Abstract
Background
Andrographis paniculata (Burm. f.) Nees (APN), a principal constituent of a famous traditional Chinese medicine Fukeqianjin tablet which is used for the treatment of pelvic inflammatory disease (PID), has been reported to have anti-inflammatory effect in vitro. However, whether it has pharmacological effect on PID in vivo is unclear. Therefore, the aim of this study is to test the anti-inflammatory effect of APN and illuminate a potential mechanism.
Methods
Thirty-six female specific pathogen-free SD rats were randomly divided into control group, PID group, APN1 group, APN2 group, APN3 group and prednisone group. Pathogen-induced PID rats were constructed. The APN1, APN2 and APN3 group rats were orally administrated with APN extract at different levels. The prednisone group rats were administrated with prednisone. Eight days after the first infection, the histological examination of upper genital tract was carried out, and enzyme-linked immunosorbent assay (ELISA) was carried out using homogenate of the uterus and fallopian tube. Furthermore, immunohistochemical evaluations of NF-κB p65 and IκB-α in uterus was conducted.
Results
APN obviously suppressed the infiltrations of neutrophils and lymphocytes, and it could significantly reduce the excessive production of cytokines and chemokines including IL-1β, IL-6, CXCL-1, MCP-1 and RANTES in a dose-dependent manner. Furthermore, APN could block the pathogen-induced activation of NF-κB pathway.
Conclusion
APN showed potent anti-inflammatory effect on pathogen-induced PID in rats, with a potential mechanism of inhibiting the NF-κB signal pathway.
Keywords: Andrographis paniculata (Burm. f.) Nees, Anti-inflammatory, Pelvic inflammatory disease, NF-κB, Rats
Background
Pelvic inflammatory disease (PID) is a common gynecological disease that usually causes ectopic pregnancy, tubal factor infertility and chronic pelvic pain, which has been deemed as a great threat for life quality of woman. It includes endometritis, salpingitis, peritonitis, etc., whose etiopathogenisis is the infection of pathogenic microorganisms in upper genital tract [1]. Infiltrations of neutrophils and lymphocytes in the upper genital tract could be employed as a criteria to diagnose PID in clinic, which could be observed on hematoxylin and eosin (H & E)-stained biopsy sections [2]. With the inflammatory cell recruitment, amount of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, etc., are released excessively in local tissue and play an important role in the pathogenesis of PID [3]. The important reason for the excessive production and release of proinflammatory cytokines is the activation of nuclear factor-kappa B (NF-κB) signaling pathway when pathogens are recognized by their receptors [4].
According to the guidelines from the Center for Disease Control and Prevention (CDC) in US, antibiotics is the first choice for the treatment of PID [5], but the bacterial drug resistance and drug side effects are shortages for clinical use of antibiotics. Therefore, new complementary medicines used for PID are needed for further improvement in clinical outcomes. Andrographis paniculata (Burm. f.) Nees (APN) is a famous traditional medicine, widely used to treat sore throat, flu, and upper respiratory tract infections in many Asian countries [6]. Phytochemical studies on APN have found the principal bioactive compound andrographolide [7] and many other constituents, including diterpenoids, flavonoids, quinic acids, xanthones [8, 9], and noriridoids [10, 11]. Based on the abundant bioactive constituents, APN showed many salutary effects, such as anticancer [12], hepatoprotective [13], antiviral [14, 15], antipyretic and analgesic [16] effects, etc. Interestingly, the immunomodulatory [17], antioxidant [18], anti-inflammatory [19] and antimicrobial [20, 21] activities of APN were also reported, which may suggest the clinical use of APN in treatment for PID. Besides, APN is the principal constituent in a famous traditional Chinese medicine Fukeqianjin tablet which is used to treat PID. However, whether APN has pharmacological effect on PID has not been eliminated yet. In this study, we test the anti-inflammatory effect of APN on PID rats, and illustrate a potential mechanism of this activity.
