Abstract
Adolescents are sensitive to the anxiolytic effect of ethanol, and evidence suggests that they may be more sensitive to stress than adults. Relatively little is known, however, about age-related differences in stress modulation of ethanol drinking or stress modulation of ethanol-induced sedation and hypnosis. We observed that chronic restraint stress transiently exacerbated free-choice ethanol drinking in adolescent, but not in adult, rats. Restraint stress altered exploration patterns of a light-dark box apparatus in adolescents and adults. Stressed animals spent significantly more time in the white area of the maze and made significantly more transfers between compartments than their non-stressed peers. Behavioral response to acute stress, on the other hand, was modulated by prior restraint stress only in adults. Adolescents, unlike adults, exhibited ethanol-induced motor stimulation in an open field. Stress increased the duration of loss of the righting reflex after a high ethanol dose, yet this effect was similar at both ages. Ethanol-induced sleep time was much higher in adult than in adolescent rats, yet stress diminished ethanol-induced sleep time only in adults. The study indicates age-related differences that may increase the risk for initiation and escalation in alcohol drinking.
Keywords: restraint stress, rat, ethanol, adolescent, adult
Preclinical studies suggest that adolescents are uniquely sensitive to ethanol's pharmacological effects and that this pattern of response may put them at risk for alcohol initiation and escalation (Spear & Swartzwelder, 2014). Adolescent rats exhibit, when compared to adults, greater sensitivity to the appetitive (Pautassi, Myers, Spear, Molina, & Spear, 2008) and social facilitating effects of ethanol (Varlinskaya & Spear, 2002). This pattern, however, is somehow different in adolescent mice, which require lengthier training or higher ethanol doses to exhibit the same magnitude of conditioned place preference (CPP) by ethanol found in their adult counterparts (Dickinson, Kashawny, Thiebes, & Charles, 2009). The adolescents from both species, however, are less sensitive than adults to the aversive motivational effects of the drug (Holstein, Spanos, & Hodge, 2011; Vetter-O'Hagen, Varlinskaya, & Spear, 2009). Moreover, compared to adults, adolescents usually consume more ethanol on a gram by kilogram (g/kg) basis (Doremus, Brunell, Rajendran, & Spear, 2005) and are much less sensitive to the hypnotic effect induced by relatively high (≥ 3.5 g/kg) doses of ethanol (Silveri & Spear, 1998).
Evidence suggests that adolescent rats may be more sensitive to stress than adults (Stone & Quartermain, 1997). An intriguing study (Song et al., 2007) observed CPP by ethanol in adult, but not in adolescent, mice. Stress exposure facilitated the expression of ethanol-induced CPP in the adolescents but did not modify its expression in the adults. Relatively little is known, however, about age-related differences in stress modulation of ethanol drinking or stress modulation of ethanol-induced sedation and hypnosis. Exposure to social isolation enhances ethanol intake in adolescent, but not in adult, mice (Lopez, Doremus-Fitzwater, & Becker, 2011; Schenk, Gorman, & Amit, 1990). More in detail, the study by Lopez et al. (2011) revealed that social isolation between weaning and adulthood, but not during adulthood, increased subsequent ethanol intake, when compared to control, group-housed, C57BL/6J mice. The stress induced by social isolation can also attenuate the duration of loss of the righting reflex, as shown in mice selectively bred for exhibiting differential sensitivity to the sedative and hypnotic effects of ethanol (i.e., long sleep [LS] and short-sleep [SS] mice) (Parker, Ponicsan, Spencer, Holmes, & Johnson, 2008). Siegmund, Vengeliene, Singer, and Spanagel (2005) reported greater alcohol intake after footshock in adult rats that had begun drinking during adolescence, but not in counterparts who experienced alcohol initiation as adults. Brunell and Spear (2005) examined modulation of ethanol intake by chronic footshock in adult and adolescent rats housed in isolation, and found a lack of stress-induced differences at any age.
The present study analyzed whether the effects of restraint stress (RS) upon ethanol intake and upon sensitivity to ethanol-induced sedation and hypnosis are similar in adolescent and adult rats. Increased ethanol intake has been found after protracted RS in Wistar rats (Lynch, Kushner, Rawleigh, Fiszdon, & Carroll, 1999; Ploj, Roman, & Nylander, 2003; Roman, Ploj, & Nylander, 2004) and sometimes in mice, although predominantly in males exposed to repeated cycles of RS and ethanol access (Chester, de Paula Barrenha, DeMaria, & Finegan, 2006). Yet rat and mice studies in which RS significantly decreased (rat: Chester, Blose, Zweifel, & Froehlich, 2004; Ng Cheong Ton, Brown, Michalakeas, & Amit, 1983) or did not affect ethanol intake (rat: Bertholomey, Henderson, Badia-Elder, & Stewart, 2011; Rockman, Hall, Hong, & Glavin, 1987; mice: Tambour, Brown, & Crabbe, 2008) have also been reported. Acute RS increased anxiety in adolescent and adult rats, and this anxiogenic effect was reversed by ethanol only in adolescents (Varlinskaya & Spear, 2012). Daily restraint sessions, for 5 days, exacerbated the social facilitating effects of ethanol in adolescents and reversed the inhibitory effects of ethanol commonly observed in non-stressed adults (Varlinskaya, Doremus-Fitzwater, & Spear, 2010). Findings concerning the effects of chronic RS upon ethanol-induced sedation revealed that it decreased and increased the duration of loss of the righting reflex, in LS and SS mice, respectively (dose: 4.1 g/kg; Jones, Connell, & Erwin, 1990).
