Abstract
We have studied the clinical picture of anti-aquaporin antibody (AQP4-Ab)– and anti-myelin oligodendrocyte glycoprotein antibody (MOG-Ab)–positive optic neuritis. However, optic neuritis associated with MOG-Abs has not been elucidated using new methods such as cell-based assay. Hence, we conducted a comprehensive investigation on its clinical profile. Serum samples from 70 patients (17 males and 53 females, mean age 43.1 years) with optic neuritis were tested for MOG-Abs by cell-based assay. In MOG-Ab seropositive patients, the disease type, recurrence status, and visual function outcome were analysed. Among 70 patients, 18 were MOG-Ab seropositive. The 18 patients comprised 2 with chronic relapsing inflammatory optic neuropathy, 2 with AQP4-Ab seropositive optic neuritis (neuromyelitis optica), 12 with idiopathic optic neuritis, and 2 with optic neuritis associated with multiple sclerosis. Excluding two cases that were also AQP4-Ab seropositive, MOG-Ab seropositive cases had relatively favourable visual acuity outcome (although not significantly different from seronegative cases) but had significant residual visual field deficit (p = 0.0015). Furthermore, the number of relapses of optic neuritis per year was significantly greater in MOG-Ab seropositive cases than in seronegative cases (0.82 vs. 0.40; p = 0.0005). MOG-Abs may contribute to the heterogeneous clinical picture of optic neuritis, and although visual acuity outcome is favourable, there is a tendency of residual visual field deficit and a possibility of repeated relapses.
Keywords: Anti-aquaporin 4 antibodies, anti-myelin oligodendrocyte glycoprotein, cell-based immunofluorescence assay, optic neuritis, recurrent rate, visual field defect
INTRODUCTION
Optic neuritis (ON) has diverse pathogenesis and a broad clinical spectrum ranging from spontaneous remission without treatment to disorders with poor prognosis, such as neuromyelitis optica (NMO) with acute onset and repeated relapses resulting in vision loss.1 In 1999, Wingerchuk et al.2 broadened the clinical criteria for diagnosing NMO to include any of the following: unilateral optic neuritis, any interval between the first events of optic neuritis and myelitis, and a relapsing course. In recent years, the relationship between anti-aquaporin 4 antibodies (AQP4-Abs) and neuromyelitis optica has been elucidated and treatment protocols have been established.3,4 In acute-phase treatment of NMO, it is important to induce remission of NMO by intravenous methylprednisolone therapy to reduce inflammation followed by plasmapheresis to remove the antibodies. AQP4 molecules are known to be present on astrocytes, a type of glial cells present in the central nervous system. As a rationale for the above treatment regimen, the specific antibodies are speculated to prevent binding of AQP4 molecules on astrocytes, which mediates complement fixation reaction and ultimately cell death. NMO consists of AQP4-Ab seropositive cases with poor prognosis, and other cases with relatively favourable prognosis that recur repeatedly.5 Recent studies have shown that among AQP4-Ab seronegative ON cases with relatively good prognosis but repeated relapses, some are seropositive for myelin oligodendrocyte glycoprotein (MOG) antibodies.6,7 In addition, MOG is known to be a causative protein of experimental autoimmune encephalitis (EAE).8 MOG derived from oligodendrocytes induces ON in mouse, suggesting that MOG antigen may be a cause of multiple sclerosis–associated ON. Furthermore, high MOG-Ab titre has been detected predominantly in patients with recurrent ON.7 We have previously reported that concomitant presence of MOG-Abs in AQP4-Ab seropositive optic neuritis is associated with severe visual function impairment. However, MOG-Ab seropositive optic neuritis has not been elucidated using new methods such as cell-based assay. Therefore, we conducted a comprehensive investigation of the clinical profile of MOG-Ab–associated optic neuritis.
MATERIALS AND METHODS
A total of 70 patients who presented with optic neuritis at the Department of Ophthalmology of Tokyo Medical University between January 2008 and December 2013 were studied. The mean observation period was 2.8 ± 1.1 years (1.5–5 years). The subjects comprised 17 males and 53 females with ages ranging from 15 to 83 years (mean: 43.1 years). Serum samples were collected and MOG-Abs in serum were measured in all the patients before initiation of treatment. MOG-Abs were measured by a cell-based assay at Kanazawa Medical University by an investigator blinded to the clinical information of the patients.
