldmann perimetry in neuro-ophthalmic practice? Arch Ophthalmol 2002;120:1162–1173 [DOI] [PubMed] [Google Scholar]
- 26.Gandolfo E. Stato-kinetic dissociation in subjects with normal and abnormal visual fields. Eur J Ophthalmol 1996;6:408–414 [DOI] [PubMed] [Google Scholar]
- 27.Pineles SL, Volpe NJ, Miller-Ellis E, Galetta SL, Sankar PS, Shindler KS, Maguire MG. Automated combined kinetic and static perimetry. Arch Ophthalmol 2006;124:363–369 [DOI] [PubMed] [Google Scholar]
- 28.King AJ, Taguri A, Wadood AC, Azuara-Blanco A. Comparison of two fast strategies, SITA Fast and TOP, for the assessment of visual fields in glaucoma patients. Graefes Arch Clin Exp Ophthalmol 2002;240:481–487 [DOI] [PubMed] [Google Scholar]