It is still unknown if the effects of RS upon ethanol intake or upon ethanol-induced sedation and hypnosis are similar in adolescent and adult rats. Yet, the age-dependent interactions between ethanol and acute and chronic RS suggest that chronic RS may enhance ethanol drinking in adolescent, but not in adult, rats. This hypothesis was analyzed in Experiment 1. Adolescent and adult rats were given RS (five daily sessions, duration: 120 min) and then assessed for ethanol-induced behavioral stimulation and ethanol drinking in two-bottle choice tests. The studies conducted in LS and SS mice, in turn, suggest that the consequences of chronic RS on ethanol-induced sedation and hypnosis may be different in adolescents and adults (Jones et al., 1990). As indicated, adolescents are much less sensitive than adults to the hypnosis induced by relatively high (≥3.5 g/kg) doses of ethanol (Silveri & Spear, 1998). Experiment 3 assessed stress modulation of this difference, which has important implications. The sedative and narcotic effects of ethanol serve as natural deterrents to alcohol drinking, and lower basal or stress-related sensitivity to these consequences constitutes a vulnerability factor for problematic alcohol use (Spear & Varlinskaya, 2010). Additionally, Experiment 2 explored the effects of chronic RS on basal and stress-induced corticosterone levels and anxiety response.
Materials and methods
Experimental designs and overview of procedures and aims
This section provides a description of the experimental designs and a brief overview of each experiment's aims and procedures, which are then described at length in the next section.
Experiments 1a and 1b
Experiment 1a analyzed acute motor-stimulating effects of ethanol and ethanol intake and employed a 2 (age: adolescent or adult) × 2 (stress condition: 120 min of restraint a day, for 5 days; or non-stressed) × 2 (ethanol dose before open-field measurement: 0.0 or 2.5 g/kg) factorial design, with 11–13 animals per group. Animals were exposed to RS on postnatal days (PD) 30-34 (adolescents) or 70-74 (adults) and were given ethanol (0.0 or 2.5 g/kg, intragastrically [i.g.]) 2 h after termination of the last stress exposure on PD34 or PD74. Five minutes after this intubation, animals were placed in the central area of an open field and tested for 10 min. Ethanol intake assessments were conducted on PD37 to PD40 (adolescent group) or PD77 to PD80 (adult group). Experiment 1b assessed metabolic processing of alcohol in naïve adolescent and adult animals (n = 6 per group).
Experiment 2
A 2 (age: adolescent or adult) × 2 (stress condition: 120 min of restraint a day, for 5 days; or non-stressed) factorial design was employed. Adolescents and adults were exposed to RS and then, on PD38 (adolescent group) or PD78 (adult group), assessed for anxiety response in a light-dark box (LDB) test (5 min), and then exposed for 5 min to inescapable stress (confinement in the white section of the light-dark box with illumination of 1200 lux). Blood samples, collected 90 min before the LDB test and immediately after termination of the inescapable stress, were used to measure corticosterone levels. Each group had 12 animals.
Experiment 3
A 2 (age: adolescent or adult) × 2 (stress condition: 120 min of restraint a day, for 5 days; or non-stressed) × 2 (ethanol dose: 4.0 or 4.5 g/kg) factorial design was employed. Each of the 8 groups included 10–12 animals. Adolescent and adult rats were either exposed or not to RS, and on PD35 or PD75 (adolescent and adult group, respectively), challenged with ethanol (4.0 or 4.5 g/kg, intraperitoneally [i.p.]) and assessed for loss of righting reflex and sleep time. Trunk blood samples were taken after recuperation from ethanol-induced sleep and processed for blood ethanol concentration (BEC) and CORT levels.
Subjects
Two-hundred forty-six male rats were used. The animals in Experiments 1a, 1b, and 2 (44, 6, and 29 adolescents; and 47, 6, and 30 adults, respectively) were Wistar rats born and reared in the animal facility of the Instituto Ferreyra (INIMEC-CONICET-UNC, Córdoba, Argentina). Rats in Experiment 3 (39 adolescents and 45 adults) were Sprague-Dawley rats (SD) born and reared in an animal facility at the Psychology Department of Binghamton University (Binghamton, NY), within an AAALAC-accredited facility. Dams were checked for births every day, and the day of delivery was considered PD0. Weaning was conducted on PD21, and unless specified, animals were housed in groups of four and given continuous ad libitum access to water and food. Both colonies were kept at an ambient temperature of 22 ± 1 °C with lights turned on and off at 8:00 AM and 8:00 PM, respectively. The rationale for using Wistar rats in Experiments 1 and 2 but SD rats in Experiment 3 was to strengthen the generalizability of the findings by assessing age-related differences in sensitivity to restraint stress in an alternative line of rats. The procedures followed the Guide for the Care and Use of Laboratory Animals of NIH (National Research Council, 2011) and were approved by the Ministry of Animal Care of INIMEC and by the Institutional Animal Care and Use Committee of Binghamton University.
