In this issue of Molecular Metabolism, Tardelli and colleagues analyzed the impact of osteopontin (OPN) on monocyte and macrophage proliferation in the context of obesity-driven adipose tissue inflammation [1]. Chronic low-grade inflammation of adipose tissue during obesity, a crucial contributor to insulin resistance, type 2 diabetes, and subsequent cardiovascular disease, is associated with abnormal cytokine production, monocyte infiltration, and activation of inflammatory signaling pathways [2]. Recent evidence could also show that, in addition to blood monocyte recruitment, in situ proliferation of adipose tissue macrophages is an important pathological feature of macrophage accumulation contributing to obesity-associated adipose tissue inflammation [3].
OPN is a secreted matricellular protein involved in many physiological and pathological processes, including biomineralization, tissue remodeling, and inflammation [4]. OPN is a proinflammatory immune regulator and has been identified as a key mediator of adipose tissue inflammation, insulin resistance, and type 2 diabetes [4]. Using OPN-neutralizing antibodies and knockout mice, the groups of Stulnig and Bruemmer showed that OPN mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in obese mice [5], [6]. Furthermore, OPN was found to stimulate inflammatory signaling pathways and secretion of cytokines in adipose tissue macrophages [7]. However, the impact of OPN on the emerging role of local macrophage proliferation remained elusive.
In the current study, Tardelli and colleagues showed for the first time that OPN directly increases proliferation of human monocytes. To address the question whether OPN might also influence proliferation of mature macrophages, human blood monocytes were differentiated into macrophages in the presence of M-CSF for 6 days. Surprisingly, using a live cell movie analyzer, the researchers were able to show that OPN also activates motility and proliferation of mature macrophages. It is known that OPN expression is drastically up-regulated in adipose tissue macrophages of high-fat diet-induced and genetically obese mice without corresponding differences in OPN plasma levels [8]. Together, these findings suggest, that OPN exercises its actions locally in adipose tissue rather than systemically. To investigate the question of whether OPN affects local adipose tissue macrophage proliferation in obesity, the authors performed diet-induced experiments using wild type (WT) and OPN-knockout mice (SPP1KO). Interestingly, in the absence of OPN, adipose tissue macrophages almost completely lost their proliferative capacity, determined by immunohistochemical Ki67+Mac-2+ double staining in mice after 12 weeks on high-fed-diet. The authors report an important and novel aspect of OPN in mediating adipose tissue inflammation, contributing to our understanding on how OPN might induce insulin resistance and metabolic diseases.
However several questions need to be clarified in future studies. What is the underlying molecular mechanism by which OPN facilitates macrophage proliferation? In the current study, adipose tissue macrophage proliferation in vivo was analyzed by Ki67 staining. Since Ki67 is an unspecific proliferation marker, it does not provide mechanistic information. Additional studies addressing the impact of OPN on cell-cycle regulators and upstream signaling pathways might provide further mechanistic insights. Another question that needs to be addressed in further studies is how inflammatory cytokines affect or even contribute to the effects of OPN on adipose tissue macrophage proliferation. Monocyte chemoattractant protein-1 (MCP-1) and Interleukin 4/Signal transducer and activator of transcription 6 (IL-4/STAT6) signaling have been demonstrated to be essential for local proliferation of macrophages in adipose tissue [3], [9]. Thus, in the future, it would be interesting to see whether OPN in the absence of MCP-1 still activates local adipose tissue proliferation in mice during the development of obesity. A recent elegant study by Zheng et al. demonstrated that adipose tissue inflammation is characterized by initial local proliferation of adipose tissue macrophages at the early stage of obesity with an increasing contribution of macrophage migration at later stages when obesity proceeds [9]. OPN was found to mediate local proliferation of adipose tissue macrophages after 12 weeks [1], while OPN-induced migration of macrophages into adipose tissue has been found to take place after 25 weeks of high-fed-diet [5]. Therefore it is tempting to speculate, that OPN initiates and maintains adipose tissue inflammation through early local proliferation of adipose tissue macrophages and facilitation of macrophages infiltration at the later stages. Further studies are needed to dissect the contribution of OPN induced macrophage proliferation and infiltration at different time points to adipose tissue inflammation.