Methods
Reagents and materials
Pentobarbital was from Xiya Reagent (Chengdu, China). Progesterone injection was obtained from Xianju Pharma (Taizhou, China). Distilled water was used in all of this experiment. Absorbable gelatin sponge was from Jinling Pharmaceutical Co. (Nanjing, China). The dried APN was purchased from Tianxiang Co. (Yueyang, China), which was identified by Prof. Zhuxin Wang (Hunan university of Chinese medicine, Changsha, China), with the total amount of andrographolide and dehydroandrographolide about 1%. A voucher specimen (No. CXL20150610) is deposited in the Key Laboratory of Hunan Province for Traditional Chinese Medicine in Obstetrics & Gynecology Research (Changsha, China). Prednisone acetate tablets were obtained from Guangdong Huanan Pharma (Guangdong, China). According to the previous method [22], a 1000 g of dried APN was extracted with 80% ethanol at room temperature and filtrated. The solution was freeze-dried to yield 62.5 g of the APN extract.
Rat PID model construction and sample collection
The animal experimental procedure was approved by the Animal Care and Use Committee of Central South University. Thirty-six female specific pathogen-free SD rats, 9-week aged and weighing 220–240 g, were randomly divided into 6 groups, including control group, PID group, APN1 group, APN2 group, APN3 group and prednisone group. Rats were acclimated for 7 days and then injected subcutaneously with 10 mg progesterone. One week later, the PID model construction was carried out referring to our previous method with some revisions [23]. Absorbable gelatin sponge, a volume of 0.125 ml, was immersed in microbe-mixing solution with U. urealyticum (t-strain mycoplasma) concentration of 1 × 108 ccu/ml and pathogenic E. coli concentration of 1 × 108 cfu/ml. Each upper genital tract of all rats except control group rats was inserted with a microbe-containing gelatin sponge, and then the rat was forced to be down for 3 min. The microbe-free gelatin sponges were implanted into the cervixes of control group rats. Four times infections were conducted with a 2-day interval. From the first infection, the APN1, APN2 and APN3 group rats were orally administrated with APN extract at a dose of 167, 334 (clinical dose) and 668 mg/kg/day, respectively. The prednisone group rats were administrated with prednisone at a dose of 1.7 mg/kg. Eight days after the first infection, rats were injected subcutaneously with pentobarbital at a dose of 30 mg/kg. The right uterus and fallopian tube were collected and restored at −80 °C, and the left uterus and fallopian tube was immersed in neutral-buffered formalin (10%). At last, rats were sacrificed by cervical dislocation.
Histological evaluation
After paraffin embedding, the left uterus and fallopian tube was cut into 2 μm sections, followed by staining with H & E. The semi-quantification was carried out according to the previous method [24]. Three parts of each slide (tissue) were checked under microscopy (×100) by a blinded observer. The inflammation of each uterus and fallopian tube was semi-scored by evaluation on the extent of inflammatory cells infiltration (graded from 0 to 3).
Enzyme-linked immunosorbent assay (ELISA)
Each right uterus and fallopian tube was weighted, immersed in physiologic saline at the ratio of 5:1 (v/w), and homogenized. The amounts of IL-1β, IL-6, CXCL-1, MCP-1 and RANTES in homogenate were determined by using ELISA kits (Neobioscience, Beijing, China). The total protein in tissue homogenate was measured with bicinchonininc acid (BCA) protein assay kit (Beyotime, Shanghai, China), and concentrations of these cytokines and chemokines were expressed as μg/g protein of homogenate.
Immunohistochemical evaluation
The paraffin embedded uterus was cut in to 2 μm sections, and then the paraffin was removed through xylene. The slide was boiled in 10 mM sodium citrate (pH 6.0) for 30 min for antigen retrieval. Non-specific binding sites were blocked by bovine serum. The specimens were incubated with NF-κB p65 or IκB-α primary antibody (Abclonal, Maryland, US) at 37 °C followed by secondary antibodies incubation. After that, the slices were treated with diaminobenzidine (DAB) reagent for color development, followed by hematoxylin counterstaining. For negative control, phosphate-buffered saline (PBS) was used instead of primary antibodies. The slices were observed under light microscopy at a magnification of × 400. Brown staining in uterus was considered as NF-κB p65 or IκB-α. The nuclear with translocation of NF-κB p65 was considered as positive. The semi-quantification of IκB-α was conducted according to a previous method with some revisions [25]. The integrated optical density (IOD) values of each slide (tissue) was obtained from its three fields with the software Image-Pro Plus 6.0. IOD index of each sample was calculated as its IOD value divided by the average IOD value of control group.
Statistic analysis
All data are expressed as means ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by Dunnett’s t test was employed for statistical analysis. Statistical analysis was conducted by using SPSS 16.0. Difference was considered as statistically significant when p < 0.05.