Apparatus and detailed description of procedures
Restraint stress procedures
RS was similar to that described elsewhere (Varlinskaya & Spear, 2012). Animals were given daily RS sessions (120 min each, from 9:00 to 11:00 AM) on PD30 to PD34, or PD70 to PD74 (adolescent and adult groups, respectively) or remained undisturbed in their home cage (non-stressed control group). Animals from the stress group were withdrawn from their home cage, weighed (portable Ohaus L2000; Ohaus, Pine Brook, NJ), transferred to a separate room, and confined in restraint tubes. Across experiments, four tube sizes were used to accommodate differences in the size of animals: 15 × 4.2 cm, 15 × 3.3 cm, 20 × 5.8 cm, and 26 × 5.8 cm, length and maximal internal diameter, respectively. Immediately after termination of stress exposure, animals were returned to their home cages.
Ethanol administration procedure
The 2.5 g/kg ethanol dose (Experiment 1 and 1b) was given i.g. in a volume equivalent to 0.015 mL per gram of body weight of a solution containing 21% ethanol. An equivalent volume of tap water was administered as vehicle (0.0 g/kg). For the i.g. administration, a 12-cm length of polyethylene-50 tubing (PE-50, Clay Adams, Parsippany, NJ) was attached to a 3-mL syringe (Becton Dickinson, Rutherford, NJ) with a 23-gauge needle. In Experiment 3, animals were given i.p. injections of ethanol (4.0 or 4.5 g/kg), at the same volume as used for the i.g. administrations. The use of different doses and routes of ethanol administration were selected based on previous studies (Acevedo, Molina, Nizhnikov, Spear, & Pautassi, 2010; Acevedo, Nizhnikov, Spear, Molina, & Pautassi, 2013; Balaszczuk, Bender, Pereno, & Beltramino, 2011; Silveri & Spear, 1998), in accord with the specific aims of each experiment. A 2.5-g/kg ethanol dose has been regularly used in our lab to assess motor effects of ethanol in adolescents. A higher dose of ethanol was used in Experiment 3, relative to Experiment 1, because ethanol-induced sleep is only found at ethanol doses ≥3.5 g/kg (Silveri & Spear, 1998).
Open-field testing
In Experiment 1, 2 h after termination of the last stress exposure on PD34 or PD74, the animals were intubated with ethanol or vehicle. Following intubation, subjects were returned to a holding cage lined with pine shavings where they remained for 5 min until testing. Five minutes after the intubation, the animals were tested for motor activity under moderate to dim light, in square chambers (60 × 60 × 60 cm; ITCOMM, Córdoba, Argentina) that recorded distance traveled (cm) during a 10-min test (i.e., post-administration time 5–14 min). The aim was to assess stress-induced differences in ethanol-induced motor stimulation in the open field.
Ethanol intake test
In Experiment 1, adolescents and adults were given daily 2-h intake sessions on PD37, 38, 39, and 40, or PD77, 78, 79, and 80, respectively; with each intake session preceded by a 22-h water deprivation period. Rats were weighed before each session and then transferred to individual wire mesh cages equipped with two graded tubes. One of the tubes contained tap water and the other an ethanol solution (3% v/v on the first day and increasing 1% on subsequent days until reaching 6% v/v on the last testing day). Ingestion from each tube (mL) was measured at termination of each session and animals were subsequently returned to their home cages. To prevent place-preference learning, the position of the tubes was switched across sessions. Ethanol intake was expressed in grams per kilogram and percent selection of ethanol ([consumption of ethanol/overall liquid ingestion] × 100).
The rationale for using this protocol is that in the absence of liquid deprivation, Wistar rats drink very little ethanol and distribute their intake across the day. We also wanted to avoid the use of mixtures of alcohol and sweeteners (e.g., sucrose), to assess response to ethanol alone without adding the caloric or sensory properties of a sweetener. The deprivation schedule also facilitated fast absorption and distribution of ethanol (Pepino, Abate, Spear, & Molina, 2004; Ponce, Pautassi, Spear, & Molina, 2004). This intake test has been used to assess the facilitative effect of pre- and post-natal alcohol exposure (Fabio et al., 2013; Pepino et al., 2004), and passive intubations (Acevedo et al., 2010), on later alcohol acceptance.
