Abstract
Normative visual field area, feasibility and repeatability using (Octopus) semi-automated kinetic perimetry are reported in 221 healthy volunteers aged 5–22 years. I4e and I2e stimuli assessed the visual field at 5°/second (°/s) or 3°/s. Blind spot was assessed with I2e at 2°/s. Reliable visual fields were plotted in 23% of participants <10 years, 64% of 10–12-year-olds, and 98% aged 13–22 years. Visual field areas were unchanged with age using 5°/s, but increased using 3°/s for I2e (p = 0.028). Blind spot area was unchanged with age. Reaction times reduced with age (p < 0.004). There was no learning effect. A test speed of 5°/s is recommended.
Keywords: Feasibility, normative peripheral vision, repeatability, semi-automated kinetic perimetry, visual development
Introduction
When making assessments of the visual field (VF) in children and young adults, knowledge of normative VFs for a given age, expected ability and reliability, is critical. In a recent unpublished survey by the authors1 of 99 UK ophthalmology clinics, 21% reported regularly using semi-automated kinetic perimetry (SKP) on the Octopus despite no normative published data being available. This study evaluates normative VF data, using SKP on the Octopus 900 (Haag-Streit, Koeniz, Switzerland) perimeter, in participants aged 5–22 years and also establishes at which age SKP can be reliably performed.
A review of the literature indicates stark disagreement on the rate of peripheral vision development and age of peripheral visual maturation. Maturation of peripheral vision has been reported to occur as young as 5 years2 and as old as 13 years of age,3 with a range of peripheral vision maturities reported between these ages.4–11 These vast maturity differences likely result from hugely different perimetric methods. For example, 20° eccentricity was used by Blumenthal et al.8 and over 90° eccentricity by other studies.6,7,9 Static or kinetic stimuli may be used and kinetic stimulus velocity and intensity also vary considerably. Therefore, assessment expectations are needed for specific instruments. With the Goldmann perimeter12 no longer commercially available, Octopus SKP is fast becoming the test of choice in many clinics and expected feasibilities and normative VF and blind spot data are needed, especially for children.
To the authors’ knowledge, the only study to report normative reaction time (RT)-corrected SKP on the Octopus in children to date is by Vonthein et al.13 They used decade sampling to report SKP results in 10–80-year-olds. Only 12 individuals aged 10–20 years were tested. Eight meridia were assessed using III4e stimuli at the high velocity of 25°/second (°/s), III4e and I3e at 5°/s, and I2e at 2°/s. There were no significant differences between VF size between 10–20-year-olds and 20–80-year-olds. However, RTs in 10–20-year-olds were comparable to the elderly participants aged 60–80 years, whereas the 20–60-year-olds had faster RTs. The stimuli and velocities reported by Vonthein et al.13 are not UK standard clinical practice, and studies employing other kinetic perimeters have suggested that an age-dependent increase is expected within this age bracket.14,15 Normative values using SKP on the Octopus for children 10 years and older is limited and younger than 10 years are unknown. Normative data for blind spot area using SKP are also not documented in children.
A recent study examined normative blind spot size using SKP and three stimulus luminosities, all at 2 °/s on the Octopus.16 The blind spot scotoma was found to decrease with increased stimulus luminosity and measurements were most repeatable using the most luminous target tested, the I4e. Blind spot scotoma size in SKP in children has not previously been reported, neither known is how potential optic nerve and fixation stability developments may affect apparent blind spot size with age. The ability to accurately plot the blind spot scotoma is often clinically recorded as a measure of reliability. Dolderer et al.17 reported the minimum size in adults to be 17 deg2. The expected age at which a blind spot can be measured accurately has not previously been reported.
No current recommendations exist regarding stimulus velocity in children or adults when performing SKP on the Octopus. A range is found in the literature. In normative VFs, Vonthein et al.13 used 25°/s for III4e and I3e stimuli and 2°/s for I2e. For assessment of the blind spot, Rhodes,16 Dolderer et al.,17 and Nevalainen et al.18 used 2°/s. In assessment of adults with advanced VF loss, 3°/s stimuli were used.18,19
Static perimetry strategies use false-positive, false-negative, and fixation loss indices to provide measures of reliability.20–22 For static perimetry, Artes et al.21 considered RTs less than 180 ms and greater than 2000 ms to represent false-positive and false-negative responses, respectively, and these criteria have also been employed in SKP.23 In children it is unknown whether these reliability criteria are suitable, as RTs to visual stimuli gradually reduce between ages 4 and 11 years and are slower than for adults.24,25 In-built measures of reliability are unavailable when performing manual perimetry and SKP; however, other factors that may provide evidence of poor reliability are the inability to plot the blind spot and overlapping isoptres.
A number of practical aspects need to be considered when testing children’s VFs. Morales and Brown11 provided recommendations for successful testing on static perimetry, which included use of a booster cushion, continuous monitoring of fixation and head position, verbal reassurance, reminders to blink, and informing the child of test progress. Previous studies have included practice stimuli for children prior to testing to ensure they understood the task.3,15,26
SKP using the Octopus is becoming the test of choice by many clinical departments due to a more standardised method compared with the Goldmann perimeter19 and good test-retest reliability.18 Despite this, there are no normative published data of children’s VF area, expected RTs, and blind spot size as a function of age. Also lacking is information regarding feasibility of testing and effect of stimulus velocity. The purpose of this study was to determine these factors, to enable greater sensitivity in the detection of abnormal VF outcomes in comparison with normative data using the cited recommendations of physical test adaptation where necessary and practice trials.