We retrospectively reviewed the medical records of MOG-Ab seropositive patients with respect to disease type, recurrence status, visual acuity before and after treatment, and status of visual field impairment.
Magnetic resonance imaging (MRI) with short T1 inversion recovery (STIR) sequence or T2-weighted fat suppressed coronal and sagittal images were used for the diagnosis and evaluation of the acute phase of optic neuritis. Chronic relapsing inflammatory optic neuropathy (CRION) was diagnosed according to Kidd et al..9 Multiple sclerosis was diagnosed according to the McDonald criteria revised in 2005.10 Neuromyelitis optica was diagnosed according to Wingerchuk criteria.1,2
Visual field was measured by Goldmann kinetic perimeter at the last follow-up. Residual visual deficit was defined as the presence of any of the visual field deficit observed at disease onset.
Antibody Assays
MOG-Abs were measured using a cell-based assay according to the method described previously.11 A full-length human MOG cDNA expression vector (a kind gift from Dr. M. Reindl, Innsbruck Medical University, Innsbruck, Austria) was transfected into human embryonic kidney (HEK) 293 cells using Lipofectamine reagent (Invitrogen Japan, Tokyo, Japan). Cell cultures were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. Twelve hours after transfection, the HEK cells were fixed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4) for 20 minutes. Non-specific binding was blocked with 10% goat serum/PBS. Thereafter, the cells were incubated with patient sera diluted 1:10 in 0.02% Triton X-100/10% goat serum in PBS for 1 hour at room temperature followed by fluorescein isothiocyanate–conjugated anti-human immunoglobulin G (IgG; dilution, 1:50; Dako Denmark, Glostrup, Denmark) for 1 hour. SlowFade Gold anti-fade reagent (Invitrogen) was then applied to the slides, which were mounted and observed using a fluorescence microscope (AxioVision; Carl Zeiss Microscopy, Jena, Germany). The presence of green fluorescence on the cells was scored as MOG-Ab seropositive.
Serum anti-AQP4 IgG antibody levels were measured by a previously reported indirect immunofluorescence assay using HEK-293 cells transfected with an expression vector containing full-length human AQP4 cDNA.12
Measurements of MOG-Abs and anti-AQP4 antibodies were conducted after obtaining informed consent from the patients. This study was approved by the Medical Research Ethical Committee of Tokyo Medical University (Approval Nos. 1983, 1545).
Treatment of Optic Neuritis
For acute exacerbation of optic neuritis, patients were treated with two courses of corticosteroid pulse therapy, consisting of 1000 mg per day of methylprednisolone administered intravenously for 3 days given at a 1-week interval, followed by oral prednisone of 40–60 mg per day. Plasma exchange was performed by double-filtration plasmapheresis (DFPP) and only in patients who did not respond to pulse steroid therapy. The therapeutic effect of DEPP was evaluated based on pre- and post-treatment visual acuity and visual field measured by a Goldmann kinetic perimeter.
Statistical Analyses
The numbers of relapses in MOG-Ab–positive and–negative cases were compared using one-way analysis of variance (Wilcoxon/Kruskal-Wallis test, chi-square approximation). Comparisons of visual field improvement and visual acuity improvement in MOG-Ab–positive and –negative cases were analysed using chi-square test followed by Fisher exact test.
RESULTS
Disease Type
The disease types of all (70) patients were CRION in 2 patients, AQP4-Ab seropositive optic neuritis (neuromyelitis optica) in 13, idiopathic optic neuritis in 34, and optic neuritis associated with multiple sclerosis in 21.
Eighteen of 70 patients (25.7%) were positive for MOG-Abs. The 18 patients comprised 7 males and 11 females. The disease types of 18 MOG-Ab seropositive patients were CRION in 2 patients, AQP4-Ab seropositive optic neuritis (neuromyelitis optica) in 2, idiopathic optic neuritis in 12, and optic neuritis associated with multiple sclerosis in 2. The disease types and patient background are shown in Table 1. Eight patients had bilateral optic neuritis (Nos. 1, 3, 4, 5, 11, 13, 16, and 17). In patients with bilateral optic neuritis, we selected the more severe eye for evaluation in the present study. Accordingly, the left eye was studied in Nos. 1, 3, 4, 5, 13, 16, and 17, and the right eye in No. 11 (Table 2).