Light-dark box test and exposure to inescapable stress
On PD38 (adolescent group) or PD78 (adult group) the animals, which had been exposed or not exposed to daily RS sessions, were removed from their home cage and a blood sample was taken from the tail. This sample provided a baseline level of CORT response. Ninety minutes later, animals were tested for anxiety-like behaviors in a 5-min light-dark box test. The apparatus (42 × 25 × 25 cm) featured two compartments made of high impact acrylic, one white (24.5 × 25 × 25 cm) and illuminated by a 60-watt white bulb lamp adjusted to generate an illumination level of 400 lux, and one black (17.5 × 25 × 25 cm) without illumination (i.e., 0 lux). A divider with an opening at floor level separated both compartments. The test began by gently placing the animal in the center of the white area, facing away from the black area. Immediately after termination of the 5-min test, the door separating the white and black section of the apparatus was closed and animals were exposed to an inescapable stress. Specifically, animals were transferred to the white section and illumination was increased to 1200 lux. The animals remained in this bright compartment for 5 min. The behavior was filmed and subsequently scored by an observer unaware of the experimental conditions. The following variables were measured: number of transfers between compartments, latency (sec) to enter the dark compartment, and time (sec) spent in the white compartment. The following variables were measured during the forced exposure to the bright compartment: locomotor activity (sec), wall climbing (sec), and number of fecal boli. Trunk blood samples were collected immediately after the 5-min stressor, and processed for CORT thereafter. Illumination of the apparatus was measured via a digital lux meter (LX1010B).
Corticosterone and blood ethanol measurements
The blood samples collected in Experiment 2 were kept at room temperature for 30 min and then centrifuged at 3000 rpm (10 min) to obtain serum aliquots. In Experiment 3, trunk blood samples were obtained when animals recovered from the sleep induced by 4.0 or 4.5 g/kg ethanol. Blood samples from both experiments were centrifuged at 4 °C for 20 min at 3000 rpm to take aliquots of the plasma and then kept at −80 °C until time of assay. Plasma or serum CORT levels were determined by radioimmunoassay using RIA kits (Experiment 2: MP Biomedicals, Solon, OH; Experiment 3: ICN Biomedicals; Orangeburg, NY). CORT and BEC values were expressed as ng/mL and mg%, respectively.
In Experiment 1b, trunk blood samples from adolescent and adult rats were collected 15 min after ethanol administration (2.5 g/kg, i.g.; Experiment 1b). Blood samples were frozen and stored at −80 °C until BELs were determined via headspace gas chromatography (HP 5890 series II Gas Chromatograph, Wilmington, DE), as previously described (Silveri & Spear, 2000). The same procedure and apparatus were used in Experiment 3 (in a separate aliquot) to determine BEC levels at time of recuperation from sleep.
Assessment of loss of righting reflex and sleep time
In Experiment 3, adolescent and adult Sprague-Dawley rats were exposed or not exposed to RS, as described earlier. One day after the last stressor they were challenged with ethanol (4.0 or 4.5 g/kg, i.p.) and returned to their home cage. Upon observation of sedation, the animal was positioned in a supine position; when the animal turned over the experimenter put him back again in a supine position. An animal was considered to have lost the righting reflex when it failed to regain a prone position three times in 30 sec. Sleep time was measured from time of loss to time of regaining the righting reflex, with recovery defined as regaining the prone position when placed supine three times within a 30-sec interval. Blood samples were obtained at recuperation.
Statistical analysis
Descriptive, in-text values indicate mean ± SEM. Across experiments, the locus of significant main effects and significant interactions yielded by ANOVAs was analyzed through post hoc or planned comparisons. Specifically, Tukey's post hoc tests were used for significant effects involving between-subject factors, whereas planned comparisons were used to analyze significant main effects or interactions involving between-by-within factors. There is no unambiguous choice of pertinent error terms for post hoc comparisons involving within-subject factors, and hence planned comparisons were chosen for use as they offer an adequate compromise between sensitivity and conservativeness (Winer, Brown, & Michels, 1991). An alpha of 0.05 was used.
Motor activity (distance traveled, cm) in Experiment 1 was analyzed using a 2 (age) × 2 (stress condition) × 2 (ethanol dose) ANOVA. Similar ANOVAs, that also included day of assessment (day 1, 2, 3, and 4) as a within-subject measure, were used to analyze intake data (g/kg ethanol consumed and % preference for ethanol, as well as overall liquid consumption per 100 g of body weight). Maximal ethanol consumption on any test day (g/kg) was analyzed through a factorial ANOVA (age × dose × stress). To obtain maximum ethanol intake values, each animal was given a single score reflecting the highest level of ethanol consumption (g/kg) achieved across days of assessment. For example, an animal that ingested 0.60, 0.20, 1.20, and 0.33 g/kg across testing days would be given a score of 1.20 in terms of maximum amount of absolute ethanol intake. Overall fluid intake and maximal ethanol consumption were independent of each other. Overall fluid intake was measured each day, whereas maximal ethanol intake on any given day is a single number, representing the highest ethanol intake score registered in each animal. This measure has already proven useful in the detection of differences in adolescent ethanol intake as a function of prior ethanol self-administration (Ponce, Pautassi, Spear, & Molina, 2008).
Body weight prior to each of the five stress sessions and prior to each of the four intake sessions was analyzed through separate ANOVAs (age × stress treatment × stress session and age × stress × ethanol dose × testing day, respectively).
