Abstract
A 34-year-old woman was hospitalised with acute onset nausea, vomiting, ataxia, nystagmus, blurred vision, and bilateral mydriasis. Toxicologic investigations and serologic tests for infectious aetiologies were negative. Demyelinating disease was suspected based on magnetic resonance imaging (MRI) findings but there were no lesions at the midbrain explaining bilateral mydriasis. Direct light, consensual light, and near responses for pupil were all negative. Biomicroscopic examination of the iris did not show any sphincter damage or tonic movements. Pupils didn’t respond to pilocarpine (0.1% and 2%) and remained unresponsive during the follow-up period. Congenital mydriasis was diagnosed because old photographs revealed that pupils were dilated previously.
Keywords: Mydriasis, pilocarpine, pupilla
INTRODUCTION
Congenital mydriasis is a rare ocular abnormality. This condition is characterised by fixed dilated pupils unresponsive to the light. We present a patient with congenital mydriasis who was diagnosed during young adulthood.
CASE REPORT
A 34-year-old female patient was hospitalised in the neurology clinic with the complaints of nausea, vomiting, vertigo, nystagmus, ataxia, and blurred vision.
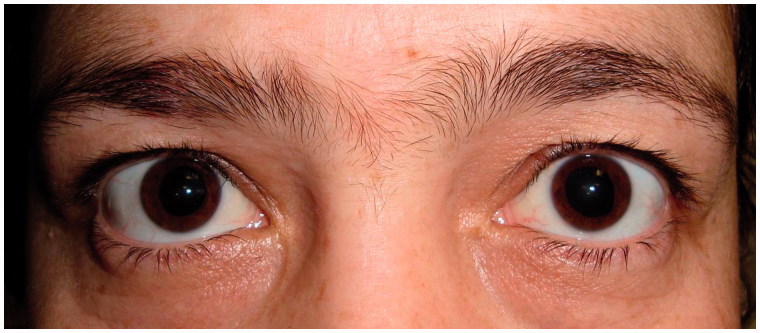
She was consulted to the ophthalmology department. In her ophthalmologic examination, distance vision was 20/100 and near vision was 20/25 in both eyes. Noncycloplegic refractive errors measured with autorefractometer were close to the emetropia and colour vision was 12/12 in both eyes. There were no ptosis or lid retraction. She was ortotropic in all diagnostic positions of gaze, the ductions were full in the fields of all muscles, and the near point of convergence was within normal limits. She had conjugate, high amplitude, upbeat nystagmus, and her pupils were dilated (Figure 1). Pupil diameters were bilateral 7 mm. Direct light, consensual light, and near responses for pupil were all negative. Biomicroscopic examination of the iris did not show any sphincter damage, transillumination defect, or tonic movements. Intraocular pressures and retinal examination were normal. The patient’s history revealed no previous pharmacologic dilatation or eye disorder.
FIGURE 1.
Photograph of the patient showing fixed, dilated pupils.
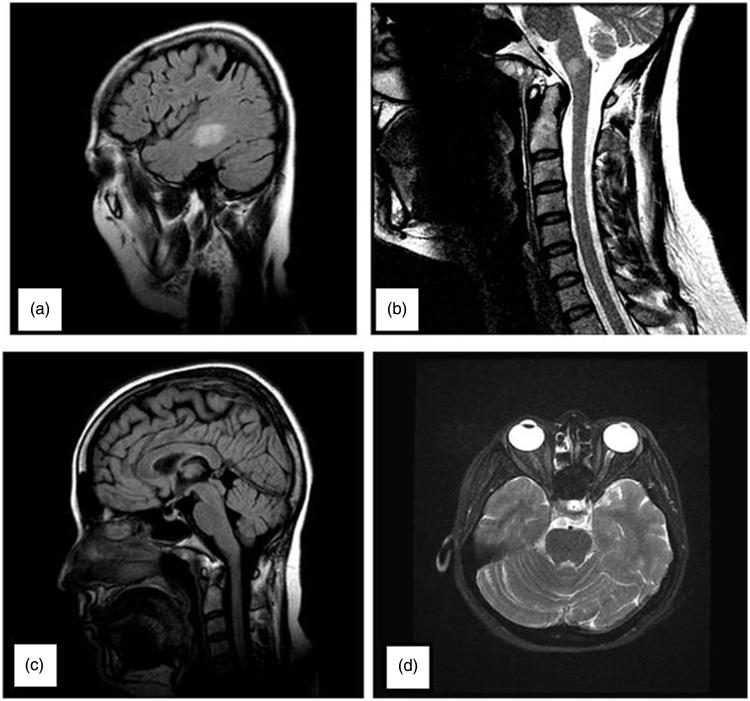
T2-weighted axial magnetic resonance imaging (MRI) showed a 2.5-cm-diameter periventricular and multiple millimetric high-intensity lesions. Cervical and thoracic MRI were normal except for a 7-mm lesion located in the medulla. These lesions could not be specified certainly as demyelination or ischaemic. There were no lesions in the dorsal midbrain that may explain pupillary involvement (Figure 2). Cerebrospinal fluid analysis was negative with respect to oligoclonal band. Immune markers for vasculitis and serologic tests for human immunodeficiency virus (HIV), syphilis, herpes, and brucella were negative.