TABLE 1. Disease types of MOG-Ab seropositive cases.
| Disease type | No. of MOG-Ab seropositive cases/No. of all cases |
|---|---|
| Chronic relapsing inflammatory optic neuropathy (CRION) | 2/2 |
| Neuromyelitis optica (NMO) | 2/13 |
| Idiopathic optic neuritis | 12/34 |
| Multiple sclerosis (MS) with optic neuritis | 2/21 |
TABLE 2. Summary of individual MOG-Ab seropositive cases.
| Case no. | Age | Gender | Disease | Vision improvement | Relapse | Visual field deficit | Ocular pain | Bilateral/ unilateral |
|---|---|---|---|---|---|---|---|---|
| 1 | 26 | F | CRION | + | 4 | + | − | B |
| 2 | 30 | M | CRION | + | 4 | + | − | U(R) |
| 3 | 41 | F | NMO | + | 4 | + | − | B |
| 4 | 48 | F | NMO | − | 8 | + | − | B |
| 5 | 28 | F | Idiopathic optic neuritis | + | 2 | + | − | B |
| 6 | 39 | F | Idiopathic optic neuritis | + | 1 | + | − | U(R) |
| 7 | 52 | F | Idiopathic optic neuritis | + | 2 | + | − | U(L) |
| 8 | 42 | F | Idiopathic optic neuritis | + | 2 | + | − | U(L) |
| 9 | 64 | F | Idiopathic optic neuritis | − | 2 | + | − | U(R) |
| 10 | 50 | F | Idiopathic optic neuritis | + | 1 | − | − | U(L) |
| 11 | 51 | M | Idiopathic optic neuritis | + | 2 | − | + | B |
| 12 | 30 | F | Idiopathic optic neuritis | + | 1 | − | + | U(R) |
| 13 | 43 | M | Idiopathic optic neuritis | + | 1 | − | + | B |
| 14 | 18 | F | Idiopathic optic neuritis | + | 1 | + | − | U(L) |
| 15 | 52 | M | Idiopathic optic neuritis | + | 1 | + | + | U(R) |
| 16 | 51 | M | Idiopathic optic neuritis | + | 2 | + | − | B |
| 17 | 33 | M | MS with optic neuritis | + | 4 | + | − | B |
| 18 | 48 | M | MS with optic neuritis | + | 1 | + | + | U(L) |
CRION = chronic relapsing inflammatory optic neuropathy; NMO = neuromyelitis optica; MS = multiple sclerosis; B = bilateral; U = unilateral; R = right; L = left.
Number of Relapses
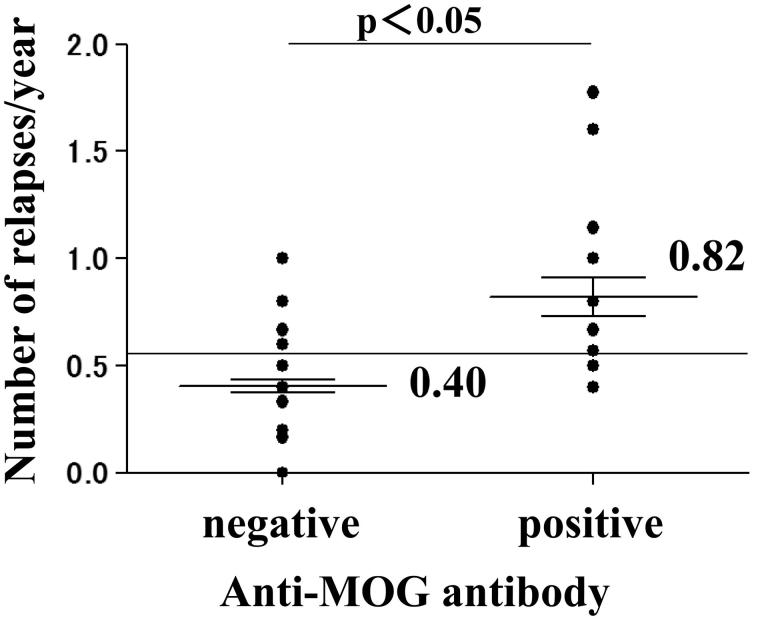
The numbers of relapses in all the MOG-Ab seropositive patients were investigated. Case 4 experienced 8 relapses, which was the largest number of all patients. The mean number of relapses per year was 0.40 in MOG-Ab seronegative patients and 0.82 in seropositive patients, and was significantly greater in MOG-Ab seropositive than in seronegative patients (Figure 1).