Materials and Methods
Participants
Two hundred and nineteen participants aged 5–17 years were recruited from four mainstream schools and 27 young adult participants aged 18–22 years were recruited from the University of Sheffield student population. Fifty-one participants had the right eye tested twice to determine the repeatability. The inclusion criteria were visual acuity of 0.150 logMAR in each eye, no previous significant ophthalmological history, epilepsy, or attention-deficit hyperactivity disorder. Participants with refractive error greater than ±3.00 spherical dipotres or ±2.00 cylindrical dioptres were excluded unless corrected with contact lenses. The study was approved by the University of Sheffield Departmental Ethics Committee and conformed to the tenets of the Declaration of Helsinki. Informed written consent was obtained from all participants aged 16 years and older and from the parent or guardian of children aged 5–15 years via a parental letter and return opt-in consent form sent via school. Additional consent was obtained from parents and children who had right eye tested twice.
Design of Study
A prospective study design was employed to quantify the normal kinetic VF in participants from the age of 5 years. Unreliable participants were identified by the number of fixation losses (>50), inability to plot the blind spot (<15 deg2), presence of overlapping isoptres, and atypical reaction times (<180 or >1500 ms). The independent variables were age, target stimulus (I2e and I4e), and velocity of stimulus (5°/s or 3°/s). Participants were randomly allocated in to the two stimulus velocity groups. The dependent variables were VF area, size of blind spot, and RT.
Equipment
The Octopus 900 perimeter was used. The I4e stimulus (328 cd/m2, 0.25 mm2) assessed the far-peripheral VF and the I2e stimulus (20 cd/m2, 0.25 mm2) assessed central VF. All stimuli appeared against a uniform white background illumination of 10 cd/m2. The light intensity of the Octopus was calibrated prior to each session of assessments.
A programme was written to ensure standardised testing of each participant, presenting the stimulus from non-seeing periphery and moving to the centre along 12 meridia (15°, 45°, 75°, 105°, 135°, 165°, 195°, 225°, 255°, 285°, 315°, and 345°). The examiner was able to repeat a meridian if the participant lost fixation during testing and could manually test the blind spot if it occurred eccentric to the position expected by the automated programme. RT-corrected VF area was determined using pre-programmed RT vectors presented within the isoptres.
Procedure
Visual acuity (VA) of each eye was assessed using the Crowded LogMAR Test. A practice programme was explained to each participant in age-appropriate language; this assessed response to three I4e stimuli and three I2e. Only after successful completion of the practice test (maximum three practice tests per person), which included the ability to maintain central fixation, was the full test administered. Participants aged 18–22 years completed the test with both right and left eyes. Statistical analyses of all test parameters showed no significant difference for eye tested; therefore, only the right eye analysis is reported. Participants under 18 years were tested with the right eye only. The left eye was occluded with a plastic, elastically secured occluder and the response button was held in the dominant hand (used for writing). Instructions were given to fixate a central green light within the perimeter bowl, whilst peripherally monitoring for a kinetic stimulus that appeared on 1 of 12 meridia presented in a random order. Participants either stood or sat at the machine, depending on age and height, with the addition of footstool or chair cushion if necessary. Small children were given an additional cleansable chin rest to elevate their head sufficiently to align the pupil centrally with the fixation target. The same examiner operated the Octopus for all tests, whilst a second examiner monitored and counted fixation losses viewed on screen via an infrared camera. A specifically written programme that tested all meridians in a randomised order ensured that the starting position of any test stimulus was identical for all participants and minimise inter-test differences.
The I4e kinetic stimulus was presented first, followed by the I2e. Static I2e stimuli were then randomly presented within each quadrant of the I2e stimulus area to identify the presence of any scotomas. Instruction was given between kinetic and static stimuli to allow participants to prepare themselves for the new stimulus. Finally, the blind spot was plotted using the I2e stimulus starting in the centre of the expected blind spot and moving the stimuli outwards at 2°/s along 4 cardinal and 4 inter-cardinal meridians. The RT vectors were assessed with 3 centripetal vectors within the I2e isoptre.
Participants who were tested at the 5°/s stimulus velocity and who had given consent for a second test undertook the same procedure within 1 week. At the end of testing participants were asked to rate their experience of performing the VF test as easy, okay, or hard.
Statistical Analysis
All data were non-normally distributed and therefore reported as median and interquartile ranges. Dependent variables were submitted to Mann-Whitney test or Kruskal-Wallis test to determine differences between target velocities, stimulus size, and age. To establish any correlation in blind spot area or RT with age, linear regression analysis was performed. The significance level was set at p < 0.05.
Repeatability analysis comprised of calculation of correlation coefficients between tests 1 and 2. Bland Altman analyses were used to determine coefficients of variability.27
Results
Of the 246 participants recruited, 221 (104 males and 117 females) fulfilled the inclusion criteria. Details of the number of participants in each age group can be found in Table 1. One hundred and sixteen were tested at 5°/s and 106 at 3°/s. Seven participants had a refractive error with spherical equivalent (SE) ranging from −1.25 to +2.25; these did not require any lens correction during testing. Four participants with myopia ≤3.50 SE wore contact lenses throughout testing.
TABLE 1.
