Abstract
Background
Malaria remains an important cause of morbidity and mortality in India. Though many comprehensive studies have been carried out in Africa and Southeast Asia to characterize and examine determinants of Plasmodium falciparum and Plasmodium vivax malaria pathogenesis, fewer have been conducted in India.
Methods
A prospective study of malaria-positive individuals was conducted at Goa Medical College and Hospital (GMC) from 2012 to 2015 to identify demographic, diagnostic and clinical indicators associated with P. falciparum and P. vivax infection on univariate analysis.
Results
Between 2012 and 2015, 74,571 febrile individuals, 6287 (8.4%) of whom were malaria positive, presented to GMC. The total number of malaria cases at GMC increased more than two-fold over four years, with both P. vivax and P. falciparum cases present year-round. Some 1116 malaria-positive individuals (mean age = 27, 91% male), 88.2% of whom were born outside of Goa and 51% of whom were construction workers, were enroled in the study. Of 1088 confirmed malaria-positive patients, 77.0% had P. vivax, 21.0% had P. falciparum and 2.0% had mixed malaria. Patients over 40 years of age and with P. falciparum infection were significantly (p < 0.001) more likely to be hospitalised than younger and P. vivax patients, respectively. While approximately equal percentages of hospitalised P. falciparum (76.6%) and P. vivax (78.9%) cases presented with at least one WHO severity indicator, a greater percentage of P. falciparum inpatients presented with at least two (43.9%, p < 0.05) and at least three (29.9%, p < 0.01) severity features. There were six deaths among the 182 hospitalised malaria positive patients, all of whom had P. falciparum.
Conclusion
During the four year study period at GMC, the number of malaria cases increased substantially and the greatest burden of severe disease was contributed by P. falciparum.
Keywords: MESA-ICEMR, Goa, Epidemiology, Diagnostics, Severity, Characteristics, Features
Background
Globally, parasites of the Plasmodium genus infect more than 200 million people and cause an estimated 438,000 deaths annually [1]. India is the second most populous country in the world with ongoing malaria transmission, with 91% of its more than 1.2 billion population living in areas of malaria risk [1]. The most recent estimates report up to 26 million malaria cases and 55,000 deaths due to malaria annually in India [1]. However, there is ongoing, vigorous debate about these figures, in part due to the vast scale of the country [2–5].
India is co-endemic for Plasmodium falciparum and Plasmodium vivax, posing challenges for malaria control and elimination planning because the two parasite species may differ in mosquito vectors, spatial distributions and transmission dynamics and because of the relapsing nature of P. vivax infection with a dormant liver stage [6–8]. Overall, approximately 66% of malaria infections in India are caused by P. falciparum and 34% are caused by P. vivax [1]. However, the proportional distribution varies across India and a wide range of clinical presentations are seen from both predominant species of malaria [6]. In contrast to Africa, malaria transmission in India is more limited, both adolescents and adults are at risk of severe malaria, and a substantial proportion of cases are infected with P. vivax rather than the traditionally more virulent P. falciparum. Although many expansive and comprehensive studies have been carried out in Africa [9–14] and Southeast Asia [15–20] to examine pathogenesis and mortality determinants in malaria-positive patients, a more limited number of such studies, generally smaller in scope, have been conducted within India [21–29].
India has one of the world’s largest and most extensive national surveillance systems to identify malaria incidence [30, 31] and is a highly heterogeneous country with more than 2000 ethnic groups and 22 official languages spread across 29 states and seven union territories. All of these states have populations in the millions and a more than ten-fold variation in average per capita income [32], which leads to important geographic variations in disease epidemiology and substantial variability in the delivery of malaria diagnosis, care and treatment across the country [33]. It would be costly and operationally difficult to measure all of the diverse malaria settings in India through community-based surveys or reactive case-detection methods [34] and challenging to comprehensively address every environmental context through national-level strategies, programmes and recommendations [35, 36].
In addition to India’s vast countrywide and state-level monitoring of malaria, rigorous hospital-based reports can provide epidemiological data relevant to local control and elimination initiatives as well as clinical data relevant to diagnosis, care and treatment efforts. Clinicians, as well as the wider public health system, may benefit from deep studies of specific patient pools in order to better understand malaria transmission and pathogenesis in local communities. Such information may also assist in the prioritization of resources for patient care and treatment.
With the aim of conducting methodical studies in a low to mid-endemicity, peri-urban setting, the Malaria Evolution in South Asia (MESA) International Center of Excellence for Malaria Research (ICEMR) established a research site at Goa Medical College and Hospital (GMC) [37–39]. Previously an overseas province of Portugal, Goa is a small, prosperous, southwestern state of India where both P. falciparum and P. vivax are endemic. GMC is the only government tertiary care centre in the state and operates under the auspices of the Government of Goa Public Health Department to provide health care to all, free-of-charge. The relatively advanced diagnostic and clinical capabilities at GMC draw a large, diverse patient pool and allow for deep clinical analysis. As a research site, GMC offers a highly heterogeneous patient population, a constant flow of febrile and malaria-positive cases, and a wide spectrum of clinical presentations of malaria in a relatively affluent, burgeoning peri-urban area. The present study provides a detailed description of the demographic, diagnostic and clinical characteristics of malaria-positive study participants at GMC from 2012 to 2015.
Methods
Institutional ethics approvals
The present work was part of the US National Institutes of Health-sponsored Program Project [40, 41] entitled Malaria Evolution in South Asia International Center of Excellence for Malaria Research (MESA-ICEMR). The activities of this centre were approved by the Government of India (GOI) Health Ministry Screening Committee (HMSC) and the Government of Goa Public Health Department. The human subjects protocol and consent forms for enrolment of Plasmodium-positive individuals presenting to Goa Medical College and Hospital (Bambolim, Goa, India) were approved by the institutional review boards (IRB) of the Division of Microbiology and Infectious Diseases (DMID) at the US National Institute of Allergy and Infectious Diseases (NIAID), GMC, and the University of Washington (UW).
Study design and enrolment
All febrile individuals presenting to the outpatient, paediatric, and casualty departments of GMC were tested by hospital staff for Plasmodium infection via finger-prick or venous blood draw and examination by microscopy of Giemsa-stained thin blood smear and/or by rapid diagnostic test (RDT), FalciVax, Zephyr Biomedicals). The hospital generally only used one method of testing per individual, usually microscopy. In rare instances, both RDT and microscopy were used. If the RDT or microscopy was positive, the individual was counted as malaria positive. If the RDT and microscopy showed different species of parasite, the individual was counted as mixed infection positive. Hospital-determined malaria positivity and species of infection results are presented for all febrile patients presenting to GMC between January 2012 and December 2015.
Individuals determined to be malaria positive by either microscopy or RDT by the hospital, who were between the ages of 12 months and 65 years and not pregnant, were referred to the MESA-ICEMR study team. Enrolment generally occurred during normal working hours and when study staff were not completing enrolment of a previous patient.