FIGURE 1.
Analysis of the number of relapses per year. The number of relapses per year was significantly greater in MOG-Ab seropositive cases than in seronegative cases (p < 0.05 by Wilcoxon/Kruskal-Wallis test, chi-square approximation).
Visual Outcome
Visual acuity and visual field deficit before and after treatment in MOG-Ab seropositive patients were analysed. In case 1 (CRION), visual acuity at disease onset was light perception negative. Subsequently, this case had 4 relapses. Although steroid pulse therapy improved visual acuity to 20/13, visual field deficit of enlarged Mariotte blind spot remained. All patients, excluding cases 4 and 9, had visual acuity improvement of two lines or more, but some kind of visual field deficit remained in all patients. The visual field at the time of acute exacerbation of optic neuritis showed diverse patterns, including central scotoma, paracentral scotoma, temporal field cut, and complete visual field cut.
The relation between MOG-Ab status and visual acuity improvement was analysed. In MOG-Ab seropositive patients, visual acuity did not improve in only 2 patients, 1 with AQP4-Ab optic neuritis and 1 with idiopathic optic neuritis, whereas visual acuity was improved in 16 of 18 patients (88.9%). In MOG-Ab seronegative patients, visual acuity was improved in 37 of 52 patients (71.2%) and was not significantly difference from the seropositive group.
Next, the relation between MOG-Ab status and visual field improvement was analysed. Visual field deficit remained after treatment in 14 of 18 (77.8%) MOG-Ab seropositive patients. In MOG-Ab seronegative patients, visual field deficit remained after treatment in 16 of 52 patients (30.8%). Comparing these figures, residual visual field defect was significantly more common in MOG-Ab seropositive patients (p = 0.0015).
Representative Cases
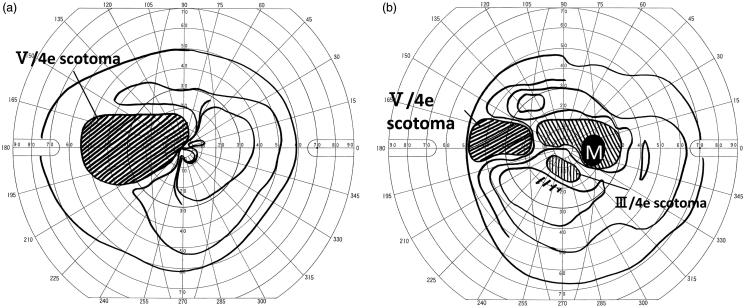
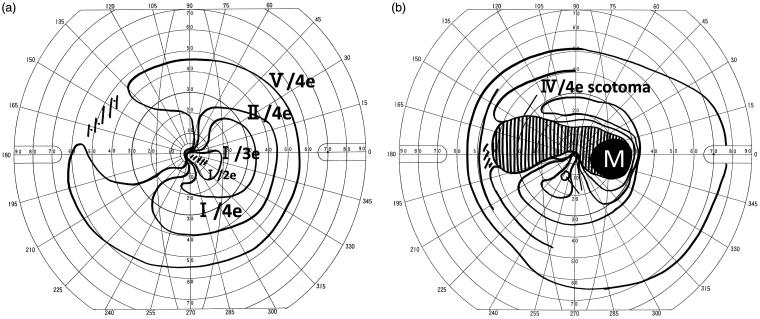
Figures 2 and 3 show the results of visual field measurements in a representative case (No. 17). A 33-year-old man with multiple sclerosis presented at our hospital because of decreased visual acuity and visual field abnormality in both eyes. Examination showed mild reddening of the optic disc in both eyes. Visual field examination showed central scotoma and nasal scotoma in the right eye and temporal scotoma in the left eye (Figure 2). Corrected visual acuity was 20/25 in the right eye and 20/66 in the left eye. MRI revealed hyperintensity in bilateral optic nerves. Optic neuritis associated with multiple sclerosis was diagnosed. In the cell-based assay for MOG-Abs, this case gave the strongest antigen-antibody reaction among the MOG-Ab seropositive cases. After steroid pulse therapy, corrected visual acuity improved to 20/22 in the right eye and 20/33 in the left eye. Thereafter the patient had 4 relapses. At each relapse, steroid pulse therapy preserved visual acuity, but Goldmann visual field test showed residual central scotoma in the right eye and temporal field cut in the left eye (Figure 3).