Participant information.
| Reliability measures |
||||||
|---|---|---|---|---|---|---|
| Age (years) | n tested | Unreliable blind spot | Overlapping isoptres | Atypical RT | n reliable | n subgroup |
| 5 | 15 | 14 | 6 | 3 | 1 | 22 (23%) |
| 6 | 19 | 11 | 2 | 4 | 5 | |
| 7 | 26 | 17 | 4 | 8 | 4 | |
| 8 | 17 | 12 | 1 | 3 | 3 | |
| 9 |
20 |
9 |
0 |
1 |
9 |
|
| 10 | 21 | 6 | 1 | 3 | 13 | 34 (64%) |
| 11 | 21 | 5 | 1 | 4 | 13 | |
| 12 |
11 |
0 |
0 |
2 |
8 |
|
| 13 | 7 | 0 | 0 | 0 | 7 | 26 (100%) |
| 14 | 11 | 0 | 0 | 0 | 11 | |
| 15 |
8 |
0 |
0 |
0 |
8 |
|
| 16 | 10 | 1 | 0 | 0 | 9 | 44 (98%) |
| 17 | 8 | 0 | 0 | 0 | 8 | |
| 18–22 |
27 |
0 |
0 |
0 |
27 |
|
| Total | 221 | 75 | 15 | 28 | 126 | 126 |
n tested = number of participants originally tested of each age. Unreliable blind spot = number of participants where SKP unable to plot the blind spot due to unsteady fixation, or where considered unreliable as the area was less than 15 deg2. Overlapping isoptres = number of participants who demonstrated overlapping isoptres (I4e and I2e). Atypical reaction time = number of participants where reaction time <180 or >1500 ms. n reliable = number remaining after exclusion criteria for unreliable data were applied. n subgroup = number of reliable participants in each age subgroups with percentage shown in brackets.
Feasibility of Testing
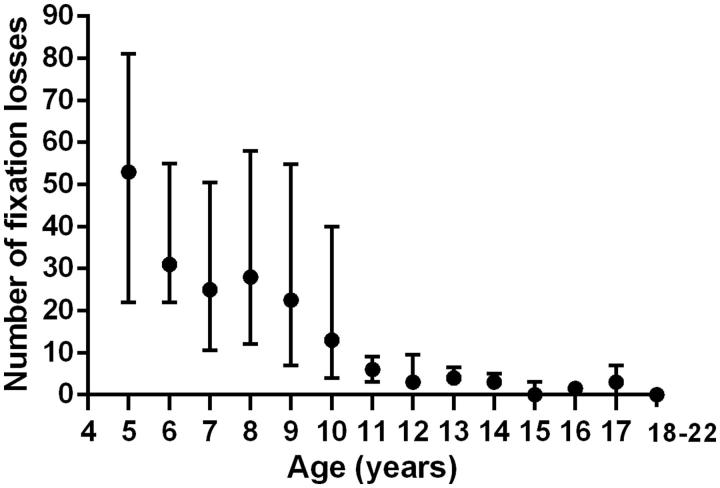
To discuss expected normative VF presentations, it is necessary to first present feasibility data for the purpose of excluding unreliable data. Figure 1 shows the median fixation loss count for each age group with upper and lower quartiles; this includes data from both stimulus velocities. There was no significant difference in fixation loss count between the two testing velocities on Mann-Whitney test (p = 0.84). A high number of fixation losses with a large quartile range were found in 5–10-year-olds. The median value for 5-year-olds was 53 gradually declining to 13 for 10-year-olds. The median values from 11 years onwards were similar and ranged between 0 and 6 fixation losses.
FIGURE 1.
Fixation loss count. Median number of fixation losses counted for each age group with upper and lower quartiles.
Table 1 shows the number of participants in each age group and the number of unreliable participants found with the three other reliability measures (inability to plot the blind spot, presence of overlapping isoptres, and atypical reaction times). Difficulty in plotting the blind spot occurred in a high proportion of the children under 12 years of age. Overlapping isoptres were less frequent, with main occurrence in 5-year-olds. Two participants had reactions times shorter than 180 ms and 26 had above 1500 ms. These atypical reaction times occurred in children aged 12 years or younger. Participants were excluded if any of the reliability criteria were not met. One hundred and twenty-six were considered reliable, 68 performed SKP with stimulus velocity of 5°/s and 58 with 3°/s. To allow for further analysis of reliable data, participants were separated into age subgroups of 5–9, 10–12, 13–15, and 16–22 years. The number of participants in each subgroup is shown in Table 1.
Normative VF Size
The median and interquartile range of RT-corrected VF area in deg2 of all participants was calculated for the I4e and I2e targets for 5°/s or 3°/s. The median VF area using the I4e stimulus was 12,837 deg2 (interquartile range 11,947–13,420) and 12,267 deg2 (interquartile range 11,462–13,613) for 5°/s and 3°/s, respectively. For the I2e stimulus, the VF area was 5025 deg2 (interquartile range 4642–6196) and 5088 deg2 (interquartile range 4041–6054) for 5°/s and 3°/s, respectively. There was no significant difference between the 5°/s and 3°/s target velocities for both I4e (p = 0.32, Mann-Whitney test) and I2e (p = 0.30).