Outpatients and individuals admitted to the Medicine, Paediatric, and Intensive Cardiac Care Unit (ICCU) wards were approached for participation in the study. Severely ill individuals requiring use of a ventilator and, therefore, admitted to the Intensive Care Unit (ICU) were not approached for inclusion in this study. Malaria-positive individuals were given oral and written descriptions of the study and were asked to provide written informed consent or, in the case of children between 12 months and 18 years, consent of a parent or guardian or, in the case of children between the ages of eight and 18 years, assent in addition to the consent of a parent or guardian. Each study participant received a unique numerical code in order to streamline data collection.
Upon enrolment, study participants provided demographic information as well as history of malaria infection, symptoms and travel to study staff. Following venous blood draw into vacutainers (ACD, BD, India), study participants received care and treatment as directed by the attending physician at GMC.
Sample processing
Each venous blood sample was transferred to the on-site MESA-ICEMR research laboratory. Research staff immediately prepared three thin and two thick blood smears. Plasmodium species and parasitaemia were determined by Giesma-stained thick smear reading by expert microscopists. Research staff then performed an additional RDT (FalciVax, Zephyr Biomedicals, India) and measured haemoglobin (Hb 201, HemoCue, USA) and haematocrit (Iris StatSpin, Beckman Coulter, USA).
In addition to the hospital determination used for initial recruitment, research study staff also made a separate, independent determination of malaria positivity and species of infection based on diagnostic tests conducted in the MESA-ICEMR laboratory. If both RDT and microscopy results were negative in the MESA-ICEMR lab, the patient was classified as an unconfirmed case. If RDT or microscopy was positive, the patient was counted as a confirmed malaria positive case. If the RDT and microscopy showed different species of parasite, the sample was identified as mixed infection positive. Individuals enroled in the study who were determined to be malaria positive by the hospital, but who were classified as malaria negative by the research study staff were excluded from statistical analysis of demographic, diagnostic and clinical characteristics. Study-determined positivity and species of infection results are presented from April 2012 through December 2015.
Clinical characteristics and severity scores
Measured features among all enroled patients with confirmed malaria infection were: high parasitaemia (>4.0% for P. falciparum, >1.5% for P. vivax); hyperparasitaemia (>10% for P. falciparum, >2.0% for P. vivax); high fever (>38.1 °C); severe fever (>38.9 °C); anaemia (haemoglobin (Hb) < 9 g/dL in those 12 years and older, Hb < 7 g/dL in those 1–11 years); and severe anaemia (Hb < 7 g/dL in those 12 years and older, Hb < 5 g/dL in those 1–11 years). Patients were admitted to GMC based on the clinical judgement of the attending physician and did not have to fulfil any WHO criteria for severe malaria classification.
Enroled P. falciparum- and P. vivax-positive patients who were hospitalised were assessed daily by trained GMC clinicians for severity of infection based on WHO criteria [42, 43]. Measured features among enroled, hospitalised patients associated with severe malaria infection were: cerebral malaria (Glasgow coma score <11 in adults or Blantyre coma score <3 in children and presence of asexual forms in blood); hypoglycaemia (blood glucose < 40 mg/dL); metabolic acidosis (plasma bicarbonate < 15 mmol/L), renal failure (serum creatinine > 3.0 ml/dL and/or blood urea nitrogen (BUN) > 17 mmol/L); abnormal bleeding (observable); respiratory distress (breathing rate > 20 breaths/min or partial oxygen (PaO2) < 75); severe jaundice (total bilirubin > 10 mg/dL); circulatory collapse/shock (systolic blood pressure (BP) < 80 mmHg with cold extremities); pulmonary oedema (observable); severe anaemia (Hb < 7 g/dL or haematocrit (HCT) < 20% in those 12 years and older, Hb < 5 g/dL or HCT < 15% in those one to 11 years); and death. Occasionally, the complete clinical laboratory investigation panel was not ordered [42, 43]. A severity score (SS) was calculated for all hospitalised patients based on the total number of WHO severe malaria criteria met at enrolment and throughout hospitalisation.
Meteorological data
Rainfall data for Goa were obtained from the India Meteorological Department (IMD) (Ministry of Earth Sciences, Government of India). Data were collected at the IMD observatory in the capital city of Goa, Panaji, located roughly 5 km from GMC.
Data and sample management
Demographic and clinical study data were collected and managed using REDCap electronic data capture tools (Nashville, TN, USA). Diagnostic study data were recorded and stored using LabKey software (Seattle, WA, USA). All samples and associated aliquots were labelled and stored using a customized FreezerPro database (RURO Inc, Frederick, MD, USA).
Statistical analysis
Data were initially entered into a customized SQL database (LabKey server) followed by independent verification against the original case report forms. Statistical differences between percent parasitaemias between species for all enroled patients, inpatients and outpatients were determined using an unequal variance t test using GraphPad Prism 6 software (La Jolla, CA, USA). All other analyses were conducted using R (Vienna, Austria).
For univariate analysis, the primary outcome was the species of malaria infection. Continuous variables were summarized with mean and standard deviation and binary variables were summarized with proportions. Between group univariate comparisons of features for P. falciparum and P. vivax were analysed with logistic regression. Demographic and diagnostic features were reported for all confirmed P. falciparum and P. vivax-infected enroled patients. Clinical laboratory tests and classifications were only reported for confirmed P. falciparum and P. vivax-infected inpatients and were included in tables only if a threshold of 20% of enroled inpatients had a particular documented clinical result or classification. Logistic regression was also used to measure differences in hospitalisation rates by species of infection between age ranges. Results were represented as odds ratios with 95% confidence intervals (OR (95%)) as well as p values. Differences were considered to be significant at p values ≤0.05.
Mixed P. falciparum and P. vivax infections were excluded from all statistical analyses due to the small sample size.
Results
Febrile and malaria-positive cases at Goa Medical College and Hospital
A total of 74,571 febrile individuals presented to GMC between January 2012 and December 2015 and all were tested for malaria. Of those, 6287 (8.4%) were determined to be positive for malaria infection (Fig. 1). Over four years of passive surveillance, the number of malaria-positive cases presenting to GMC steadily and significantly increased, from 889 cases in 2012 to 2261 cases in 2015 (Fig. 2). While the traditional malaria season in Goa is June to December, P. falciparum and P. vivax cases were recorded throughout the year at GMC with the peak coinciding with the rainy season (Fig. 3). In 2015, the increase in malaria cases preceded the annual rains and case numbers remained high from April through December. The number of monthly cases observed at GMC during the height of the malaria season (September) was approximately five times greater than at the lowest point during the middle of the dry season (February).
Fig. 1.