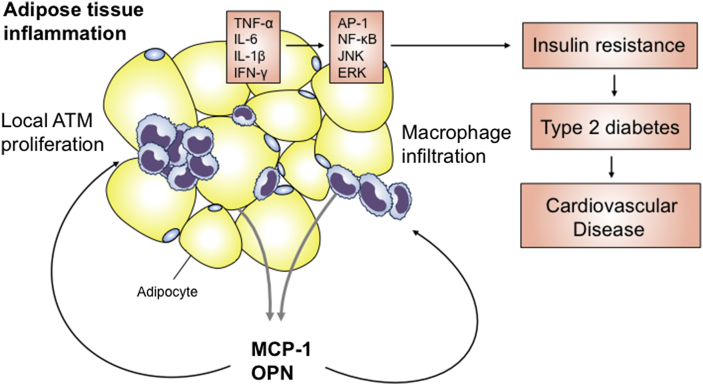
In conclusion, the study by Tardelli et al. provides convincing evidence that adipose tissue macrophage proliferation is regulated by OPN. This work further highlights the role of OPN as one of the most crucial inflammatory mediators of adipose tissue inflammation, insulin resistance and type 2 diabetes (Figure 1). Pharmacological targeting and neutralizing OPN might open new therapeutic avenues for the prevention of obesity-related metabolic disorders like type 2 diabetes and its life threatening cardiovascular complications.
Figure 1.
During the development of diet-induced weight gain OPN and MCP-1 are upregulated and mediate macrophage infiltration and local proliferation of adipose tissue macrophages (ATM) leading to enhanced adipose tissue inflammation, insulin resistance, type 2 diabetes and cardiovascular complications.
Footnotes
This commentary refers to “Osteopontin is a key player for local adipose tissue macrophage proliferation in obesity by Tardelli Matteo et al.”, http://dx.doi.org/10.1016/j.molmet.2016.09.003.
References
- 1.Tardelli M., Moreno-Viedma V., Wanko B., Grün N.G., Staffler G., Zeyda M. Osteopontin is a key player for local adipose tissue macrophage proliferation in obesity. Molecular Metabolism. 2016 doi: 10.1016/j.molmet.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 3.Amano S.U., Cohen J.L., Vangala P., Tencerova M., Nicoloro S.M., Yawe J.C. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metabolism. 2014;19(1):162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahles F., Findeisen H.M., Bruemmer D. Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Molecular Metabolism. 2014;3(4):384–393. doi: 10.1016/j.molmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nomiyama T., Perez-Tilve D., Ogawa D., Gizard F., Zhao Y., Heywood E.B. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. The Journal of Clinical Investigation. 2007;117(10):2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiefer F.W., Zeyda M., Gollinger K., Pfau B., Neuhofer A., Weichhart T. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes. 2010;59(4):935–946. doi: 10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeyda M., Gollinger K., Todoric J., Kiefer F.W., Keck M., Aszmann O. Osteopontin is an activator of human adipose tissue macrophages and directly affects adipocyte function. Endocrinology. 2011;152(6):2219–2227. doi: 10.1210/en.2010-1328. [DOI] [PubMed] [Google Scholar]
- 8.Kiefer F.W., Zeyda M., Todoric J., Huber J., Geyeregger R., Weichhart T. Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology. 2008;149(3):1350–1357. doi: 10.1210/en.2007-1312. [DOI] [PubMed] [Google Scholar]
- 9.Zheng C., Yang Q., Cao J., Xie N., Liu K., Shou P. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death & Disease. 2016;7:e2167. doi: 10.1038/cddis.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]