In Experiment 2 and 3, body weight prior to each stress session (and prior to the loss of righting reflex test in Experiment 3) was analyzed through an age × stress × day RM ANOVA. Each of the variables recorded in the LDB test and during exposure to the inescapable stress (Experiment 2) were analyzed through an age × stress factorial ANOVA. CORT values at baseline and immediately after the acute stress exposure were analyzed through a two-way RM ANOVA. Time of measurement (baseline or after acute stress) was the repeated measure. Duration of loss of the righting reflex, ethanol-induced sleep time, and CORT and BEC values were analyzed in Experiment 3 via 2 (age) × 2 (stress condition) × 2 (ethanol dose: 4.0 or 4.5 g/kg) ANOVAs. Data from an adolescent, non-stressed animal were lost in Experiment 3 due to procedural errors.
Results
Experiment 1
Body weights
The ANOVAs for body weight scores during stress days indicated, besides the obvious age difference (F[1,85] = 1752.4, p < 0.001), a significant interaction between stress, age, and day of assessment (F[1,83] = 4.28, p < 0.001). The ANOVA for body weight at the beginning of each intake session revealed significant main effects of age (F[1,83] = 1293.10, p < 0.001), stress (F[1,83] = 5.15, p < 0.05), and a significant age × session interaction (F[3,249] = 74.09, p < 0.001). To understand these interactions, follow-up ANOVAs (stress × days) were conducted for each age. The analysis for adolescent's body weight over the stress exposure days indicated a significant increase across days (F[4,168] = 632.59, p < 0.05), whereas body weights on intake session days 1 and 4 were significantly greater than weights on session days 2 and 3, F[3,120] = 35.39, p < 0.05.
In adults, the analysis during stress exposure days indicated a significant interaction between stress treatment and days, F[4,172] = 18.22, p < 0.001. Planned comparisons indicated significantly lower body weights in stressed than in non-stressed adult animals on the last stress exposure day. During intake test days, the ANOVA indicated a significant effect of stress, with stressed animals weighing significantly less than their non-stressed counterparts, F[1,43] = 4.69, p < 0.05. These scores are depicted in Table 1 (upper section).
Table 1.
Body weights in Experiments 1, 2, and 3.
| Experiment 1 | Phase of Experiment | Stress Group | ||||
|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | ||
| Adolescents | Stress Sessions (1 to 5) | 115.82±2.91 | 124.77±2.72 | 132.09±2.64 | 139.09±2.76 | 146.23±2.74 |
| Intake test sessions (1 to 4) | 147.05±2.61 | 144.95±2.61 | 145.50±2.61 | 148.18±2.61 | ||
| Adults | Stress Sessions (1 to 5) | 417.39±8.29 | 415.62±8.15 | 413.17±8.34 | 413.96±8.39 | 415.26±8.36 |
| Intake test sessions (1 to 4) | 391.35±8.53 | 383.70±8.16 | 380.39±8.53 | 379.83±8.26 | ||
| Experiment 1 | Phase of Experiment | Non-Stressed Group | ||||
|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | ||
| Adolescents | Stress Sessions (1 to 5) | 115.86±3.68 | 124.27±3.60 | 132.27±3.55 | 141.22±3.57 | 151.68±3.61 |
| Intake test sessions (1 to 4) | 151.59±3.49 | 148.55±3.39 | 149.00±3.43 | 151.36±3.53 | ||
| Adults | Stress Sessions (1 to 5) | 426.42±9.52 | 432.86±10.39 | 436.81±10.36 | 438.18±10.61 | 445.09±10.81 |
| Intake test sessions (1 to 4) | 420.46±10.16 | 411.96±9.78 | 409.88±9.84 | 409.29±9.85 | ||
| Experiment 2 | Stress Group | |||||
|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | ||
| Adolescents | Stress Group | 92.85±3.21 | 96.89±3.10 | 102.86±3.26 | 110.52±3.44 | 117.00±3.68 |
| Non-stressed | 90.97±5.01 | 98.66±5.50 | 106.40±5.53 | 114.04±5.69 | 122.93±5.82 | |
| Adults | Stress Group | 424.58±14.63 | 423.83±14.63 | 422.33±14.78 | 422.83±14.67 | 421.92±14.41 |
| Non-stressed | 410.25±11.60 | 414.00±11.63 | 423.00±12.71 | 420.58±12.08 | 427.83±12.75 | |
| Experiment 3 | Stress Group | |||||
|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | ||
| Adolescents | Stress Group | 122.85±2.22 | 129.25±2.41 | 135.85±2.40 | 140.75±2.32 | 144.85±2.67 |
| Non-stressed | 126.11±3.44 | 134.05±3.46 | 141.26±3.63 | 149.11±3.60 | 157.68±3.89 | |
| Adults | Stress Group | 415.50±9.30 | 415.90±9.18 | 417.46±9.08 | 417.05±9.18 | 417.55±9.02 |
| Non-stressed | 424.30±8.18 | 430.78±8.05 | 430.30±8.07 | 436.96±8.26 | 437.83±8.27 | |
Body weights (g) during stress exposure (5 daily sessions of 2 h of restraint stress, or no stress exposure, during postnatal days (PD) 30–34 or 70–74, for adolescents and adults, respectively), in Experiments 1, 2, and 3, as a function of age and stress treatment. The section for Experiment 1 also depicts body weight prior to two-bottle intake test sessions (daily 2-h sessions on postnatal days 37–40 or 77–80, for adolescents and adults, respectively). In Experiment 1, data have been collapsed across ethanol treatment at PD34 or 74 (2 h after termination of the last stress, session animals were challenged with 0.0 or 2.0 g/kg ethanol). The latter factor did not significantly alter body weights. Values express mean ± SEM.