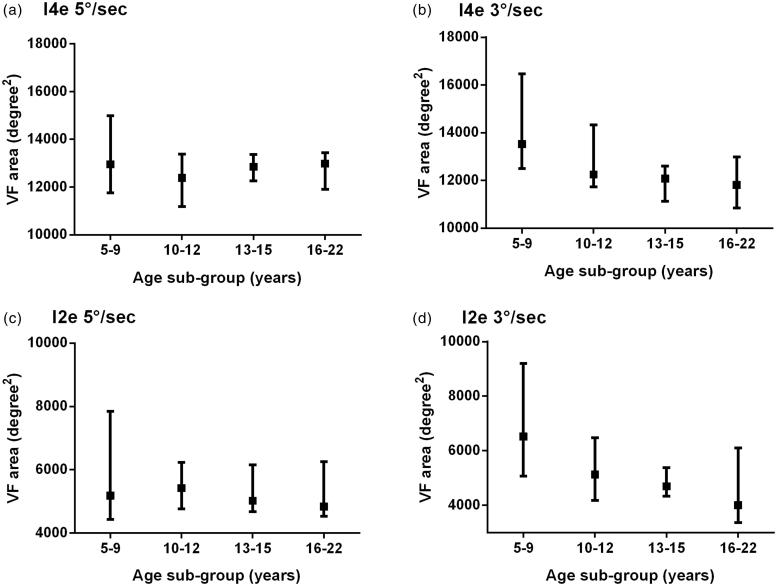
The data were divided into age subgroups for further analysis (Figure 2). Figure 2(a–d) show larger interquartile ranges for the youngest age group. The median VF area shows no obvious changes with age when tested with the 5°/s stimulus velocity; however, with 3°/s the VF area for the 5–9 age group is notably larger. There was no significant difference in VF area with age using 5°/s stimulus velocity for I2e (p = 0.90) and I4e (p = 0.63). However, there was a significant difference for I2e tested at 3°/s (p = 0.028, Kruskal-Wallis test) and close to significance for I4e at 3°/s (p = 0.051). To determine the age at which VF area was significantly different at 3°/s, each age subgroup were compared. For I2e, a significant difference was found between 5–9 and 13–15 years (p = 0.013, Mann-Whitney test) and 5–9 against 16–22 years (p = 0.013), comparison between other age groups showed no significant difference. For I4e, a significant difference was found between 5–9 and 13–15 years (p = 0.029, Mann-Whitney test) and 5–9 against 16–22 years (p = 0.022).
FIGURE 2.
The effect of age on visual field area. Median VF area (deg2) with upper and lower quartiles using I4e stimulus (a, b) and I2e stimulus for 5°/s (a, c) and 3°/s (b, d), respectively.
Normative Blind Spot Area
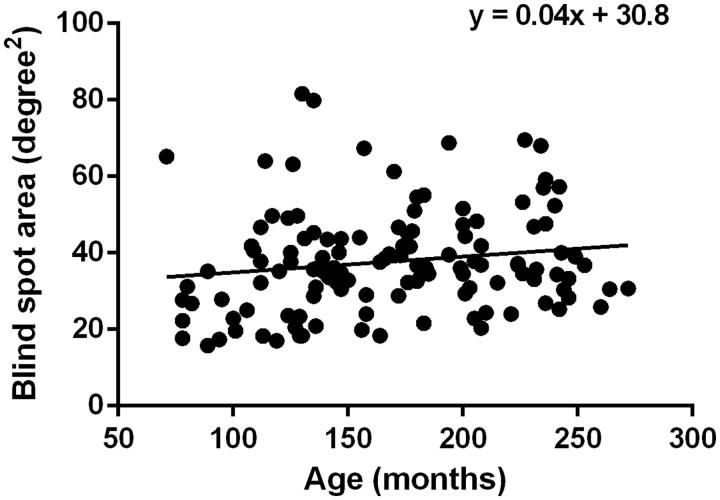
The median blind spot area plotted with the I2e target at 2°/s was 36.0 deg2 (interquartile range 28.8–44.6 deg2). Figure 3 shows wide variation between participants. No relationship was found between blind spot area size and age (r = 0.15, p = 0.08, Pearson correlation coefficient).
FIGURE 3.
The effect of age on blind spot area. Scatter plot of the blind spot area (deg2) on the y-axis and age in months on the x-axis including data measured at both testing velocities. The correlation coefficient was r = 0.15.
Reaction Times (RTs)
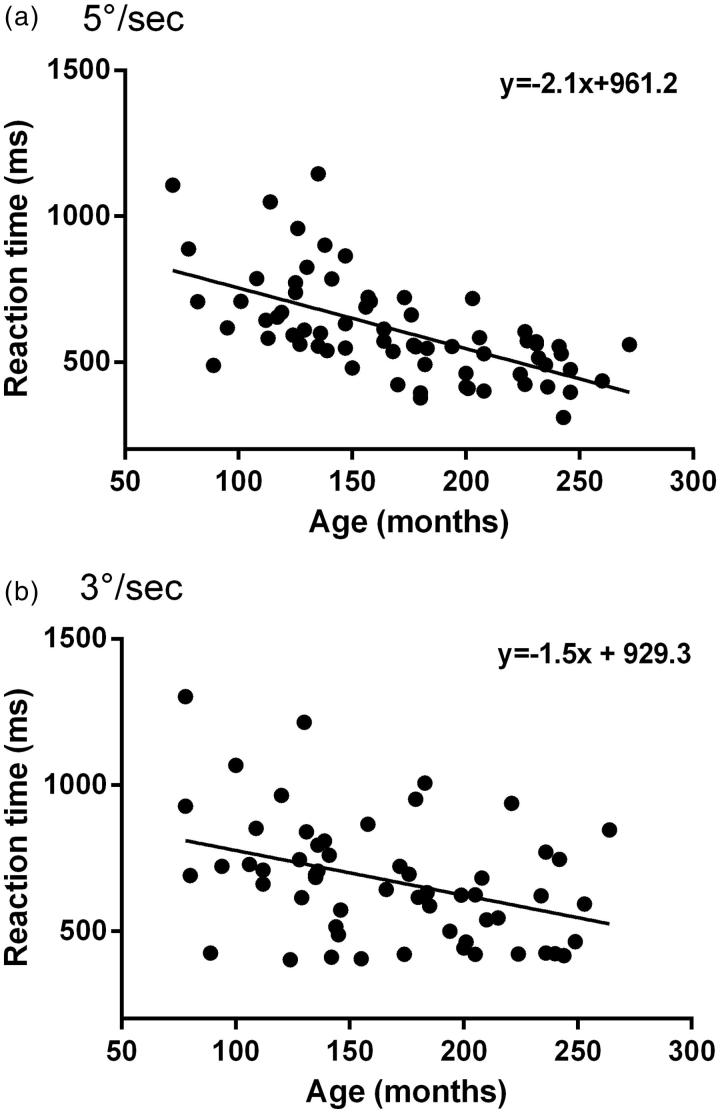
The median RTs for all participants and age subgroups for both stimulus velocities are shown in Table 2. Median RTs at 5°/s testing velocity were less than 3°/s but this did not quite reach significance (p = 0.05, Mann-Whitney test). A reduction in RT with increasing age group is evident (Table 2); therefore, the RT for each participant was plotted against age in months (Figure 4). A significant reduction in RT with increasing age was found for both 5°/s (r = 0.61, p < 0.001) and 3°/s (r = 0.38, p = 0.004). Figure 4(b) shows a large variation between participants at similar ages for 3°/s.