Results
APN attenuated the infiltration of inflammatory cells
The results of histological evaluation are showed in Fig. 1. Mass inflammatory cells, including neutrophils and lymphocytes, infiltrated into the uterus and fallopian tube of the PID group rat, indicating the occurrence of inflammation in upper genital tract. After administration of APN, the infected rats showed decreased infiltrations of neutrophils and lymphocytes, compared to PID group rats. Therefore, APN could suppress the infiltration of inflammatory cells in the upper genital tract of PID rats.
Fig. 1.
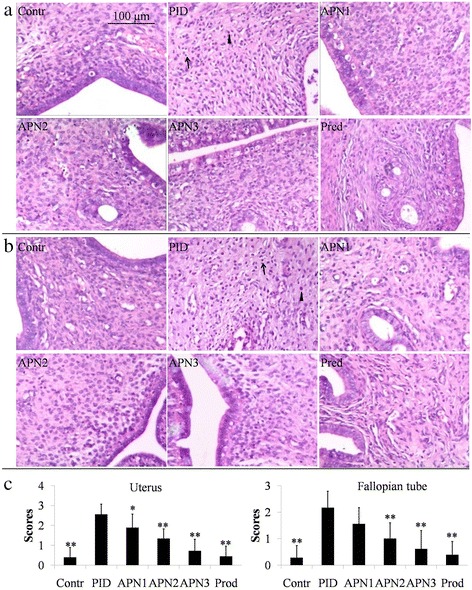
Effect of APN on pathogen-induced infiltration of neutrophils and lymphocytes in uterus and fallopian tube. Representative micrographs of uterus a and fallopian tube b stained with H & E are showed at a magnification of × 100. The infiltration of neutrophil is indicated as ▲, and the infiltration of lymphocyte is indicated as ↑. c Histological semi-quantitative scores of inflammatory cell. Each bar represents the mean ± SD (*P < 0.05, **P < 0.01, significantly different from PID group; n = 6). Contr and Pred represent control group and prednisone group, respectively
APN reduced the excessive production of cytokines and chemokines
To observe the inflammatory response and investigate the reason of inflammatory cells infiltration in the uterus and fallopian tube, the IL-1β, IL-6, CXCL-1, MCP-1, and RANTES in tissue homogenate were determined by using ELISA kits. As showed in Fig. 2 all these cytokines or chemokines were excessively produced in PID group compared to control group, and APN significantly reduced this pathogens-induced excessive production in a dose-dependent manner.
Fig. 2.
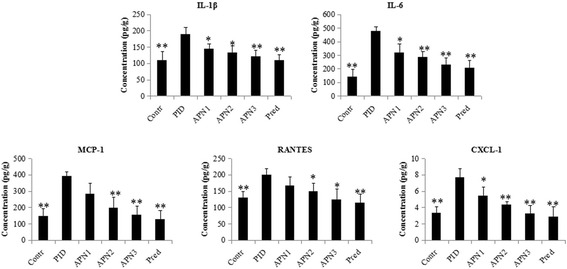
Effect of APN on pathogen-induced elevated production of IL-1β, IL-6, CXCL-1, MCP-1 and RANTES in uterus and fallopian tube. Each bar represents the mean ± SD (*P < 0.05, **P < 0.01, significantly different from PID group; n = 6)
APN exerted its anti-inflammatory effect via down-regulating the NF-κB pathway
To illustrate a potential mechanism of anti-inflammatory effect of APN, NF-κB p65 and IκB-α, two important signaling molecules in NF-κB signaling pathway, were determined by immunohistochemical method (presented in Figs. 3 and 4, respectively). In PID group, NF-κB p65 was translocated to cell nucleus, and IκB-α showed a lower content than that in the other groups. In control, APN and prednisone groups, more NF-κB p65 was distributed in cytoplasm. These results indicated that the NF-κB pathway was up-regulated in PID group, and that APN could suppress the up-regulation of signaling pathway when the upper genital tract was infected with pathogen.
Fig. 3.