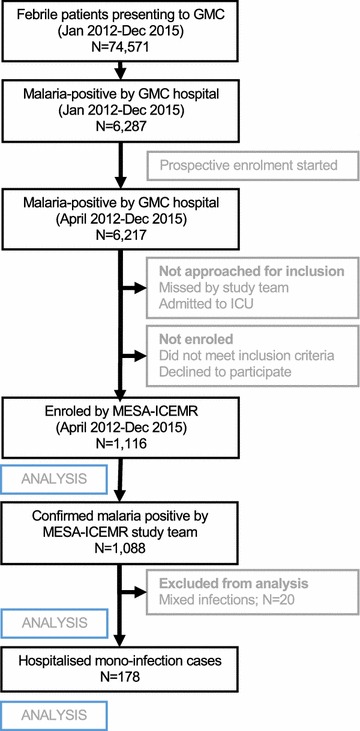
Diagram of study enrolment at Goa Medical College and Hospital. Of the 74,517 febrile patients who presented to GMC and were tested for malaria, 6287 were positive by hospital diagnosis. Of the 6287 cases identified by the hospital between January 2012 and December 2015, 6217 were referred to the MESA-ICEMR study team between April 2012 and December 2015. Of 6217, 1116 study participants were enroled by the MESA-ICEMR and 1088 were confirmed by the MESA-ICEMR to be malaria positive. Excluding mixed infections, 178 confirmed malaria cases were hospitalised by GMC
Fig. 2.
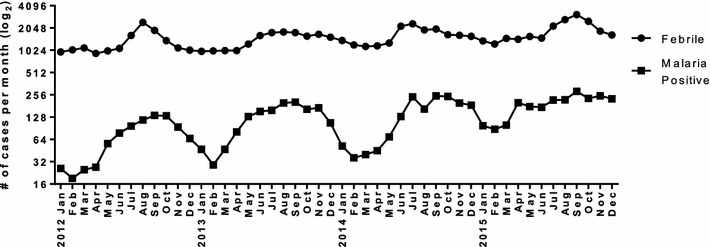
Increasing febrile and malaria positive cases at Goa Medical College and Hospital over four years. In 2012, 889 malaria cases were identified out of 15,589 febrile cases. In 2013, 1477 malaria cases were identified out of 17,017 febrile cases. In 2014, 1660 malaria cases were identified out of 19,454 febrile cases. In 2015, 2261 malaria cases were identified out of 22,511 febrile cases
Fig. 3.
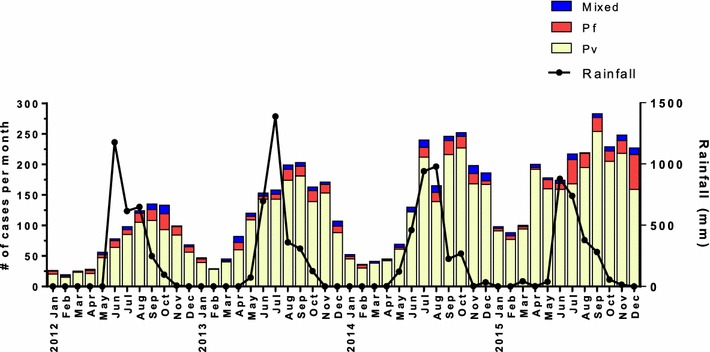
Monthly malaria positive cases by species with rainfall data over four years. In 2012, 721 P. vivax, 120 P. falciparum and 48 mixed malaria cases were diagnosed for a total of 889 annual malaria infections at GMC. In 2013, 1297 P. vivax, 116 P. falciparum and 64 mixed malaria cases were diagnosed for a total of 1477 annual malaria infections at GMC. In 2014, 1467 P. vivax, 111 P. falciparum and 82 mixed malaria cases were diagnosed for a total of 1660 annual malaria infections at GMC. In 2015, 1972 P. vivax, 223 P. falciparum and 66 mixed malaria cases were diagnosed for a total of 2261 annual malaria infections at GMC
Of the malaria-positive individuals presenting to GMC, the majority (86.8%) were diagnosed by the hospital with P. vivax mono-infection. Plasmodium falciparum infections accounted for 9.1%, and mixed infections for 4.1% of the total cases over four years (Fig. 3). Month-wise, the number of P. vivax infections was always greater than P. falciparum and mixed infections. The seasonality of P. vivax and P. falciparum infections was very similar, with case numbers of both increasing after the start of the rains. At the peak of the malaria transmission season as many as 10% of fever cases at GMC were malaria positive (Fig. 2).
Demographic characteristics of study participants
A total of 1116 of 6217 (18%) febrile individuals identified by GMC as malaria-positive via microscopy or RDT were enroled in the present study between April 2012 and December 2015 (Fig. 1). Enrolment of this subset of patients was based on the logistical capabilities of the study team. All study participants had a mean age of 27 years (median 24 years; IQR 20–32 years) and were predominantly male (91.0%). The male-to-female ratio of study participants was roughly similar to the gender distribution of febrile cases presenting to GMC (86.7% male and 13.3% female). The mean age of male study participants was 27 years (median 24 years, IQR 20–31), while the mean-age of non-pregnant female study participants was slightly older at 30 years (median 31 years, IQR 19–40). A small minority of malaria-positive cases (51, 4.6%) were children under the age of 16 years, of whom 64.7% were male and 35.3% were female.
Study participants at GMC represented a very heterogeneous Indian population. Approximately one-tenth of study participants (11.8%) were born in Goa and 88.2% were born in 26 other Indian states or outside of India (Fig. 4). The states with the greatest representation of study participants were the eastern states of Bihar with 186 (16.7%) individuals, West Bengal with 179 (16.0%), Uttar Pradesh with 152 (13.6%), and Jharkhand with 111 (9.9%). The majority of study participants (51.5%) self-identified as construction workers while 4.7% self-identified as students, 3.0% as housewives, 1.7% as soldiers or police, 1.5% as office workers, 1.3% as factory workers, and 33.2% selected the option other.
Fig. 4.
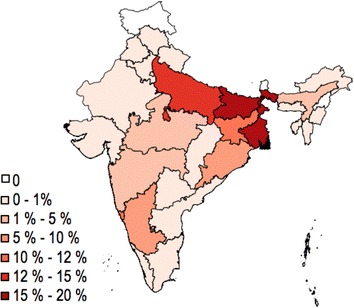
Geographic heterogeneity of Goa Medical College malaria-positive study participants. Study participants at GMC represented a very heterogeneous Indian population and most study participants were born outside of Goa. The proportion of patients that were born in each state is shown
Some 97.5% of study participants were confirmed to be Plasmodium positive by the MESA-ICEMR study team, with 77.0% of those cases being P. vivax, 21.0% P. falciparum and 2.0% mixed-infection positive. The majority of malaria-positive individuals (83.3%) were classified as having uncomplicated malaria and treated on an outpatient basis based on the clinical judgement of the attending physician at GMC. While P. vivax infections were much more common than P. falciparum, a higher proportion of hospitalised cases were P. falciparum positive (107/182, 58.8%) versus P. vivax positive (71/182, 39.0%) and mixed-infection positive (4/182, 2.2%). All analyses presented below includes only patients with mono-infections.
Demographic and diagnostic characteristics of confirmed malaria patients
As expected, malaria patients infected with P. falciparum only were significantly more likely to have a higher parasitaemia than those infected with P. vivax mono-infection among both outpatient and inpatient groups (Table 1). The mean parasitaemia value among P. vivax cases was slightly higher for outpatients as compared to inpatients, though the difference was not significant.
Table 1.