TABLE 2.
Reaction times.
| Stimulus velocity |
||
|---|---|---|
| Age subgroup (years) | 5°/s | 3°/s |
| 5–9 | 688 (624–862) | 725 (683–962) |
| 10–12 | 631 (557–844) | 693 (502–801) |
| 13–15 | 557 (491–688) | 694 (615–866) |
| 16–22 | 502 (416–561) | 542 (429–668) |
| All participants | 570 (491–706) | 672 (504–768) |
Median reaction times (ms) with interquartile range shown in brackets for each age subgroup and all participants.
FIGURE 4.
The effect of age on reaction time (RT). Scatter plot of RT (ms) on the y-axis and age in months on the x-axis, measured at testing velocity of (a) 5°/s and (b) 3°/s. The correlation coefficient was r = 0.61 at 5°/s and r = 0.38 at 3°/s.
Test Duration
The median test duration using 5°/s was 3.4 minutes (range 2.2–6.4) and using 3°/s was 4.3 minutes (range 2.2–6.2). Testing at 5°/s stimulus velocity was significantly faster than 3°/s (p < 0.0001, Mann-Whitney test).
Repeatability
Thirty of 51 participants who repeated the test fulfilled the reliability criteria on both VF assessments and their data were included in further analysis. Of the 21 classified as unreliable, 12 were unreliable on both VF assessments, 4 were unreliable on the first and 5 were unreliable on the second test.
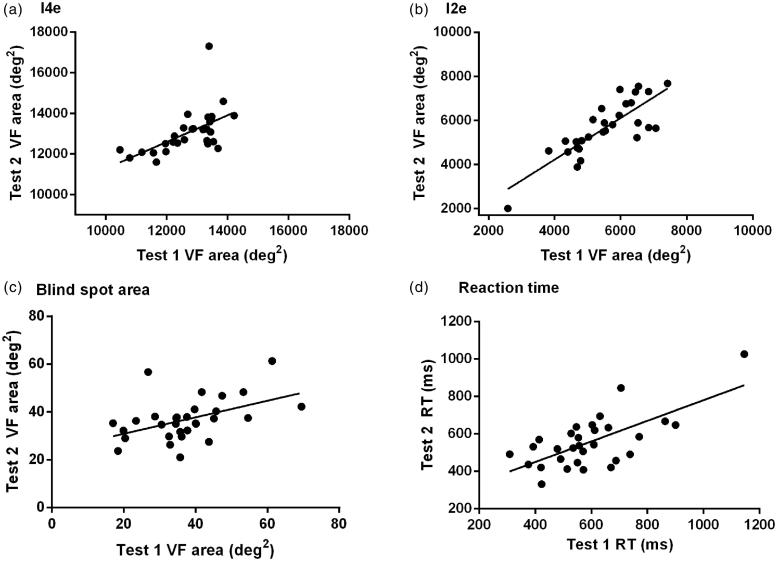
The I4e, I2e, blind spot area, and RT for each participant were plotted for test 1 against test 2 (Figure 5). Whilst the majority of participants showed close agreement between tests 1 and 2 for I4e, one participant demonstrated a large increase in VF area in test 2 that appears to be outside normative values (Figure 5a). There was, however, significant correlation between tests 1 and 2 for all of the parameters (I4e: r = 0.57, p = 0.001, I2e: r = 0.82, p > 0.0001; blind spot area: r = 0.47, p = 0.008; RT: r = 0.68, p < 0.0001; Pearson correlation coefficient).
FIGURE 5.
Repeatability: Scatter plot of test 1 on x-axis against test 2 on y-axis. (a) VF area using I4e; (b) VF area using I2e; (c) blind spot area; (d) RT.
To determine a clinical value of expected variability between repeated testing, Bland Altman analyses were used to determine coefficients of variability. The outlier for I4e was removed for this analysis (Figure 5a). The coefficients of variability were 1303 deg2 for I4e, 1392 deg2 for I2e, 22 deg2 for blind spot area, and 250 ms for RT. The median test duration was 3.4 minutes (range 2.4–6.4) for test 1 and 3.1 minutes (range 2.6–6.3) for test 2.