FIGURE 2.
Case 17: Goldmann visual field in left eye (a) and right eye (b) at initial onset. Pre-treatment visual field test showed scotoma from the centre to temporal side in the left eye (a), and central scotoma and nasal scotoma in the right eye (b). Corrected visual acuity was 20/66 in the left eye and 20/25 in the right eye.
FIGURE 3.
Case 17: Goldmann visual field of the left eye (a) and right eye (b) at the last follow-up. After the initial onset, the lesion recurred four times, and was treated with steroid pulse therapy every time. Visual field test at the last follow-up showed temporal field cut remaining in the left eye (a), and central scotoma remaining in the right eye (b). Corrected visual acuity was 20/22 in the right eye and 20/33 in the left eye, with improvement of 2 lines or more, which has been maintained until the present.
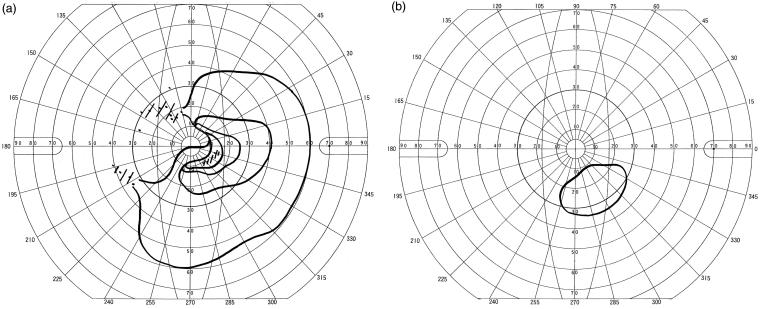
Figure 4 shows the visual field findings of the case (No. 4) that had the largest number of relapses. A 48-year-old woman had AQP4-Ab seropositive optic neuritis (neuromyelitis optica). Corrected visual acuity at onset was 20/500. She was treated with steroid pulse therapy and plasmapheresis, but subsequently had a total of 8 relapses (seven times in the left eye, once in the right eye). Visual field test at the last follow-up showed residual visual field in only a part of the inferior field (Figure 4), with corrected visual acuity of 20/2000 in the left eye.
FIGURE 4.
Case 4: Goldmann visual field of the left eye at initial onset (a) and at the last follow-up (b). After the initial onset, the patient had 8 subsequent relapses. At onset, widespread temporal field cut was observed (a), and corrected visual acuity was 20/500. Steroid pulse therapy and plasmapheresis were conducted at every relapse. Visual field test at the last follow-up showed residual visual field in only a part of the inferior field, and corrected visual acuity was 20/2000.
DISCUSSION
A recent study has recommended measurement of MOG-Abs in patients with neuromyelitis optica negative for anti-AQP4 antibodies considered to be specific for neuromyelitis optica.13 In the present study, MOG-Abs were positive in 18 of 70 patients with optic neuritis. The disease types found in MOG-Ab seropositive optic neuritis included CRION, neuromyelitis optica, idiopathic optic neuritis, and multiple sclerosis. Among them, idiopathic optic neuritis was the prominent type. Other report has also indicated that patients with MOG-Ab–positive neuromyelitis optica or neuromyelitis optica spectrum disorder tend to relapse.14 On the other hand, MOG-Abs are also found in multiple sclerosis patients, although the titres are low. In our series, MOG-Abs were positive in two cases of optic neuritis associated with multiple sclerosis. The disease with the largest number of MOG-Ab–positive cases was idiopathic optic neuritis. We also observed that MOG-Abs were not detected in anterior ischaemic optic neuropathy, suggesting a weak association of MOG-Abs with non-inflammatory ocular disease (data not shown). In addition, high MOG-Ab titres have been reported to be prominently detected in patients with recurrent optic neuritis.7 Case 17 in our series was strongly positive for MOG-Abs, and this case had 4 relapses with residual visual field deficit. Furthermore, in our previous report, cases double positive for MOG-Abs and AQP4-Abs were particularly refractory to treatment, progressed rapidly, and tended to become resistant to treatment.6 Case 4 in the present series was positive for both MOG-Abs and AQP4-Abs. In this case, despite courses of treatments, optic neuritis recurred repeatedly with residual visual field deficit resulting in no improvement in visual acuity.