FIGURE 2.
On the patient’s cranial and cervical MRI, T2-weighted axial shows one 2.5-cm-diameter periventricular high-intensity lesion (a) and 7-mm lesion located in the medulla (b). There were no lesions in the dorsal midbrain on axial (c) and sagittal (d) views.
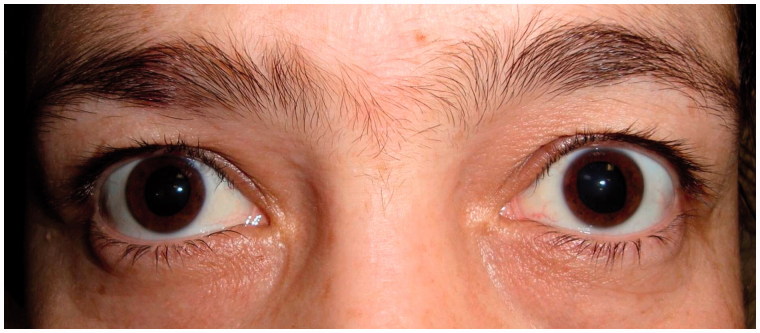
Unresponsiveness of the pupils to pilocarpine (0.1% and 2%) eliminated the possibility of central or peripheral pupilloparesis and denervation supersensitivity (Figure 3). We did a toxicologic investigation with the suspicion of exposure to a reversible or irreversible cholinergic receptor blocking agent. Toxicologic investigation of urine by using gas chromatography–mass spectrometer (GC-MS) showed no sympathomimetic (amphetamine, cocaine, etc.) or anticholinergic (atropine, tricyclic antidepressants, etc.) agents that may lead to mydriasis. Botulinum toxin investigation of stool was also negative.
FIGURE 3.
Diameters of pupils remained unchanged after pilocarpine (2%) test.
Neurologists started pulse steroid treatment with the suspicion of multiple sclerosis based on MRI findings. Patient’s nystagmus showed improvement and her distance vision reached to the level of 20/25 after 1 week of treatment, but the pupillary findings did not change, including pharmacologic tests. There was no evidence for optic neuritis and her vision reduction at distance was closely related to nystagmus. She had a similar second attack that responded to steroid treatment 5 months after the initial one.
After a 6-month follow-up period, biomicroscopic evaluation showed no change of pupil dimensions, and no specific aetiologic reason could be identified to explain mydriasis. The only other possibility for bilateral dilated unresponsive pupil was congenital mydriasis; therefore, family history was questioned. Pupillary response to a cycloplegic agent (cyclopentolate 1%) was also tested and no response was observed. Requested old photographs of the patient demonstrated bilateral large pupil diameters.
DISCUSSION
There were no midbrain lesions that can explain bilateral fixed dilated pupils unresponsive to light and near stimulus in our case. Additionally, unresponsiveness to topical pilocarpine eliminated the possibility of pupilloparesis. Toxicologic investigation of urine showed no sympathomimetic (amphetamine, cocaine, etc.) or anticholinergic (atropine, tricyclic antidepressants, etc.) agents that may lead to mydriasis.