Ethanol-induced motor activity in the open field
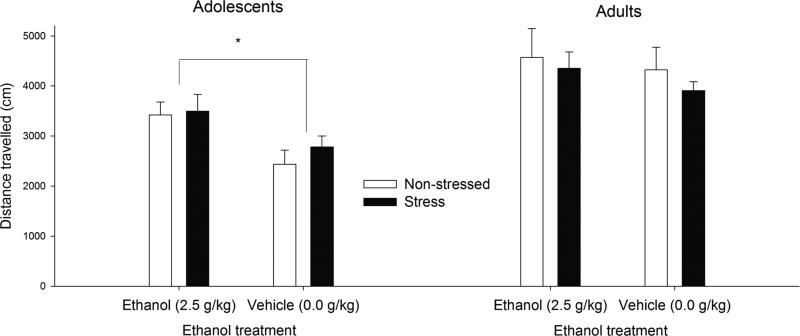
The ANOVA for ethanol-induced motor activity yielded significant main effects of age (F[1,83] = 24.77, p < 0.001) and dose (F[1,83] = 5.62, p < 0.001). Overall locomotion was significantly greater in adults than in adolescents (4290.31 ± 181.09 vs. 3035.32 ± 175.64) and significantly greater after ethanol treatment than after vehicle treatment. A planned comparison indicated significant age-related differences in motor activity in the basic control (i.e., vehicle-treated) condition (F[1,83] = 17.97, p < 0.001). Given this baseline difference, separate dose × stress ANOVAs were conducted at each age. The ANOVA for adolescents revealed a significant main effect of ethanol dose, F[1,40] = 4.38, p < 0.05. Motor activity was significantly greater in ethanol-treated than in vehicle-treated subjects. This stimulatory effect of ethanol was similar in adolescents exposed to stress or not. The ANOVA for adult subjects indicated a lack of significant main effects or significant interactions. These results are depicted in Fig. 1.
Fig. 1. Ethanol-induced behavioral stimulation activity in adolescent and adult rats.
Locomotor activity (distance traveled, cm) in adolescent and adult Wistar rats exposed to 5 days of restraint stress (120 min per day), or non-stressed during postnatal days 30–34 or 70–74, for each age, respectively. Two hours following termination of the last stress session on postnatal day 34 or 74, the rats were given ethanol (2.5 g/kg, i.g.) or its vehicle (tap water) before locomotor activity was measured during 5–9 min post-administration time in an open field. The asterisk sign indicates a significant difference between adolescents given ethanol vs. those given vehicle (p < 0.05). The vertical bars indicate SEM.
Ethanol-intake scores
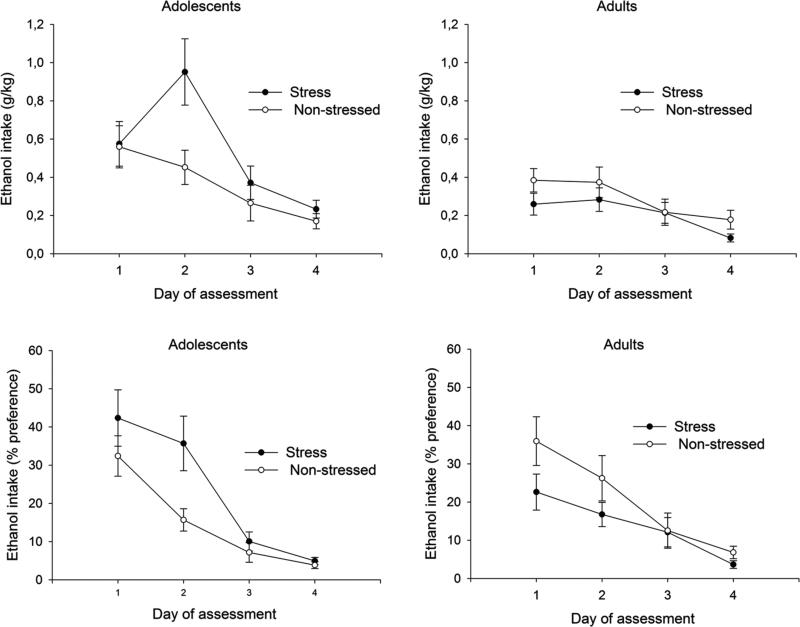
Fig. 2 depicts mean ethanol intake scores across days of assessment, and Fig. 3 presents ethanol mean intake scores across days. The ANOVA for g/kg of ethanol consumed revealed a significant main effect of age and day of assessment (F[1,83] = 13.77, p < 0.005; F[3,249] = 19.72, p < 0.001), and a significant age × stress interaction (F[1,83] = 5.42, p < 0.05). Post hoc analysis indicated that ethanol intake was greater in the first two days than in the last two days. Perhaps more important, the post hoc tests also indicated that stress exposure enhanced ethanol intake (g/kg) in adolescents but did not affect this behavior in adults. Planned comparisons conducted between stressed and non-stressed rats confirmed that the effect of stress on adolescent ethanol intake was significant only on the second day of assessment. The analysis for percent preference ethanol scores indicated significant main effects of day of assessment and a significant interaction comprising age and stress exposure (F[3,249] = 40.63, p < 0.001; F[1,83] = 8.09, p < 0.01). Post hoc analyses indicated that percent preference declined significantly across days. Even more important is that, according to the planned comparisons, stressed adolescents exhibited significantly greater ethanol percent preference than non-stressed adolescents during the second day of assessment. Ethanol percent preference in adult animals was similar in stressed and control subjects.