Participant Difficulty Rating
Participants less than 18 years of age were asked to rate the difficulty of performing the test. This included 99 participants of which only 4 rated the test as difficult and these were all in the 5–9-year group. Forty-eight participants rated the test okay and 47 rated the test easy to perform. Of those asked who repeated the test (n = 27), 41% found the test to be easy on the first test, whereas this increased to 85% on the second test.
Discussion
This study evaluated the age at which SKP on the Octopus perimeter can be reliably performed. Normative VF area and RT data using two testing speeds were determined and repeatability of the test evaluated. This provides the largest sample of reported normative and feasibility data in children and young adults, from age 5 to 22 years. None of the participants in the study had significant ophthalmic disorders or refractive errors; therefore, findings cannot be generalised to a patient population.
Feasibility of Testing Children
One of the main findings of this study are that the feasibility of testing children, using SKP on the Octopus 900, increases markedly from the age of 11–12 years. This is reflected in the stark decline in number of fixation losses from age 11 (Figure 1), and adult-like performance on all of the other reliability measures from the age of 12 years (Table 1). Whilst static perimetry incorporates fixation loss catch trials as a standard clinical measure of reliability, SKP using the Octopus does not provide this. Therefore, an experienced perimetrist is required to monitor fixation, particularly in the assessment of young children. Additional reliability measures of unreliable blind spot, overlapping isoptres, and atypical reaction times were derived in this study to ensure true representation of normative VF data.
Only one other study has examined automated kinetic perimetry from age 5 years, using the Twinfield perimeter.15 Of 50 children tested, only 1 could not complete the test; however, reliability measures were not considered.
The age of reliability in this current study is similar to that found by Wabbels and Wilscher3 who reported static perimetry in 28 children aged 5–14 years on the Twinfield perimeter. They found that reliable results and ability to fully complete the testing strategies could be obtained in all children from 13 years. Blumenthal et al.,8 using frequency-doubling perimetry, found that on the best VF of two trials, in children younger than 8 years of age, 43% of VFs were unreliable, compared with 23% unreliable for those older than 8 years. Morales and Brown11 performed short automated static perimetry on the Octopus in 50 normal children aged 6–12 years and suggested reliable results can be obtained after 7 years of age. When examining their results further, mean sensitivity values are variable until 11–12 years. Safran et al.,28 also using the Octopus perimeter, used a static testing strategy on the central 15°, and found good reliability in 8-year-olds. They noted that a training programme improved results, especially in the younger children. In this current study using kinetic perimetry, all children successfully completed a practice programme. The later age of reliability found may reflect the longer test duration and greater eccentricity than some of the studies mentioned above. The significant influence of conceptual and attentional factors on peripheral vision testing has been previously reported29 and may differentially influence results depending on the level of difficulty required for the test used.
Normative VF Area
Normative VF areas have been presented for two isoptres (I4e and I2e) at two test speeds (5°/s and 3°/s). There is controversy in the literature regarding developmental change in VF area. Wilscher et al.15 using automated kinetic perimetry found no significant change with age. This disagrees with studies using manual kinetic testing on the Goldmann perimeter6,7,14 where a developmental increase in VF area has been reported. This current study found no increase in VF area with age with the I4e and I2e stimuli using 5°/s test velocity. However, 3°/s target velocity produced contradictory results where VF area was larger in the youngest age group. The Octopus makes a significant noise, produced simultaneously with stimulus onset, which may have distracted some younger children such that they pressed the button at the onset of sound rather than when they first perceived the light. This may well explain the seemingly larger fields for the 3°/s target velocity over the faster testing speed in 5–9-year-old children. This additional sound cue may be masking true developmental improvements in VF area as reported by other authors. The test’s predecessor, the Goldmann, did not produce any stimulus-related noise; therefore, this difference might explain the different results found. However, the Goldmann did not make RT corrections to isoptres; therefore, seeming VF developments with age on the Goldmann could represent RT decreases with age found in this study.
Reaction Time (RT)
RT decreased significantly with age in this study, which is consistent with previous reports using general RT tasks.30,31 A larger variation in RT between participants of similar ages was shown when testing with 3°/s than 5°/s. This finding may suggest that more reliable measures can be obtained when testing with a stimulus velocity of 5°/s. SKP using the Octopus allows RT vectors to be plotted in addition to the VF test outer limits, and uses an average RT to adjust the VF for where the peripheral stimulus was truly identified. Decreasing RT with age therefore has implications on how the VF is adjusted, as older children will have less VF area added to compensate for RT and younger children will have more VF area to compensate for RT. Thus, RT correction could explain the absence of age-related VF increases in this study in comparison to age related VF increases previously reported on the Goldmann6,7,14.
Blind Spot Area
Median RT-corrected blind spot area from age 5 to 22 years was 36 deg2, ranging from 16 to 82 deg2, showing wide variation between participants. Rhodes16 reported a slightly larger mean scotoma area of 63 deg2 using the I2e target with high variability (±21.7°). Dolderer et al.17 also reported large variation in scotoma size with a similar stimulus, the II2e. The authors have not found any previously reported data on whether the blind spot area increases during childhood. There was no correlation in blind spot area with age.