MOG-Abs are a marker of demyelination in the central nervous system and not an indicator of astrocyte damage.11,15 MOG-Ab seropositive optic neuritis probably involves demyelination between the optic nerve and optic tract, which responds well to a sequence of early treatment resulting in improvement of central visual field but leaving residual peripheral visual field deficits. If regeneration of the damaged myelin sheath occurs, nervous function would recover with improvement of symptoms. However, when demyelination occurs repeatedly, delay in treatment may lead to axonal damage, and improvement in symptoms cannot be expected. In our series also, a significant number of cases had some kinds of residual visual field deficit.
The clinical characteristics of MOG-Ab seropositive optic neuritis include widespread involvement of the optic nerve and damages extending from the optic chiasma to the optic tract, which are similar to those of AQP4-Ab seropositive optic neuritis. Clinically, this disease responds to high-dose steroid therapy, but although visual acuity outcome is relatively good, visual field deficits remain, and the lesions tend to recur. This clinical picture suggests that MOG-Ab seropositive disease resembles CRION.
In recent studies, MOG-Abs are measured commonly by cell-based assays.2,6,13,16–19 Generally, measurement by enzyme-linked immunosorbent assay (ELISA) is influenced by the quantity and property of the protein, which may result in poor quantification and inadequate specificity. On the other hand, cell-based assay is performed by immunohistochemical staining and antigen-antibody reaction can be observed as fluorescence or coloration under a microscope, allowing confirmation of the presence of MOG-Abs. For this reason, we used a cell-based assay in the present study.
In this report and our recent report,19 we identified two cases double positive for MOG-Abs and anti-AQP4 antibodies. In our past study of 23 cases of optic neuritis, in which we used ELISA to measure MOG-Abs, the positive rates for both MOG-Abs and anti-AQP4 antibodies were higher than the results obtained from cell-based assays, due to false-positive results from ELISA.6 However, regardless of the method of measurement, double-positive cases were found to have significantly poorer visual outcome, suggesting that anti-AQP4 antibodies and MOG-Abs may indicate the prognosis of visual function in optic neuritis. A recent study has reported that MOG-Ab seropositive patients tend to relapse but respond to treatment better than AQP4-Ab seropositive optic neuritis.6 Cases positive for MOG-Abs detected by ELISA tend to relapse, have residual visual field deficit, and respond to treatment.
At present, the methods of determining MOG-Abs by cell-based assay have been gradually consolidated in several research groups. Therefore, a standardized method of measuring MOG-Abs should select the cell-based assay in the future.
MOG-Ab seropositive optic neuritis responds to high-dose steroid therapy and plasmapheresis.15 For AQP4-Ab seropositive cases, since steroid resistance is common, usually two courses of steroid pulse therapy are given, and if visual acuity or visual field does not improve, plasmapheresis is conducted.20 However, for treatment of neuromyelitis optica, regardless of whether the case is AQP4-Ab seropositive or MOG-Ab seropositive, initial treatment with high-dose steroid therapy is important. Although the pathophysiology of MOG-Abs is unknown, it is clear that the choice of anti-inflammatory and antibody-eliminating treatments is important for optic neuritis. Therefore, in the case of acute onset of optic neuritis, initiating treatment without waiting for the result of MOG-Ab test is appropriate, and clinical diagnosis and characteristic MRI findings become important. In our series, early high-dose steroid therapy and DFPP for acute exacerbation of MOG-Ab seropositive optic neuritis resulted in significant improvement of visual acuity by 2 lines or more in many patients. Therefore, the status of MOG-Abs is highly relevant in deciding treatment strategy and visual outcome.
Acknowledgements
We thank Ms. Teresa Nakatani for critical revision of the manuscript.
Declaration of interest. This work was supported in part by Grant-in-Aid for Scientific Research (C), Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS), and Health and Labour Sciences Research Grants for research on intractable diseases from the Ministry of Health, Labour, and Welfare of Japan.