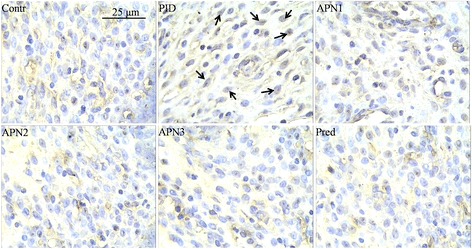
Effect of APN on pathogen-induced translocation of NF-κB p65. Representative immunohistochemistry micrographs of uterus are showed at a magnification of × 400. Arrows indicate the representative cells with nuclear translocation of NF-κB p65 (positive nuclear)
Fig. 4.
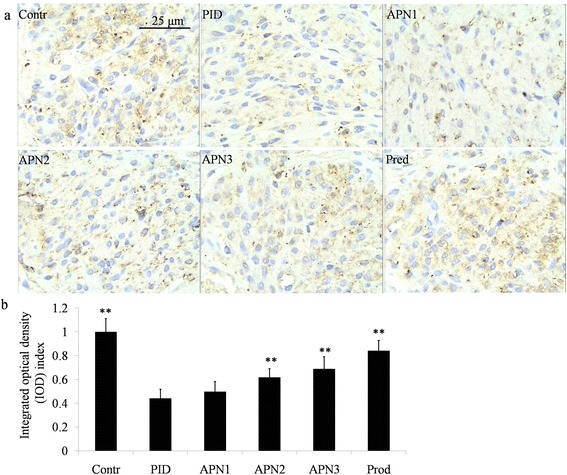
Effect of APN on pathogen-induced degradation of IκB-α. Representative immunohistochemistry micrographs of uterus a are showed at a magnification of × 400. b Semi-quantitative scores of IκB-α. Each bar represents the mean ± SD (**P < 0.01, significantly different from PID group; n = 6)
Discussion
In clinic, the common pathogens, whose infections in upper genital tract can lead to PID, include Chlamydia trachomatis, Neisseria gonorrhoeae, genital mycoplasmas, some gram-negative and gram-positive bacteria, etc. [26–29]. Each pathogen could be recognized by one or more members of Toll-like receptor (TLR) family and then initiate inflammation. TLR2 and TLR4 are two important members of TLR family in upper genital tract [30], and U. urealyticum and E. coli can be recognized by TLR2 and TLR4, respectively [31, 32]. Therefore, we attempted to use U. urealyticum and E. coli mixed solution to provoke an augmented inflammation in upper genital tract.
Neutrophils in blood circulation could firmly adhere to the endothelium cell barrier, cross it, and are recruited into sites of inflammation in different pathogen-infected tissues, which is the first line of innate immune defense against pathogens [33]. Recently, study also showed the infiltration of neutrophils into the endometrium of mice with lipopolysaccharides (LPS)-induced endometritis [34]. When mass neutrophils reach the tissue at the site of infection, they will release abundant inflammatory factors, oxygen free radical and proteolytic enzyme to kill pathogens. However, the excessive products will also cause tissue damage and lead to structural disease in upper genital tract. Infiltration of lymphocyte in genital tract of mice were observed after chronic pathogen genital infection, and this infiltration play a significant role in controlling the infection [35]. Additionally, researchers found more T lymphocyte and fewer plasma cells throughout the stroma and within the epithelium [36]. Unfortunately, Patton et al. reported that the tissue damage including epithelial cell degeneration occurred close approximation to lymphocytes [37]. In the present study, after chronic pathogen infections, large number of neutrophils and lymphocytes infiltrated into the epithelium of upper genital tract, and APN showed a good activity in attenuating the infiltration of these inflammatory cells, avoiding tissue damages which is due to the inflammation.
The proinflammatory cytokines, such as IL-1β and IL-6, play essential roles on the initiation and propagation of inflammatory response, whose level in upper genital tract were increased with the pathogen infection and the recognition of immunogens by local TLRs [38–40]. At the site of inflammation, these proinflammatory cytokines stimulate the proliferation and activation of leukocyte, and enhance the production of chemokines (i.e., CXCL-1, MCP-1, and RANTES) leading to the recruitment of hematopoietic immune cells. Then, activated neutrophils will release inflammatory cytokines and chemokines, and further intensify the inflammatory response [41]. At the same time, various proinflammatory cytokines will enhance the survival or function of neutrophil [42]. In this case, the tissue damage and structural disease in upper genital tract may occur due to the intensified inflammatory response. In the present study, elevated productions of cytokines and chemokines including IL-1β, IL-6, CXCL-1, MCP-1 and RANTES were observed in upper genital tract of PID rats, indicating an obvious local inflammatory response. Besides, APN could significantly lower the levels of these cytokines and chemokines in a dose-dependent manner, suggesting a potent anti-inflammatory effect of APN for PID. As andrographolide is the major bioactive substance and has a critical effect on inhibiting the release of an important proinflammatory factor TNF-α in APN [43], it may also contribute importantly to suppressing the inflammatory response in this study.