Monaco et al. reported that botulinum type B toxin caused unreactive mydriasis following 5-day constipation in a 29-year-old woman. Unreactive mydriasis improved following antitoxin treatment. Eye symptoms disappeared entirely in 5 weeks.1 In the literature, it is reported that botulism is one of the causes for acquired mydriasis that could be transient or persistent.2 Stool examination for botulinum toxin in our patient was negative.
The only remaining possibility for bilateral dilated unresponsive pupil was congenital mydriasis for our case and old photographs helped us in diagnosis. Congenital mydriasis is described as a condition with fixed, dilated pupils from birth. Additionally, no structural changes in iris and ciliary body have been observed in congenital mydriasis. It is a very rare condition and is generally diagnosed during early childhood. This condition was first described in monozygotic twins by White and Fulton.3 It is generally bilateral and most of the patients are female. Hereditary type is inherited autosomal dominantly or X-linked dominantly. X-linked dominant form is incompatible with life in male gender. Only two male patients with unilateral congenital mydriasis have been reported in the literature.4,5
Buys et al. reported a case having congenital mydriasis associated with patent ductus arteriosus (PDA) in 1993.6 Graf and Jungherr reported congenital mydriasis, accommodation failure, and patent ductus arteriosus in two patients.7 Lemire et al. and Lindberg and Brunvand reported congenital mydriasis and PDA associated with aortic arch anomalies.8,9 Cardiologists had been consulted to investigate the pathologies of our patient; however, no pathology was detected. Pathologic mechanism for congenital myriasis is not clear yet. Possible mechanisms are a receptor defect for smooth muscle of the eye and the media of the ductus arteriosus, aplasia of iris sphincter and dilator muscles, abnormality of neurotransmitter production, and a variant of the entity of aniridia.6,7,10
Congenital mydriasis can be diagnosed easily in ordinary conditions, but the diagnosis can be quite challenging when it presents as a coincidental finding of another disease spectrum. It can remain unnoticed throughout childhood and adulthood, as occurred in our case. To be able to diagnose, it should be remembered in the differential diagnosis list of bilateral fixed dilated pupil. Pharmacologic tests and old photos are helpful in diagnosis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Note: Figure 1 of this article is available in colour online at www.informahealthcare.com/oph
References
- 1.Monaco S, Freddi N, Francavilla E, Meneghetti F, Fenicia L, Franciosa G, Cadrobbi P. Transient tonic pupils in botulism type B. J Neurol Sci 1998;156:96–98 [DOI] [PubMed] [Google Scholar]
- 2.Caglayan HZ, Colpak IA, Kansu T. A diagnostic challenge: dilated pupil. Curr Opin Ophthalmol 2013;24:550–557 [DOI] [PubMed] [Google Scholar]
- 3.White BV, Fulton MN. A rare pupillary defect inherited by identical twins. J Hered 1937;28:177–179 [Google Scholar]
- 4.Laor N, Korczyn AD. Waardenburg syndrome with a fixed dilated pupil. Br J Ophthalmol 1978;62:491–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki T, Obara Y, Fujita T, Shoji E. Unilateral congenital mydriasis. Br J OPhthalmol 1994;78:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buys Y, Buncic JR, Enzenauer RW, Mednick E, O'Keefe M. Congenital aplasia of the iris sphincter and dilator muscles. Can J Ophthalmol 1993;28:72–75 [PubMed] [Google Scholar]
- 7.Graf MH, Jungherr A. Clinicopathologic reports, case reports, and small case series: congenital mydriasis, failure of accommodation, and patent ductus arteriosus. Arch ophthalmol 2002;120:509–510 [PubMed] [Google Scholar]
- 8.Lemire BD, Buncic JR, Kennedy SJ, Dyack SJ, Teebi AS. Congenital mydriasis, patent ductus arteriosus, and congenital cystic lung disease: new syndromic spectrum? Am J Med Genet A 2004;131:318–319 [DOI] [PubMed] [Google Scholar]
- 9.Lindberg K, Brunvand L. Congenital mydriasis combined with aneurysmal dilatation of a persistent ductus arteriosus Botalli: a rare syndrome. Acta Ophthalmol Scand 2005;83:508–509 [DOI] [PubMed] [Google Scholar]
- 10.Bregstrom CB, Lambert SR, Hutchinson AK. Bilateral congenital mydriasis [abstract]. J Am Assoc Pediatr Ophthalmol Strab 2004;8:102 [Google Scholar]