Fig. 2. Ethanol intake in adolescent and adult rats after restraint stress.
Absolute ethanol intake (g/kg) and percent ethanol preference scores (upper and lower panels, respectively) in adolescent and adult Wistar rats as a function of day of assessment (intake test sessions 1, 2, 3, and 4) and stress treatment experienced during postnatal days 30–34 or 70–74 (5 days of restraint stress [120 min per day] or non-stressed). In each daily intake test (duration: 120 min), animals had access to a bottle of water and a bottle of ethanol (ethanol concentration: 3, 4, 5, or 6% v/v, sessions 1 to 4; respectively). Please refer to the text for an account of significant differences across groups. Vertical lines indicate SEM.
Fig. 3.
Mean ethanol intake (g/kg ingested and percent preference, left and right panels, respectively), across the 4 intake sessions conducted in Experiment 1, in adolescent and adult Wistar rats as a function of stress treatment experienced during postnatal days 30–34 or 70–74 (5 days of restraint stress [120 min per day] or non-stressed). The asterisk indicates that stress-exposed adolescents drank significantly more ethanol (g/kg) than any of the other groups (p<0.05). The pound sign indicates that stress-exposed adolescents exhibit significantly more ethanol percent preference than either non-stressed adolescents or stressed adults (p<0.05). Vertical lines indicate SEM.
The ANOVA for maximal daily ethanol intake (g/kg) revealed a similar pattern relative to that provided by absolute or relative ethanol intake. The analysis revealed a significant main effect of age and a significant age × stress interaction (F[1,83] = 13.23, p < 0.001; F[1,83] = 13.23, p < 0.001). Stressed adolescents exhibit significantly greater maximal daily ethanol intake (1.16 ± 0.15 g/kg) when compared to non-stressed adolescents (0.72 ± 0.12 g/kg), or stressed (0.49 ± 0.07 g/kg) or non-stressed adults (0.60 ± 0.08 g/kg).
The ANOVA for overall liquid intake (mL/100 g) indicated significant main effects of session and age (F[3,249] = 20.38, p < 0.001; F[1,83] = 334.40, p < 0.001), and a significant age × session interaction, F[3,249] =10.69, p < 0.001. Fluid intake was lower in session 1 than in subsequent sessions and, particularly in sessions 2 to 4, significantly greater in adolescents than in adults (see Table 2). There were no significant effects of stress, nor significant stress × age or stress × dose interactions, on overall fluid intake on the day in which the maximal ethanol intake value for that animal was selected (all p > .10). This analysis is important to rule out the possibility that stress effects on ethanol intake may have been selective depending on whether overall fluid intakes were also altered on the particular day of maximal ethanol intake.
Table 2.
Overall fluid consumption
| Age | Treatment at PD33 or PD73 | Stress Group | Non-stressed Control Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Test day 1 | Test day 2 | Test day 3 | Test day 4 | Test day 1 | Test day 2 | Test day 3 | Test day 4 | ||
| Adolescent | 0.0 g/kg ethanol | 7.41±1.25 | 9.35±0.37 | 9.38±0.38 | 9.95±0.35 | 7.50±0.90 | 9.06±0.30 | 9.32±0.43 | 9.46±0.50 |
| 2.5 g/kg ethanol | 6.20±1.12 | 9.47±0.80 | 9.83±0.51 | 9.49±0.24 | 7.72±0.81 | 8.69±0.30 | 9.40±0.30 | 9.71±0.27 | |
| Adults | 0.0 g/kg ethanol | 4.46±0.27 | 5.17±0.25 | 4.88±0.26 | 5.00±0.29 | 4.70±0.21 | 4.79±0.22 | 5.21±0.21 | 5.15±0.30 |
| 2.5 g/kg ethanol | 4.79±0.81 | 5.24±0.24 | 5.42±0.22 | 5.35±0.32 | 5.24±0.45 | 5.28±0.34 | 4.84±0.29 | 5.52±0.22 | |
Overall fluid consumption (mL/100 g of body weight) during 2-h, two-bottle intake tests, in Experiment 1 as a function of age (adolescent or adult), stress treatment (5 daily sessions of 2 h of restraint stress, or no stress exposure, during postnatal days (PD) 30–34 or 70–74, for adolescents and adults, respectively), and ethanol treatment at PD34 or PD74 (2 h after termination of the last stress session animals were challenged with 0.0 or 2.5 g/kg ethanol). Values express mean ± SEM.