NF-κB is a pivotal factor in promoting the transcription of genes involved in inflammatory and immune responses [44]. In most resting cells, NF-κB family members are covalently bound to IκB family members and located in the cytoplasm with no activity [45]. When the TLRs on surface of these cells recognize pathogens, resulting in the phosphorylation and degradation of IκB members, the NF-κB members will translocate to the nucleus and bind to the cis-acting NF-κB enhancer element of genes, promoting the expression of inflammatory mediators, such as IL-1β, IL-6, etc. [31]. These produced proinflammatory cytokines act as a positive autocrine feedback so as to the further activation of NF-κB, and subsequently more proinflammatory mediators are produced [46]. NF-κB p65 and IκB-α are representative members of NF-κB and IκB family in uterus and fallopian tube, respectively [47], and therefore were chosen as indexes to test whether the NF-κB pathway was activated in the present study. Our results demonstrated the activation of NF-κB signal pathway after multi-infection of pathogens in uterus. In the rat PID model, APN exerted its anti-inflammatory with a potential mechanism of blocking the activation of NF-κB pathway. Previous studies reporting that both of APN and its main substance andrographolide showed the effect on inhibiting NF-κB pathway in vitro support this result [48, 49]. On the other hand, andrographolide also showed the other anti-inflammatory effects, such as inhibiting JAK/STAT signaling [49], inhibiting p38 MAPK pathway [50], suppressing TLRs family expressions [49], etc., and whether APN have the same or more effects in upper genital tract should be verified in the further studies to facilitate its clinical use.
Conclusions
In this study, oral administration of APN showed significant anti-inflammatory activity in pathogen-induced PID rats, including suppressing the infiltration of neutrophils and lymphocytes and reducing excessive production of cytokines or chemokines. A potential mechanism of this effect was involved in inhibiting the activation of NF-κB pathway.
Acknowledgements
We sincerely thank Dr. Mimi Tang, Miss Yi Zheng and Miss Hui Li for assistances in animal experiment, and thank Prof. Zhuxin Wang for botanical identification.
Funding
This research was supported by National Natural Science Foundation (No.81501218), China Postdoctoral Science Foundation (No. 2015 M582330), and Science and Technology Project of Hunan Province (No. 2015RS4054).
Availability of data and materials
Data are included in paper.
Authors’ contributions
JL, SC and XW carried out the design of the study. WZ, ZX, XW and JH carried out the experiments. ZC and DX performed the statistical analysis. XW and WZ wrote the manuscript and made revisions. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no conflict of interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The animal experimental procedure was approved by the Animal Care and Use Committee of Central South University.
Abbreviations
- APN
Andrographis paniculata (Burm. f.) Nees
- BCA
Bicinchonininc acid
- CDC
Center for disease control and prevention
- DAB
Diaminobenzidine
- ELISA
Enzyme-linked immunosorbent assay
- H & E
Hematoxylin and eosin
- IL
Interleukin
- IOD
Integrated optical density
- LPS
Lipopolysaccharides
- NF-κB
Nuclear factor-kappa B
- PBS
Phosphate-buffered saline
- PID
Pelvic inflammatory disease
- SD
Standard deviation
- TLR
Toll-like receptor
Contributor Information
Xiaoke Wen, Email: wxk19631214@sina.com.
Jingyu He, Email: jy.he@giat.ac.cn.