Parasitaemia profiles by unequal variances t test of Plasmodium falciparum and Plasmodium vivax patients
| Number of cases$ | Per cent parasitaemia (Mean ± SD) | p value | |||
|---|---|---|---|---|---|
| P. falciparum | P. vivax | P. falciparum | P. vivax | ||
| Outpatients | 121 | 767 | 1.30 ± 1.94% | 0.56 ± 0.67% | <0.001*** |
| Inpatients | 107 | 71 | 1.35 ± 3.31% | 0.41 ± 0.65% | 0.005**# |
| All patients | 228 | 838 | 1.32 ± 2.67% | 0.55 ± 0.67% | <0.001*** |
** p < 0.01; *** p < 0.001, # Welch correction, $ excludes mixed-infection cases
To better understand the P. falciparum and P. vivax patient populations at GMC, univariate analyses using logistic regression with a primary outcome of malaria parasite species were performed. The demographic and basic diagnostic features associated with species of infection for all malaria-confirmed cases are shown in Table 2. Occupation as a construction worker was weakly correlated with P. vivax infection only (55%), albeit a significant proportion of P. falciparum cases (47%) self-identified as construction workers. Higher parasitaemia, lower Hb, anaemia, severe anaemia and hospitalisation were all significantly associated with P. falciparum infection. Conversely, gender, age, geographic origin, temperature, high fever, and severe fever did not significantly differ between P. falciparum and P. vivax cases.
Table 2.
Univariate logistic regression of demographic and diagnostic features by species of malaria infection
| Feature | P. falciparum $ | P. vivax $ | OR (95% CI) | p value |
|---|---|---|---|---|
| Male, n/N (%) | 210/228 (92.1) | 768/838 (91.6) | 1.1 (0.6–1.9) | 0.806 |
| Age, y, Mean ± SD | 27.0 ± 11.2 | 27.4 ± 10.5 | 1.0 (1.0–1.0) | 0.659 |
| Construction workers, n/N (%) | 107/228 (46.9) | 461/838 (55.0) | 0.7 (0.6–1.0) | 0.046* |
| Born in Goa, n/N (%) | 30/228 (13.2) | 96/838 (11.5) | 1.2 (0.8–1.9) | 0.337 |
| Born in SW India, n/N (%) | 56/228 (24.6) | 187/838 (22.3) | 1.1 (0.8–1.6) | 0.475 |
| Born in E India, n/N (%) | 134/228 (58.8) | 545/838 (65.0) | 0.8 (0.6–1.1) | 0.103 |
| % Parasitaemia, Mean ± SD | 1.3 ± 2.7 | 0.6 ± 0.7 | 1.6 (1.4–1.9) | <0.001*** |
| Temperature, C, Mean ± SD | 38.1 ± 1.6 | 38.2 ± 1.4 | 1.0 (0.9–1.0) | 0.642 |
| High fever (>38.1 °C), n/N (%) | 104/228 (45.6) | 380/838 (45.3) | 1.0 (0.8–1.4) | 0.769 |
| Severe fever (>38.9 °C), n/N (%) | 71/228 (31.1) | 235/838 (28.0) | 1.2 (0.9–1.7) | 0.266 |
| Haemoglobin, g/dL, Mean ± SD | 10.9 ± 2.6 | 11.6 ± 2.1 | 0.9 (0.8–0.9) | <0.001*** |
| Anaemia, n/N (%) | 42/219 (19.2) | 90/837 (10.8) | 2.0 (1.3–2.9) | 0.001** |
| Severe anaemia, n/N (%) | 15/219 (6.8) | 14/837 (1.7) | 4.3 (2.0–9.2) | <0.001*** |
| Hospitalisation, n/N (%) | 107/228 (46.9) | 71/838 (8.5) | 9.1 (6.4–13.0) | <0.001*** |
* p < 0.05; ** p < 0.01; *** p < 0.001, $ excludes mixed-infection cases
Clinical characteristics of hospitalised confirmed malaria patients
Overall, 46.9% (107/228) of enroled P. falciparum patients were hospitalised compared with 8.5% (71/838) of enroled P. vivax patients, demonstrating an expected strong skew toward inpatient care and treatment of P. falciparum versus P. vivax (logistic regression; p < 0.001). Furthermore, there was a significant age-dependent increase in hospitalisations. All malaria-positive patients over 40 years of age infected with either P. falciparum or P. vivax were significantly more likely to be hospitalised than those between the ages of one and 20 and ages 21 and 40 with the same species of infection (logistic regression; P. vivax p < 0.001, P. falciparum p = 0.033).
Among inpatients with malaria-confirmed infections at GMC, those with P. falciparum were more severely ill according to the WHO severe malaria criteria (Table 3) [42, 43]. Approximately equal percentages of hospitalised P. falciparum (76.6%) and P. vivax (78.9%) cases presented with at least one severity indicator. Of these criteria, respiratory distress, jaundice and renal failure were the most common symptoms present among both P. falciparum (51, 40.7, 42%) and P. vivax (42.9, 32.1, 19.7%). However, a significantly greater percentage of P. falciparum inpatients presented with at least two (43.9%, p < 0.05) and at least three (29.9%, p < 0.01) severity features compared with P. vivax inpatients, indicating that multi-organ involvement was much more common in hospitalised P. falciparum cases. There were six deaths among the 182 enroled malaria positive inpatients at GMC (6/182, 3.3%), all of which were P. falciparum cases (6/107, 5.6%).
Table 3.