References
- 1.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489 [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 1999;53:1107–1114 [DOI] [PubMed] [Google Scholar]
- 3.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112 [DOI] [PubMed] [Google Scholar]
- 4.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of opticspinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005;202:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kezuka T. Optic neuritis-immunological approach to elucidate pathogenesis and develop innovative therapy [in Japanese]. Nihon Ganka Gakkai Zasshi 2013;117:270–291 [PubMed] [Google Scholar]
- 6.Kezuka T, Usui Y, Yamakawa N, Matsunaga Y, Matsuda R, Masuda M, Utsumi H, Tanaka K, Goto H. Relationship between NMO antibody and MOG-Ab in optic neuritis. J Neuroophthalmol 2012;32:107–110 [DOI] [PubMed] [Google Scholar]
- 7.Rostasy K, Mader S, Schanda K, Huppke P, Gärtner J, Kraus V, Karenfort M, Tibussek D, Blaschek A, Bajer-Kornek B, Leitz S, Schimmel M, Di Pauli F, Berger T, Reindl M. Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch Neurol 2012;69:752–756 [DOI] [PubMed] [Google Scholar]
- 8.Shao H, Huang Z, Sun SL, Kaplan HJ, Sun D. Myelin/oligodendrocyte glycoprotein-specific T-cells induce severe optic neuritis in the C57BL/6 mouse. Invest Ophthalmol Vis Sci 2004;45:4060–4065 [DOI] [PubMed] [Google Scholar]
- 9.Kidd D, Burton B, Plant GT, Graham EM. Chronic relapsing inflammatory optic neuropathy (CRION). Brain 2003;126:276–284 [DOI] [PubMed] [Google Scholar]
- 10.Dalton CM, Brex PA, Miszkiel KA, Hickman SJ, MacManus DG, Plant GT, Thompson AJ, Miller DH. Application of the new McDonald criteria to patients with clinically isolated syndromes suggestive of multiple sclerosis. Ann Neurol 2002;52:47–53 [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Tanaka K. MOG-Abs in adult patients with demyelinating disorders of the central nervous system. J Neuroimmunol 2014;270:98–99 [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Tani T, Tanaka M, Saida T, Idezuka J, Yamazaki M, Tsujita M, Nakada T, Sakimura K, Nishizawa M. Antiaquaporin 4 antibody in selected Japanese multiple sclerosis patients with long spinal cord lesions. Mult Scler 2007;13:850–855 [DOI] [PubMed] [Google Scholar]
- 13.Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J, Küker W, Chandratre S, Vincent A, Palace J. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014;71:276–283 [DOI] [PubMed] [Google Scholar]
- 14.Tsuburaya RS, Miki N, Tanaka K, Kageyama T, Irahara K, Mukaida S, Shiraishi K, Tanaka M. Anti-myelin oligodendrocyte glycoprotein (MOG) antibodies in a Japanese boy with recurrent optic neuritis. Brain Dev 2015;37:145–148 [DOI] [PubMed] [Google Scholar]
- 15.Ikeda K, Kiyota N, Kuroda H, Sato DK, Nishiyama S, Takahashi T, Misu T, Nakashima I, Fujihara K, Aoki M. Severe demyelination but no astrocytopathy in clinically definite neuromyelitis optica with anti-myelin-oligodendrocyte glycoprotein antibody. Mult Scler 2015;21:656–659 [DOI] [PubMed] [Google Scholar]
- 16.Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haidar Jorge FM, Takahashi T, Nakashima I, Apostolos-Pereira SL, Talim N, Simm RF, Lino AM, Misu T, Leite MI, Aoki M, Fujihara K. Distinction between MOG antibody seropositive and AQP4 antibody seropositive NMO spectrum disorders. Neurology 2014;82:474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinshenker BG, Wingerchuk DM. The two faces of neuromyelitis optica. Neurology 2014;82:466–467 [DOI] [PubMed] [Google Scholar]
- 18.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815 [DOI] [PubMed] [Google Scholar]
- 19.Kezuka T, Tanaka K, Matsunaga Y, Goto H. Distinction between MOG antibody seropositive and AQP4 antibody seropositive NMO spectrum disorders. Neurology 2014;83:475–476 [DOI] [PubMed] [Google Scholar]
- 20.Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, Palace J, Vincent A. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 2012;79:1273–1277 [DOI] [PubMed] [Google Scholar]