References
- 1.Soper DE. Pelvic inflammatory disease. Obstet gynecol. 2010;116(2 Pt 1):419–428. doi: 10.1097/AOG.0b013e3181e92c54. [DOI] [PubMed] [Google Scholar]
- 2.Vicetti Miguel RD, Chivukula M, Krishnamurti U, Amortegui AJ, Kant JA, Sweet RL, Wiesenfeld HC, Phillips JM, Cherpes TL. Limitations of the criteria used to diagnose histologic endometritis in epidemiologic pelvic inflammatory disease research. Pathol res pract. 2011;207(11):680–685. doi: 10.1016/j.prp.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SA, Tsai HT, Ou HC, Han CP, Tee YT, Chen YC, Wu MT, Chou MC, Wang PH, Yang SF. Plasma interleukin-1beta, −6, −8 and tumor necrosis factor-alpha as highly informative markers of pelvic inflammatory disease. Clin chem lab med. 2008;46(7):997–1003. doi: 10.1515/CCLM.2008.196. [DOI] [PubMed] [Google Scholar]
- 4.Epstein FH, Barnes PJ, Karin M. Nuclear factor-κB-a pivotal transcription factor in chronic inflammatory diseases. N engl j med. 1997;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 5.Control CFD. Prevention: sexually transmitted diseases treatment guidelines, 2010. Ann emerg med. 2011;58(1):67–68. doi: 10.1016/j.annemergmed.2011.04.006. [DOI] [Google Scholar]
- 6.Jayakumar T, Hsieh CY, Lee JJ, Sheu JR. Experimental and clinical pharmacology of andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid based complement alternat med. 2013;2013:846740. doi: 10.1155/2013/846740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SHARMA M, SHARMA R. Identification, purification and quantification of andrographolide from andrographis paniculata (burm. F.) nees by HPTLC at different stages of life cycle of crop. J curr chem pharm sci. 2013;3(1):23–32. [Google Scholar]
- 8.Subramanian R, Asmawi MZ, Sadikun A. A bitter plant with a sweet future? a comprehensive review of an oriental medicinal plant: andrographis paniculata. Phytochem rev. 2012;11(1):39–75. doi: 10.1007/s11101-011-9219-z. [DOI] [Google Scholar]
- 9.Zhou K-L, Chen L-X, Zhuang Y-L, Wang N-L, Yao X-S, Qiu F. Two new ent-labdane diterpenoid glycosides from the aerial parts of andrographis paniculata. J asian nat prod res. 2008;10(10):939–943. doi: 10.1080/10286020802217432. [DOI] [PubMed] [Google Scholar]
- 10.Sareer O, Ahad A, Umar S. Prophylactic and lenitive effects of Andrographis paniculata against common human ailments: an exhaustive and comprehensive reappraisal. J Pharm Res Opin. 2014;2(10):138–62. [Google Scholar]
- 11.Xu C, Chou G-X, Wang C-H, Wang Z-T. Rare noriridoids from the roots of andrographis paniculata. Phytochemistry. 2012;77:275–279. doi: 10.1016/j.phytochem.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Sheeja K, Kuttan G. Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by andrographis paniculata extract and andrographolide. Immunopharmacol immunotoxicol. 2007;29(1):81–93. doi: 10.1080/08923970701282726. [DOI] [PubMed] [Google Scholar]
- 13.Rana A, Avadhoot Y. Hepatoprotective effects ofAndrographis paniculata against carbon tetrachloride-induced liver damage. Arch pharm res. 1991;14(1):93–95. doi: 10.1007/BF02857822. [DOI] [PubMed] [Google Scholar]
- 14.Chang RS, Ding L, Gai-Qing C, Qi-Choa P, Ze-Lin Z, Smith KM. Dehydroandrographolide succinic acid monoester as an inhibitor against the human immunodeficiency virus. Exp biol med. 1991;197(1):59–66. doi: 10.3181/00379727-197-43225. [DOI] [PubMed] [Google Scholar]
- 15.Lin T-P, Chen S-Y, Duh P-D, Chang L-K, Liu Y-N. Inhibition of the Epstein-Barr virus lytic cycle by andrographolide. Biol pharm bull. 2008;31(11):2018–2023. doi: 10.1248/bpb.31.2018. [DOI] [PubMed] [Google Scholar]
- 16.Madav S, Tripathi H, Mishra S. Analgesic, antipyretic and antiulcerogenic effects of andrographolide. Indian j pharm sci. 1995;57(3):121. [Google Scholar]
- 17.Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S. Anticancer and immunostimulatory compounds from andrographis paniculata. J ethnopharmacol. 2004;92(2):291–295. doi: 10.1016/j.jep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Verma N, Vinayak M. Antioxidant action of andrographis paniculata on lymphoma. Mol biol rep. 2008;35(4):535–540. doi: 10.1007/s11033-007-9119-x. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Wang Z-T, Ji L-L, Ge B-X. Inhibitory effects of neoandrographolide on nitric oxide and prostaglandin E2 production in LPS-stimulated murine macrophage. Mol cell biochem. 2007;298(1–2):49–57. doi: 10.1007/s11010-006-9349-6. [DOI] [PubMed] [Google Scholar]