Univariate logistic regression of clinical features by species of malaria infection among hospitalised patients
| Feature | P. falciparum $ | P. vivax $ | OR (95% CI) | p value |
|---|---|---|---|---|
| Glasgow coma score, Mean ± SD | 13.8 ± 2.5 | 14.5 ± 1.5 | 0.8 (0.7–1.0) | 0.040* |
| Coma, n/N (%) | 10/107 (9.3) | 2/71 (2.8) | 3.7 (0.8– 17.9) | 0.099 |
| Blood urea nitrogen, mg %, Mean ± SD | 75.7 ± 82.0 | 38.3 ± 24.7 | 1.0 (1.0–1.0) | 0.003** |
| Serum creatinine, mg/dL, Mean ± SD | 2.2 ± 3.2 | 1.1 ± 0.5 | 1.4 (1.1–2.0) | 0.027* |
| Renal failure, n/N (%) | 42/100 (42.0) | 12/61 (19.7) | 3.0 (1.4–6.3) | 0.004** |
| Respiration rate (breaths/m), Mean ± SD | 23.2 ± 6.7 | 21.8 ± 8.7 | 1.0 (1.0–1.1) | 0.395 |
| Respiratory distress, n/N (%) | 50/98 (51.0) | 27/63 (42.9) | 1.5 (0.8–2.8) | 0.219 |
| Total bilirubin, mg/dL, Mean ± SD | 5.9 ± 8.6 | 3.5 ± 4.1 | 1.0 (1.0–1.0) | 0.812 |
| Jaundice, n/N (%) | 35/86 (40.7) | 18/56 (32.1) | 1.5 (0.8–3.1) | 0.225 |
| Severe jaundice, n/N (%) | 15/86 (17.4) | 5/56 (8.9) | 2.3 (0.8–6.7) | 0.136 |
| Systolic BP, mm Hg, Mean ± SD | 99.0 ± 13.0 | 95.8 ± 13.4 | 1.0 (1.0–1.0) | 0.107 |
| Shock, n/N (%) | 3/105 (2.9) | 5/70 (7.1) | 0.4 (0.9–1.8) | 0.217 |
| Pulmonary oedema, n/N (%) | 9/106 (8.5) | 5/72 (6.9) | 1.5 (0.8–3.1) | 0.225 |
| Abnormal bleeding, n/N (%) | 7/107 (6.5) | 1/71 (1.4) | 5.0 (0.6–43.4) | 0.136 |
| % Parasitaemia, Mean ± SD | 1.4 ± 3.3 | 0.4 ± 0.7 | 1.5 (1.0–2.1) | 0.024* |
| Temperature, C, Mean ± SD | 37.7 ± 1.5 | 37.5 ± 1.4 | 1.0 (0.9–1.2) | 0.481 |
| High fever (>38.1 °C), n/N (%) | 35/107 (32.7) | 22/71 (31.0) | 1.2 (0.6–2.3) | 0.599 |
| Severe fever (>38.9 °C), n/N (%) | 19/107 (17.8) | 13/71 (18.3) | 1.0 (0.5–2.3) | 0.918 |
| Haemoglobin, g/dL, Mean ± SD | 10.1 ± 2.7 | 10.6 ± 2.5 | 0.9 (0.8–1.0) | 0.159 |
| Anaemia, n/N (%) | 33/107 (30.8) | 16/71 (22.5) | 1.6 (0.8–3.3) | 0.157 |
| Severe anaemia, n/N (%) | 12/107 (11.2) | 2/71 (2.8) | 4.6 (1.0–21.9) | 0.049* |
| Days in hospital, Mean ± SD | 6.0 ± 5.5 | 4.6 ± 2.0 | 1.1 (1.0–1.2) | 0.044* |
| Long hospital stay (>8 days), n/n (%) | 18/107 (16.8) | 3/71 (4.2) | 4.7 (1.3–17.2) | 0.016* |
| Severity, Mean ± SD | 2.0 ± 2.02 | 1.2 ± 1.14 | 1.3 (1.1–1.67) | 0.005** |
| Severe malaria (SS > 0), n/N (%) | 82/107 (76.6) | 56/71 (78.9) | 1.1 (0.5–2.2) | 0.818 |
| Severe malaria (SS > 1), n/N (%) | 47/107 (43.9) | 19/71 (26.8) | 2.2 (1.2–4.3) | 0.014* |
| Severe malaria (SS > 2), n/N (%) | 32/107 (29.9) | 9/71 (12.7) | 3.0 (1.3–7.0) | 0.007** |
* p < 0.05; ** p < 0.01, $ excludes mixed-infection cases, SS # of WHO severe malaria criteria met at admission and during hospitalisation
In addition to increasing clinical severity (p < 0.01), lower Glasgow coma score, higher blood urea nitrogen, higher serum creatinine, renal failure, per cent parasitaemia, severe anaemia, more days in the hospital and hospitalisation for more than eight days were all significantly correlated (p < 0.05) with P. falciparum infection. Shock or circulatory collapse was the only feature that showed a greater frequency among hospitalised P. vivax cases as compared to P. falciparum, albeit numbers were low in both species of infections. Though hypoglyemia and acidosis were not routinely measured, one case of hypoglycaemia was observed in a P. falciparum patient (1/45, 2.2%), while it was not seen among any of the tested P. vivax patients (0/13, 0%). Metabolic acidosis, when tested for, was observed more frequently in P. falciparum cases (12/33, 36.4%) as compared to P. vivax (2/19, 10.5%).
Discussion
The present four year study was undertaken to better understand malaria disease burden in a peri-urban setting in India. The study points to a complex malaria situation in Goa with both P. vivax and P. falciparum cases year-round, a recent, substantial increase in the number of malaria-positive patients annually, a greater burden of disease from P. falciparum, and a highly diverse population of Indian patients from 26 of 29 states.
Between 2012 and 2015, GMC saw 6277 malaria-positive cases, with the number of P. falciparum and P. vivax patients increasing more than two-fold, from 889 in 2012 to 2261 in 2015. In contrast, between 2012 and 2015, the Indian National Vector Disease Control Programme (NVBDCP) reported 4854 cases of malaria in the entire state of Goa, 443 of which were caused by P. falciparum, and showed a more than two-fold decline in cases between 2012 (1714 cases) and 2015 (786 cases) [44]. Whether this discrepancy in malaria cases is due to an increase in the number of patients from other states, higher local transmission or some other cause remains to be determined. At the same time, these figures suggest that a large proportion of the total malaria cases in the state of Goa may be presenting to GMC and, thus, GMC may provide an approximation of malaria transmission and disease burden in the local community.
Despite the expected increase in number of malaria cases during and after the monsoon season, both P. vivax and P. falciparum patients were seen year-round at the GMC. While the year-round presence of P. vivax cases may be explained by the ability of P. vivax parasites to lie dormant as hypnozoites in liver stages with small numbers re-emerging during the dry season, this would not be the case for P. falciparum [8, 45]. Although it remains to be established, a more perennial transmission cycle of both P. vivax and P. falciparum may be aided by the recent discovery of a previously unsuspected vector in this region, Anopheles subpictus [46]. This vector has been shown to peak in numbers and transmission capacity both after the monsoons when the traditional urban malaria vector Anopheles stephensi peaks, but also in the dry season when An. stephensi numbers are low.
Although P. vivax infections were much more common at GMC, P. falciparum infections were associated with greater severity. Although approximately equal proportions of hospitalised patients with P. falciparum and P. vivax had at least one WHO severe malaria feature, patients with two or more severity criteria were more common to P. falciparum cases. The six deaths that occurred among study participants were all P. falciparum patients. In recent years, there have been a number of studies pointing to the importance of severe P. vivax in Asia, with some suggesting that P. vivax may be as or nearly as virulent as P. falciparum [47–52]. In the present study, though P. vivax caused some severe illness, multi-organ involvement was not typical. P. falciparum infections showed greater severity with substantial multi-organ involvement.
There was a strikingly greater proportion of adult males (86.7%) presenting to GMC with P. falciparum and P. vivax malaria than adult females. The same gender bias was smaller for children enroled in the study, with 64.7% male children and 35.3% female children. Such findings are consistent with previous reports of age-dependent sex-bias in clinical malarial disease in hypo-endemic regions [53] and with the gender distribution of malaria-positive patients at other MESA-ICEMR government tertiary hospital sites in India (unpublished data). It is also important to consider the profile of individuals who commonly seek care at government hospitals, which may be a contributing factor.
Both P. falciparum and P. vivax patients over 40 years of age were significantly more likely to be hospitalised by GMC than those between the ages of one and 20 years and 21 and 40 years, which points to a possible increase in the susceptibility of older populations to severe malaria in lower transmission settings. Future analysis will explore age-related symptomology and outcomes as well as parasite and gametocyte carriage to better understand if malaria virulence is increased in older populations in Goa. Other studies have shown that presenting syndromes in severe malaria depend on age and that age is an independent risk factor for a fatal outcome of malaria [16, 54, 55].