Repeatability
Of the 51 participants who repeated the test, 21 (41%) produced unreliable measures on one or both tests, all of which were younger than 12 years. A learning effect was not demonstrated, as 12 were unreliable on both tests and 5 unreliable on test 2 only. On questioning, more participants found the test easier to perform on the second test. A learning effect has previously been reported for static and kinetic perimetry.29,32,33 Horani et al.33 suggested that the second VF test, in patients who are unfamiliar with testing, should be taken as the baseline rather than the first VF test. All children in our study completed between one and three practice trials. Therefore, the introduction of these practice trials may have lessened the learning effects seen.
In the small sample of participants who reliably repeated the test, all measures were found to be correlated between tests 1 and 2. However, the coefficient of variability was relatively large for all parameters, which may be a reflection of the small sample size. A larger study is required to establish clinical values of expected variability and any learning effect between visits.
Limitations
Whilst initial recruitment for the study was successful, elimination of participants due to unreliable responses significantly reduced the final sample size for age subgroups. Of the 97 children recruited under the age of 10 years, only 23% provided reliable data. In order to gain sufficient data to provide normative values and study development of the visual field, a larger sample should be recruited. However, this does provide evidence of the limited accuracy that can be expected in this age group of children with SKP on the Octopus.
The oldest participant in our study was 22 years old and no participants had ocular pathology; therefore, we cannot comment on the feasibility of testing elderly or those with ocular pathology. It is worth noting that a significant proportion of children who attend clinics for VF testing have serious underlying pathologies affecting their health, for example, optic nerve pathway glioma with neurofibromatosis type I. As such, these patients may not feel well at time of testing and may additionally be undergoing therapeutic radiotherapy/chemotherapy or both, which affect their well-being and ability to concentrate for this test. Our results, unfortunately, cannot be generalised to give expectations for such patients. However, having more normative data available for comparison may promote earlier detection of some VF abnormalities secondary to pathology.
In the youngest age group, physical adaptation of the testing method had to be implemented that included children standing, sitting on a cushion, and use of an additional chin cushion. These adaptations could have impacted on concentration and performance.
Conclusion
In summary, this study found that participants aged 13 years or older and approximately 2/3 aged 10–12 years can reliably perform SKP on the Octopus perimeter. With physical adaptations to the machine such as additional chin rest and administration of a practice programme, reliable VFs are possible in a minority of children aged 5–9 years. The authors recommend exercising caution when interpreting the VF results of children younger than 13 years. Employing reliability measures such as high fixation loss count, inability to plot the blind spot, overlapping isoptres, and atypical RTs can help to filter unreliable fields. Using 3°/s stimuli velocity produced larger variation in VF and RT data than 5°/s. Therefore, a test velocity of 5°/s is recommended for healthy children and adults.
Blind spot area using 2°/s and VF area using 5°/s stimuli velocity were unaffected by age; however, RT significantly decreased with age. It can be concluded that similar sized VF and blind spot areas are to be expected in children and young adults when using RT vector adjustment.
Acknowledgements
We are very grateful to the staff and students at Brunswick Community Primary School, Dobcroft Infant School, and Tapton Secondary School and to children and parents from Greystones Primary School who came to The University of Sheffield to participate. We kindly thank Orthoptists Clare Hockley and Nana Theodorou for help in data collection, and The University of Sheffield Estates Team for all their help in transporting the Octopus between schools. The authors have no financial, commercial, or propriety interests in the equipment used.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Griffiths H. (2013). UK orthoptic survey to the British and Irish Orthoptic Society (BIOS) on the usage of semi-automated kinetic perimetry on the Octopus perimeter. [unpublished]
- 2.Cummings MF, van Hof-van Duin J, Mayer DL, Hansen RM, Fulton AB. Visual fields of young children. Behav Brain Res 1988;29:7–16 [DOI] [PubMed] [Google Scholar]
- 3.Wabbels BK, Wilscher S. Feasibility and outcome of automated static perimetry in children using continuous light increment perimetry (CLIP) and fast threshold strategy. Acta Ophthalmol Scand 2005;83:664–669 [DOI] [PubMed] [Google Scholar]
- 4.Aspinall PA. Peripheral vision in children. Ophthalmologica 1976;173:364–374 [DOI] [PubMed] [Google Scholar]
- 5.Lakowski R, Aspinall PA. Static perimetry in young children. Vision Res 1969;9:303–312 [DOI] [PubMed] [Google Scholar]
- 6.Liao F. Perimetry in young children. Jpn J Ophthalmol 1973;17:277–289 [Google Scholar]