- 20.Voravuthikunchai SP, Limsuwan S. Medicinal plant extracts as anti-Escherichia coli O157: H7 agents and their effects on bacterial cell aggregation. J food protect. 2006;69(10):2336–2341. doi: 10.4315/0362-028x-69.10.2336. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Marshall RL, Mukkur TK. An investigation on the antimicrobial activity of andrographis paniculata extracts and andrographolide in vitro. Asian j plant sci. 2006;5:527–530. doi: 10.3923/ajps.2006.527.530. [DOI] [Google Scholar]
- 22.Zhang XF, Tan BKH. Antihyperglycaemic and anti‐oxidant properties of andrographis paniculata in normal and diabetic rats. Clin exp pharmacol physiol. 2000;27(5–6):358–363. doi: 10.1046/j.1440-1681.2000.03253.x. [DOI] [PubMed] [Google Scholar]
- 23.Zou W, Wen X, Zheng Y, Xiao Z, Luo J, Chen S, Wang Y, Cheng Z, Xiang D, Nie Y. Metabolomic study on the preventive effect of patrinia scabiosaefolia fisch on multipathogen induced pelvic inflammatory disease in rats. Evid based complement alternat med. 2015;501:170792. doi: 10.1155/2015/170792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo YL, Zhang CC, Li PB, Nie YC, Wu H, Shen JG, Su WW. Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int immunopharmacol. 2012;13(3):301–307. doi: 10.1016/j.intimp.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Jiang R, Wang H, Deng L, Hou J, Shi R, Yao M, Gao Y, Yao A, Wang X, Yu L, et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC cancer. 2013;13:59. doi: 10.1186/1471-2407-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections: 2008. vol. 20. In: World Health Organization, editor. Geneva: Reproductive Health Matters; 2012. p. 207–9.
- 27.Quentin R, Verdon R. Microbiologic basis of diagnosis and treatment of pelvic inflammatory disease. J gynecol obstet biol reprod. 2012;41(8):850–863. doi: 10.1016/j.jgyn.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Saini S, Gupta N, Batra G, Arora DR. Role of anaerobes in acute pelvic inflammatory disease. Indian j med microbiol. 2003;21(3):189–192. [PubMed] [Google Scholar]
- 29.Zhang D, Wen J, Zhou W, Wu X. Pathogenic bacteria distribution and drug resistance isolated from women with pelvic inflammatory disease. Chinese j nosocomiolog. 2009;19(13):1747–1750. [Google Scholar]
- 30.Pioli PA, Amiel E, Schaefer TM, Connolly JE, Wira CR, Guyre PM. Differential expression of toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect immun. 2004;72(10):5799–5806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He J, You X, Zeng Y, Yu M, Zuo L, Wu Y. Mycoplasma genitalium-derived lipid-associated membrane proteins activate NF-κB through toll-like receptors 1, 2, and 6 and CD14 in a MyD88-dependent pathway. Clin vaccine immunol. 2009;16(12):1750–1757. doi: 10.1128/CVI.00281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheldon IM, Rycroft AN, Dogan B, Craven M, Bromfield JJ, Chandler A, Roberts MH, Price SB, Gilbert RO, Simpson KW. Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice. Plos one. 2010;5(2):e9192. doi: 10.1371/journal.pone.0009192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rebordão M, Carneiro C, Alexandre-Pires G, Brito P, Pereira C, Nunes T, Galvão A, Leitão A, Vilela C, Ferreira-Dias G. Neutrophil extracellular traps formation by bacteria causing endometritis in the mare. J reprod immunol. 2014;106:41–49. doi: 10.1016/j.jri.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Lv X, Fu K, Li W, Wang Y, Wang J, Li H, Tian W, Cao R. TIIA attenuates LPS-Induced mouse endometritis by suppressing the NF-κB signaling pathway. Can J Physiol Pharmacol. 2015;93(11):967–71. doi: 10.1139/cjpp-2015-0003. [DOI] [PubMed] [Google Scholar]