Construction sites in India are commonly suggested as potential transmission hot-spots, especially of severe P. falciparum, in low prevalence, relatively prosperous areas such as Goa [6, 56]. Though construction workers, who live and work at these sites and traditionally hail from the east and northeast states of India, accounted for roughly half of the malaria-positive study participants at GMC, they were nearly equally likely to be infected with P. vivax as P. falciparum. These findings may have implications for the conventional understanding of risk factors as well as the basis of targeted control measures at and around construction sites in the low transmission state of Goa. In the future, investigation to determine the possible effect of pre-existing P. falciparum and P. vivax immunity, compared across age, gender, origin and occupation, on transmission in Goa will be carried out.
There are a number of limitations to the present study. Due to logistical limitations and the high number of cases, only 18% of the hospital-identified malaria positive cases that presented to GMC over the study period were enroled. To date, there is not a complete, widely agreed-upon definition of what clinical features constitute severe P. vivax disease, as compared to severe P. falciparum disease, globally or on the Indian subcontinent [52, 57]. As such, the WHO definition of severe malaria for P. falciparum infection was used for both species, as is generally the case, which may bias or weaken associations seen among P. vivax cases. The present study does not include data for splenomegaly, spleen rupture or thrombocytopaenia, which have been reported to be common among severe P. vivax cases and are not reflected in the WHO criteria [22, 24, 52]. Lactate, hypoglycaemia, and metabolic acidosis, all of which are important indicators of severe malaria, were not routinely measured due to existing hospital practices. Malaria patients admitted to the GMC who required use of a ventilator, presumably due to respiratory distress [29, 47], which also has been commonly reported in severe P. vivax, were not included in the present study. This may impact the disease burden being reported among P. vivax and/or P. falciparum cases. It was also not always possible to accurately determine whether a patient had co-infections and/or had been treated with anti-malarials prior to presentation at GMC, a tertiary care centre. Co-infections and/or prior treatment may have impacted the measurement of clinical severity and/or course of malaria infection [58]. Future studies will seek to present the dynamic course of infection among severe malaria patients at GMC.
Conclusion
The present study highlights the demographic, diagnostic and clinical profiles of P. falciparum and P. vivax patients at the only government tertiary care facility in a peri-urban setting in southwestern India. The number of malaria cases presenting to GMC increased more than two-fold from 2012 to 2015, which may be of interest in the context of elimination efforts. The data from GMC show a greater burden of disease contributed by P. falciparum than P. vivax and suggest a potential age-dependent increase in susceptibility that will be further investigated.
Authors’ contributions
LC, AM, ND, EG, and PR designed and administered the study. AM, JTW, PG, AA, MF, MV, RR, DS, LP, AK, AK, MS, and EG collected the data. MB, SKM, RC, PB, JWIII, DGM, SK, KMS, UK, CL, KSS, AK, NV, VNJ, AK, PN, SA, ST, MD, JS, ND, and RGWP trained study staff, processed the samples and facilitated the research. LC and JNM designed the data management systems. LC, JNM and WZ analysed the data. LC wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank all the study participants at the Goa Medical College and Hospital who assisted with this work and Dr Sachin Shinde, Special Secretary (Health) and Administrator (GMC), for his support. The authors are most grateful for the administrative and scientific guidance provided by the MESA-ICEMR Scientific Advisory Group, including the Government of India representatives Dr Rashmi Arora, Dr Shiv Lal and Dr P. Joshi and US NIH Programme Officer Dr Malla Rao. This manuscript was approved by the publication committee of NIMR and bears approval No. 038/2016.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available in order to protect the confidentiality and privacy of study participants, but are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The human subjects protocol and consent forms for enrolment of Plasmodium positive individuals presenting to Goa Medical College and Hospital were approved by the institutional review boards of the Division of Microbiology and Infectious Diseases at the US National Institute of Allergy and Infectious Diseases (DMID 11-0074), GMC (no number assigned), and the University of Washington (42271).
Funding
This work was supported by the US NIAID MESA-ICEMR Program Project U19 AI089688 to PKR of the University of Washington, Seattle, WA, USA, and by the Government of India (Indian Council of Medical Research and the National Institute of Malaria Research). The REDCap data management resources were developed through support from a University of Washington Institute of Translational Health Science (ITHS) US NIH grant (UL1 RR02514).
Abbreviations
- ACD
acid citrate dextrose
- BP
blood pressure
- BUN
blood urea nitrogen
- DNA
deoxyribonucleic acid
- DMID
Division of Microbiology and Infectious Diseases
- GMC
Goa Medical College and Hospital
- GOI
Government of India
- Hb
haemoglobin
- HCT
haematocrit
- HMSC
Health Ministry Screening Committee
- ICEMR
International Center of Excellence for Malaria Research
- IMD
India Meteorological Department
- IRB
Institutional Review Board
- IQR
interquartile range
- MESA
Malaria Evolution in South Asia
- NIAID
National Institute of Allergy and Infectious Diseases
- NIH
US National Institutes of Health
- NVBDCP
National Vector Borne Diseases Control Programme
- OR
odds ratio
- RDT
rapid diagnostic test
- SS
severity score
- UW
University of Washington
- WHO
World Health Organization
Contributor Information
Laura Chery, Email: chery@chem.washington.edu.
Jennifer N. Maki, Email: maki@chem.washington.edu
Anjali Mascarenhas, Email: anjali.m@mesa-icemr.org.
Jayashri T. Walke, Email: jayashri.w@mesa-icemr.org
Pooja Gawas, Email: pooja.g@mesa-icemr.org.
Anvily Almeida, Email: anvily.a@mesa-icemr.org.
Mezia Fernandes, Email: mezia.f@mesa-icemr.org.
Marina Vaz, Email: marinavaz18@yahoo.com.
Rakesh Ramanan, Email: rakush_star@yahoo.com.
Diksha Shirodkar, Email: diksha_shirodkar@yahoo.co.in.
Maria Bernabeu, Email: maria.bernabeu@cidresearch.org.
Suresh Kumar Manoharan, Email: suresh.k@mesa-icemr.org.
Ligia Pereira, Email: ligia.p@mesa-icemr.org.
Rashmi Dash, Email: rashmi.d@mesa-icemr.org.
Ambika Sharma, Email: ambika.s@mesa-icemr.org.
Riaz Basha Shaik, Email: riaz.b@mesa-icemr.org.
Rimi Chakrabarti, Email: rimi.c@mesa-icemr.org.
Prasad Babar, Email: prasad.b@mesa-icemr.org.
John White, III, Email: jwhite3@uw.edu.
Devaraja G. Mudeppa, Email: devmg@uw.edu
Shiva Kumar, Email: shiva121@uw.edu.
Wenyun Zuo, Email: margaretzxy@gmail.com.
Kristen M. Skillman, Email: kmskillm@hsph.harvard.edu
Usheer Kanjee, Email: ukanjee@hsph.harvard.edu.