- 7.Wilson M, Quinn G, Dobson V, Breton M. Normative values for visual fields in 4- to 12-year-old children using kinetic perimetry. J Pediatr Ophthalmol Strabismus 1991;28:151–153 [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal EZ, Haddad A, Horani A, Anteby I. The reliability of frequency doubling perimetry in very young children. Ophthalmology 2004;111:435–439 [DOI] [PubMed] [Google Scholar]
- 9.Bowering ER, Maurer D, Lewis TL, Brent HP. Constriction of the visual field of children after early visual deprivation. J Pediatr Ophthalmol Strabismus 1997;34:347–356 [DOI] [PubMed] [Google Scholar]
- 10.Codina CJ, Buckley D, Port M, Pascalis O. Deaf and hearing children, a comparison of peripheral vision development. Dev Sci 2010;14:725–737 [DOI] [PubMed] [Google Scholar]
- 11.Morales J, Brown SM. The feasibility of short automated static perimetry in children. Ophthalmology 2001;108:157–162 [DOI] [PubMed] [Google Scholar]
- 12.Goldmann H. Ein selbstregistrierendes Projektionskugelperimeter. Ophthalmologica 1945;109:71–79 [Google Scholar]
- 13.Vonthein R, Rauscher S, Paetzold J, Nowomiejska K, Krapp E, Hermann A, Sadowski B, Chaumette C, Wild JM, Schiefer U. The normal age-corrected and reaction time-corrected isopter derived by semi-automated kinetic perimetry. Ophthalmology 2007;114:1065–1072 [DOI] [PubMed] [Google Scholar]
- 14.Quinn GE, Fea AM, Minguini N. Visual fields in 4 to 10 year old children using the Goldmann and double-arc perimeters. J Pediatr Ophthalmol Strabismus 1991;28:314–319 [DOI] [PubMed] [Google Scholar]
- 15.Wilscher S, Wabbels B, Lorenz B. Feasibility and outcome of automated kinetic perimetry in children. Graefes Arch Clin Exp Ophthalmol 2010;248:1493–1500 [DOI] [PubMed] [Google Scholar]
- 16.Rhodes MR. Prospective pilot study looking at the size and variation of the blind spot scotoma in adults measured on the Octopus 900 Field Analyser. Ophthalmol Res 2013;1:38–50 [Google Scholar]
- 17.Dolderer J, Vonthein R, Johnson CA, Scheifer U, Hart W. Scotoma mapping by semi-automated kinetic perimetry: the effects of stimulus properties and the speed of subjects’ responses. Acta Ophthalmol Scand 2006;84:338–344 [DOI] [PubMed] [Google Scholar]
- 18.Nevalainen J, Paetzold J, Krapp E, Vonthein R, Johnson CA, Schiefer U. The use of semi-automated kinetic perimetry (SKP) to monitor advanced glaucomatous visual field loss. Graefes Arch Clin Exp Ophthalmol 2008;246:1331–1339 [DOI] [PubMed] [Google Scholar]
- 19.Nowomiejska K, Vonthein R, Paetzold J, Zagorski Z, Kardon R, Schiefer U. Comparison between semiautomated kinetic perimetry and conventional Goldmann manual kinetic perimetry in advanced visual field loss. Ophthalmology 2005;112:1343–1354 [DOI] [PubMed] [Google Scholar]
- 20.Olsson J, Asman P, Heijl A. A perimetric learner’s index. Acta Ophthalmol Scand 1997;75:665–668 [DOI] [PubMed] [Google Scholar]
- 21.Artes PH, McLoed D, Henson DB. Response time as a discriminator between true- and false-positive responses in supra-threshold perimetry. Invest Ophthalmol Vis Sci 2002;43:129–132 [PubMed] [Google Scholar]
- 22.Becker ST, Vonthein R, Volpe NJ, Schiefer U. Factors influencing reaction time during automated kinetic perimetry on the Tübingen computer campimeter. Invest Ophthalmol Vis Sci 2005;46:2633–2638 [DOI] [PubMed] [Google Scholar]
- 23.Nowomiejska K, Vonthein R, Paetzold J, Zagorski Z, Kardon R, Schiefer U. Reaction time during semi-automated kinetic perimetry (SKP) in patients with advanced field loss. Acta Ophthalmol 2010;88:65–69 [DOI] [PubMed] [Google Scholar]
- 24.Kiselev S, Espy KA, Sheffield T. Age-related differences in reaction time task performance in young children. J Exp Child Psychol 2009;102:150–166 [DOI] [PubMed] [Google Scholar]
- 25.Venker CC, Goodwin JL, Roe DJ, Kaemingk KL, Mulvaney S, Quan SF. Normative psychomotor vigilance task performance in children ages 6 to 11—the Tucson Children’s Assessment of Sleep Apnea Study. Sleep Breath 2007;11:217–224 [DOI] [PubMed] [Google Scholar]
- 26.Brown SM, Bradley JC, Monhart MJ, Baker DK. Normal values for Octopus tendency orientated perimetry in children 7 through 13 years old. Graefes Arch Clin Exp Ophthalmol 2005;243:886–893 [DOI] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310 [PubMed] [Google Scholar]
- 28.Safran AB, Laffi GL, Bullinger A, Viviani P, de Weisse C, Désangles D, Tschopp C, Mermoud C. Feasibility of automated visual field examination in children between 5 and 8 years of age. Br J Ophthalmol 1996;80:515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiteside JA. Peripheral vision in children and adults. Child Dev 1976;47:290–293 [PubMed] [Google Scholar]
- 30.Rose SA, Feldman JF, Jankowski JJ, Caro DM. A longitudinal study of visual expectation and reaction time in the first year of life. Child Dev 2002;73:47–61 [DOI] [PubMed] [Google Scholar]
- 31.Der G, Deary IJ. Age and sex differences in reaction time in adulthood: results from the United Kingdom health and lifestyle survey. Psychol Aging 2006;21:62–73 [DOI] [PubMed] [Google Scholar]
- 32.Wood JM, Wild JM, Hussey MK, Crews SJ. Serial examination of the normal visual field using Octopus automated projection perimetry: evidence for a learning effect. Acta Ophthalmol 1987;65:326–333 [DOI] [PubMed] [Google Scholar]
- 33.Horani A, Frenkel S, Yahalom C, Farber MD, Ticho U, Blumenthal EZ. The learning effect in visual field testing of healthy subjects using frequency doubling technology. J Glaucoma 2002;11:511–516 [DOI] [PubMed] [Google Scholar]