- 35.Rank R, Soderberg L, Barron A. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect immun. 1985;48(3):847–849. doi: 10.1128/iai.48.3.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiviat NB, Wølner-Hanssen P, Eschenbach DA, Wasserheit JN, Paavonen JA, Bell TA, Critchlow CW, Stamm WE, Moore DE, Holmes KK. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am j surg pathol. 1990;14(2):167–175. doi: 10.1097/00000478-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Patton DL, Askienazy-Elbhar M, Henry-Suchet J, Campbell LA, Cappuccio A, Tannous W, Wang SP, Kuo CC. Detection of Chlamydia trachomatis in fallopian tube tissue in women with postinfectious tubal infertility. Am j obstet gynecol. 1994;171(1):95–101. doi: 10.1016/S0002-9378(94)70084-2. [DOI] [PubMed] [Google Scholar]
- 38.Tortorella C, Piazzolla G, Matteo M, Pinto V, Tinelli R, Sabbà C, Fanelli M, Cicinelli E. Interleukin-6, interleukin-1β, and tumor necrosis factor α in menstrual effluents as biomarkers of chronic endometritis. Fertil steril. 2014;101(1):242–247. doi: 10.1016/j.fertnstert.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 39.Cronin JG, Turner ML, Goetze L, Bryant CE, Sheldon IM. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol reprod. 2012;86(2):51. doi: 10.1095/biolreprod.111.092718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner ML, Cronin JG, Healey GD, Sheldon IM. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via toll-like receptors TLR2, TLR1, and TLR6. Endocrinology. 2014;155(4):1453–1465. doi: 10.1210/en.2013-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gasperini S, Zambello R, Agostini C, Trentin L, Tassinari C, Cadrobbi P, Semenzato G, Cassatella MA. Impaired cytokine production by neutrophils isolated from patients with AIDS. Aids. 1998;12(4):373–379. doi: 10.1097/00002030-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Milot E, Filep JG. Regulation of neutrophil survival/apoptosis by Mcl-1. Scientific world journal. 2011;11:1948–1962. doi: 10.1100/2011/131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Low M, Khoo CS, Munch G, Govindaraghavan S, Sucher NJ. An in vitro study of anti-inflammatory activity of standardised andrographis paniculata extracts and pure andrographolide. BMC complement altern med. 2015;15:18. doi: 10.1186/s12906-015-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helenius M, Hänninen M, Lehtinen SK, Salminen A. Aging-induced up-regulation of nuclear binding activities of oxidative stress responsive NF-kB transcription factor in mouse cardiac muscle. J mol cell cardiol. 1996;28(3):487–498. doi: 10.1006/jmcc.1996.0045. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu rev immunol. 1998;16(1):225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 46.Bours V, Bonizzi G, Bentires-Alj M, Bureau F, Piette J, Lekeux P, Merville M-P. NF-κB activation in response to toxical and therapeutical agents: role in inflammation and cancer treatment. Toxicology. 2000;153(1):27–38. doi: 10.1016/S0300-483X(00)00302-4. [DOI] [PubMed] [Google Scholar]
- 47.Dai L, Gu L, Di W. MiR-199a attenuates endometrial stromal cell invasiveness through suppression of the IKKβ/NF-κB pathway and reduced interleukin-8 expression. Mol hum reprod. 2012;18(3):136–145. doi: 10.1093/molehr/gar066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chao WW, Kuo YH, Hsieh SL, Lin BF. Inhibitory effects of ethyl acetate extract of andrographis paniculata on NF-kappaB trans-activation activity and LPS-induced acute inflammation in mice. Evid based complement alternat med. 2011;2011:254531. doi: 10.1093/ecam/nep120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parichatikanond W, Suthisisang C, Dhepakson P, Herunsalee A. Study of anti-inflammatory activities of the pure compounds from andrographis paniculata (burm.f.) nees and their effects on gene expression. Int immunopharmacol. 2010;10(11):1361–1373. doi: 10.1016/j.intimp.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Shao ZJ, Zheng XW, Feng T, Huang J, Chen J, Wu YY, Zhou LM, Tu WW, Li H. Andrographolide exerted its antimicrobial effects by upregulation of human beta-defensin-2 induced through p38 MAPK and NF-kappaB pathway in human lung epithelial cells. Can j physiol pharmacol. 2012;90(5):647–653. doi: 10.1139/y2012-050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are included in paper.