Caeul Lim, Email: clim0205@gmail.com.
Kathryn Shaw-Saliba, Email: kshawsal@hsph.harvard.edu.
Ashwani Kumar, Email: ashwani07@gmail.com.
Neena Valecha, Email: neenavalecha@gmail.com.
V. N. Jindal, Email: jindalvn@gmail.com
Anar Khandeparkar, Email: anar1662@gmail.com.
Pradeep Naik, Email: dr.naik.p@gmail.com.
Sunanda Amonkar, Email: sunandaamonkar@yahoo.co.in.
Manoj T. Duraisingh, Email: mduraisi@hsph.harvard.edu
Shripad Tuljapurkar, Email: tulja@stanford.edu.
Joseph D. Smith, Email: joe.smith@cidresearch.org
Nagesh Dubhashi, Email: nageshdubhashi@yahoo.com.
Roque G. W. Pinto, Email: wisemanpinto@gmail.com
Maria Silveria, Email: mimisil5@hotmail.com.
Edwin Gomes, Email: e_jj_gomes@hotmail.com.
Pradipsinh K. Rathod, Email: rathod@chem.washington.edu
References
- 1.WHO. World Malaria Report 2015. Geneva, World Health Organization, 2015.
- 2.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 3.Valecha N, Staedke S, Filler S, Mpimbaza A, Greenwood B, Chandramohan D. Malaria-attributed death rates in India. Lancet. 2011;377:992–993; author reply 994–995. [DOI] [PubMed]
- 4.Kumar A, Dua VK, Rathod PK. Malaria-attributed death rates in India. Lancet. 2011, 377:991–992; author reply 994–995. [DOI] [PMC free article] [PubMed]
- 5.White NJ, Dondorp AM, Faiz A, Mishra S, Hien TT. New global estimates of malaria deaths. Lancet. 2012;380:559–560. doi: 10.1016/S0140-6736(12)61321-X. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2007;77:69–78. [PubMed] [Google Scholar]
- 7.Singh N, Mishra AK, Chand SK, Bharti PK, Singh MP, Nanda N, et al. Relative abundance and Plasmodium infection rates of malaria vectors in and around Jabalpur, a malaria endemic region in Madhya Pradesh State. Central India. PLoS One. 2015;10:e0126932. doi: 10.1371/journal.pone.0126932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 9.Bejon P, Berkley JA, Mwangi T, Ogada E, Mwangi I, Maitland K, et al. Defining childhood severe falciparum malaria for intervention studies. PLoS Med. 2007;4:e251. doi: 10.1371/journal.pmed.0040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh K, English M, Crawley J, Peshu N. The pathogenesis of severe malaria in African children. Ann Trop Med Parasitol. 1996;90:395–402. doi: 10.1080/00034983.1996.11813068. [DOI] [PubMed] [Google Scholar]
- 11.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 12.Mogeni P, Williams TN, Fegan G, Nyundo C, Bauni E, Mwai K, et al. Age, Spatial, and Temporal variations in hospital admissions with malaria in Kilifi County, Kenya: a 25-year longitudinal observational study. PLoS Med. 2016;13:e1002047. doi: 10.1371/journal.pmed.1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snow RW, Bastos de Azevedo I, Lowe BS, Kabiru EW, Nevill CG, Mwankusye S, et al. Severe childhood malaria in two areas of markedly different falciparum transmission in east Africa. Acta Trop. 1994;57:289–300. doi: 10.1016/0001-706X(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 14.von Seidlein L, Olaosebikan R, Hendriksen IC, Lee SJ, Adedoyin OT, Agbenyega T, et al. Predicting the clinical outcome of severe falciparum malaria in african children: findings from a large randomized trial. Clin Infect Dis. 2012;54:1080–1090. doi: 10.1093/cid/cis034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dondorp AM, Chau TT, Phu NH, Mai NT, Loc PP, Chuong LV, et al. Unidentified acids of strong prognostic significance in severe malaria. Crit Care Med. 2004;32:1683–1688. doi: 10.1097/01.CCM.0000132901.86681.CA. [DOI] [PubMed] [Google Scholar]
- 16.Dondorp AM, Lee SJ, Faiz MA, Mishra S, Price R, Tjitra E, et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis. 2008;47:151–157. doi: 10.1086/589287. [DOI] [PubMed] [Google Scholar]
- 17.Hanson J, Lee SJ, Mohanty S, Faiz MA, Anstey NM, Charunwatthana P, et al. A simple score to predict the outcome of severe malaria in adults. Clin Infect Dis. 2010;50:679–685. doi: 10.1086/649928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton PN, Stepniewska K, Dondorp A, Silamut K, Chierakul W, Krishna S, et al. Prognostic indicators in adults hospitalized with falciparum malaria in Western Thailand. Malar J. 2013;12:229. doi: 10.1186/1475-2875-12-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaung M, Kyi TT, Aung NM, Kyaw MP, Min M, Htet ZW, et al. The prognostic utility of bedside assessment of adults hospitalized with malaria in Myanmar: a retrospective analysis. Malar J. 2015;14:63. doi: 10.1186/s12936-015-0549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning L, Laman M, Law I, Bona C, Aipit S, Teine D, et al. Features and prognosis of severe malaria caused by Plasmodium falciparum, Plasmodium vivax and mixed Plasmodium species in Papua New Guinean children. PLoS ONE. 2011;6:e29203. doi: 10.1371/journal.pone.0029203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitra S, Abhilash K, Arora S, Miraclin A. A prospective study from south India to compare the severity of malaria caused by Plasmodium vivax, P. falciparum and dual infection. J Vector Borne Dis. 2015;52:281–286. [PubMed] [Google Scholar]
- 22.Kochar DK, Das A, Kochar A, Middha S, Acharya J, Tanwar GS, et al. Thrombocytopenia in Plasmodium falciparum, Plasmodium vivax and mixed infection malaria: a study from Bikaner (Northwestern India) Platelets. 2010;21:623–627. doi: 10.3109/09537104.2010.505308. [DOI] [PubMed] [Google Scholar]
- 23.Kochar DK, Das A, Kochar A, Middha S, Acharya J, Tanwar GS, et al. A prospective study on adult patients of severe malaria caused by Plasmodium falciparum, Plasmodium vivax and mixed infection from Bikaner, northwest India. J Vector Borne Dis. 2014;51:200–210. [PubMed] [Google Scholar]
- 24.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–198. [PubMed] [Google Scholar]
- 25.Kochar DK, Pakalapati D, Kochar SK, Sirohi P, Khatri MP, Kochar A, et al. An unexpected cause of fever and seizures. Lancet. 2007;370:908. doi: 10.1016/S0140-6736(07)61417-2. [DOI] [PubMed] [Google Scholar]
- 26.Kochar DK, Tanwar GS, Khatri PC, Kochar SK, Sengar GS, Gupta A, et al. Clinical features of children hospitalized with malaria–a study from Bikaner, northwest India. Am J Trop Med Hyg. 2010;83:981–989. doi: 10.4269/ajtmh.2010.09-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohanty S, Mishra SK, Pati SS, Pattnaik J, Das BS. Complications and mortality patterns due to Plasmodium falciparum malaria in hospitalized adults and children, Rourkela, Orissa, India. Trans R Soc Trop Med Hyg. 2003;97:69–70. doi: 10.1016/S0035-9203(03)90027-7. [DOI] [PubMed] [Google Scholar]
- 28.Mohanty S, Mishra SK, Patnaik R, Dutt AK, Pradhan S, Das B, et al. Brain swelling and mannitol therapy in adult cerebral malaria: a randomized trial. Clin Infect Dis. 2011;53:349–355. doi: 10.1093/cid/cir405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain V, Agrawal A, Singh N. Malaria in a tertiary health care facility of Central India with special reference to severe vivax: implications for malaria control. Pathog Glob Health. 2013;107:299–304. doi: 10.1179/204777213X13777615588180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Integrated Disease Surveillance Project (IDSP) [http://www.idsp.nic.in].
- 31.Dehal N, Krishan K, Kanchan T, Unnikrishnan B, Singh J. Integrated disease surveillance in India - progress and pitfalls. Perspect Public Health. 2015;135:290. doi: 10.1177/1757913915606657. [DOI] [PubMed] [Google Scholar]
- 32.Census Organization of India. Indian Population Census 2011. 2011. [http://www.census2011.co.in. Accessed 10 Nov 2015.
- 33.Singh N. Decentralization and public delivery of health care services in India. Health Aff (Millwood). 2008;27:991–1001. doi: 10.1377/hlthaff.27.4.991. [DOI] [PubMed] [Google Scholar]
- 34.van Eijk AM, Ramanathapuram L, Sutton PL, Kanagaraj D, Sri Lakshmi Priya G, Ravishankaran S, et al. What is the value of reactive case detection in malaria control? A case-study in India and a systematic review. Malar J. 2016;15:67. doi: 10.1186/s12936-016-1120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NVBDCP. National Framework for Malaria Elimination in India 2016–2030. Delhi: National Vector Borne Disease Control Program. 2016.
- 36.NIMR. Guidelines for Diagnosis and Treatment of Malaria in India 2011. Delhi: National Institute of Malaria Research. 2011.
- 37.Kumar A, Chery L, Biswas C, Dubhashi N, Dutta P, Dua VK, et al. Malaria in South Asia: prevalence and control. Acta Trop. 2012;121:246–255. doi: 10.1016/j.actatropica.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narayanasamy K, Chery L, Basu A, Duraisingh MT, Escalante A, Fowble J, et al. Malaria evolution in South Asia: knowledge for control and elimination. Acta Trop. 2012;121:256–266. doi: 10.1016/j.actatropica.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson ML, Krogstad DJ, Arinaitwe E, Arevalo-Herrera M, Chery L, Ferreira MU, et al. Urban malaria: understanding its epidemiology, ecology, and transmission across seven diverse ICEMR network sites. Am J Trop Med Hyg. 2015;93:110–123. doi: 10.4269/ajtmh.14-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao MR. Foreword: International Centers of Excellence for Malaria Research. Am J Trop Med Hyg. 2015;93:1–4. doi: 10.4269/ajtmh.15-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foreword Rao M. The international centers of excellence for malaria research. Acta Trop. 2012;121:157. doi: 10.1016/j.actatropica.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Severe malaria. Trop Med Int Health 2014;19 Suppl 1:7-131. [DOI] [PubMed]
- 43.Severe falciparum malaria World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–S90. [PubMed] [Google Scholar]
- 44.NVBDCP. Malaria Situation in India.
- 45.Howes RE, Battle KE, Mendis KN, Smith DL, Cibulskis RE, Baird JK, et al. Global epidemiology of Plasmodium vivax. Am J Trop Med Hyg. 2016; pii: 16-0141. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 46.Kumar A, Hosmani R, Jadhav S, de Sousa T, Mohanty A, Naik M, et al. Anopheles subpictus carry human malaria parasites in an urban area of Western India and may facilitate perennial malaria transmission. Malar J. 2016;15:124. doi: 10.1186/s12936-016-1177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baird JK. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev. 2013;26:36–57. doi: 10.1128/CMR.00074-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaikh S, Memon H, Iohano B, Shaikh A, Ahmed I, Baird JK. Severe disease in children hospitalized with a diagnosis of Plasmodium vivax in south-eastern Pakistan. Malar J. 2012;11:144. doi: 10.1186/1475-2875-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valecha N, Pinto RG, Turner GD, Kumar A, Rodrigues S, Dubhashi NG, et al. Histopathology of fatal respiratory distress caused by Plasmodium vivax malaria. Am J Trop Med Hyg. 2009;81:758–762. doi: 10.4269/ajtmh.2009.09-0348. [DOI] [PubMed] [Google Scholar]
- 50.Beg MA, Khan R, Baig SM, Gulzar Z, Hussain R, Smego RA., Jr Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg. 2002;67:230–232. doi: 10.4269/ajtmh.2002.67.230. [DOI] [PubMed] [Google Scholar]
- 51.Lacerda MV, Fragoso SC, Alecrim MG, Alexandre MA, Magalhaes BM, Siqueira AM, et al. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis. 2012;55:e67–e74. doi: 10.1093/cid/cis615. [DOI] [PubMed] [Google Scholar]
- 52.Rahimi BA, Thakkinstian A, White NJ, Sirivichayakul C, Dondorp AM, Chokejindachai W. Severe vivax malaria: a systematic review and meta-analysis of clinical studies since 1900. Malar J. 2014;13:481. doi: 10.1186/1475-2875-13-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pathak S, Rege M, Gogtay NJ, Aigal U, Sharma SK, Valecha N, et al. Age-dependent sex bias in clinical malarial disease in hypoendemic regions. PLoS ONE. 2012;7:e35592. doi: 10.1371/journal.pone.0035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baird JK. Age-dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol. 1998;92:367–390. doi: 10.1080/00034989859366. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz E, Sadetzki S, Murad H, Raveh D. Age as a risk factor for severe Plasmodium falciparum malaria in nonimmune patients. Clin Infect Dis. 2001;33:1774–1777. doi: 10.1086/322522. [DOI] [PubMed] [Google Scholar]
- 56.Srivastava HC, Chandrashekar P, Kurien G, Sreehari U, Yadav RS. Malaria in seasonal migrant population in Southern Gujarat. India. Trop Biomed. 2011;28:638–645. [PubMed] [Google Scholar]
- 57.Anstey NM, Price RN. Improving case definitions for severe malaria. PLoS Med. 2007;4:e267. doi: 10.1371/journal.pmed.0040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lampah DA, Yeo TW, Hardianto SO, Tjitra E, Kenangalem E, Sugiarto P, et al. Coma associated with microscopy-diagnosed Plasmodium vivax: a prospective study in Papua, Indonesia. PLoS Negl Trop Dis. 2011;5:e1032. doi: 10.1371/journal.pntd.0001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available in order to protect the confidentiality and privacy of study participants, but are available from the corresponding author on reasonable request.