The ANOVAs for absolute (g/kg) and relative (%) ethanol intake also revealed a significant interaction between day of assessment, ethanol dose given during the motor activity assessment, and age (F[3,249] = 4.48, p < 0.001; F[3,249] = 2.72, p < 0.001, respectively). Planned comparisons indicated that, during the second intake test, the adolescent animals that had been treated with 2.5 g/kg ethanol during the motor assessment consumed significantly more ethanol (g/kg and % preference) than their adult counterparts. Adolescent ethanol consumption (g/kg) across sessions was 0.66 ± 0.12, 0.57 ± 0.12, 0.29 ± 0.09, and 0.20 ± 0.04 in animals that had been given vehicle, and 0.47 ± 0.10, 0.83 ± 0.17, 0.35 ± 0.10, and 0.21 ± 0.05 in animals that had been given 2.5 g/kg i.g. ethanol on the earlier motor assessment test. Adults given vehicle drank 0.29 ± 0.06, 0.43 ± 0.09, 0.15 ± 0.05, and 0.17 ± 0.05 g/kg across intake sessions, whereas those treated with 2.5 g/kg ethanol drank 0.35 ± 0.06, 0.22 ± 0.04, 0.28 ± 0.07, and 0.09 ± 0.02 g/kg in sessions 1 to 4.
A separate group of adolescent and adult animals was given 2.5 g/kg ethanol, and trunk blood samples were obtained 15 min post-administration. A t test indicated similar BECs in adult (150.92 ± 10.97) and adolescent rats (142.67 ± 22.03).
Experiment 2
Body weights
The ANOVAs for body weight scores during stress days indicated a significant sex difference (F[1,44] = 1004, p < 0.001), and a significant interaction between stress, age, and day of assessment (F[4,176] = 3.86, p < 0.001).To understand these effects, stress × days ANOVAs were run for each age. These analyses only indicated a significant increase across days (significant effect of days, F[4,88] = 9.91, F[4,88] = 1296.2; p < 0.05; for adolescents and adults, respectively). Body weights are indicated in Table 1 (middle section).
Exploration of the light-dark box and acute reactivity to stress
Although latency to enter the black compartments was not affected by stress or age, the stressed animals exhibited significantly more time in the bright compartment (F[1,44] = 5.03, p < 0.05) and moved significantly more often between compartments (F[1,44] = 5.23, p < 0.05) than control, non-stressed counterparts (Fig. 4, upper panels).
Fig. 4. Baseline and acute-stress induced anxiety response in adolescent and adult rats.
Upper panels: response in a 5-min light-dark box (LDB) test (time spent in the white area [sec], latency to escape to dark area [sec], and frequency of transfers between compartments, panel A, B, and C, respectively), in adolescent and adult Wistar rats as a function of stress treatment experienced during postnatal days 30–34 or 70–74 (5 days of restraint stress [120 min per day] or un-manipulated). Lower panels: immediately after the light-dark test, animals were exposed for 5 min to inescapable acute stress (confinement in the white area of the LDB, illuminated with 1200 lux). The panels depict locomotor activity (sec), frequency of wall climbing, and number of fecal boli during acute stress exposure (panels D, E, and F, respectively). The asterisks in panels A and C indicate that the stressed animals, both adolescents and adults, exhibited significantly more time in the bright compartment and moved significantly more often between compartments than control, non-stressed counterparts. The asterisk in panel E indicates that adolescents displayed significantly lower frequency of wall-climbing behaviors than adults. The asterisk in panel D indicates that adults, but not adolescents, with a previous history of stress exhibited lower levels of motor activity than their non-stressed age-matched controls (p<0.025). Vertical lines indicate SEM.
During the acute stress exposure (Fig. 4, lower panels), there were no differences between stressed and control adolescent or adult groups in the number of fecal boli (p > 0.60) (Fig. 4, panel F). Adolescents, however, displayed significantly lower frequency of wall-climbing behaviors than adults (F[1,44] = 5.03, p < 0.05) (Fig. 4, panel E), and the ANOVA for locomotor activity revealed significant main effects of age and stress (F[1,44] = 19.19, p < 0.05 and F[1,44] = 4.69, p < 0.05, respectively) and a significant age × stress interaction (F[1,44] = 4.61, p < 0.05). Adults, but not adolescents, with a history of stress exposure exhibited lower levels of motor activity than their non-stressed age-matched controls (Fig. 4, panel D).
Corticosterone response
The ANOVA yielded a significant main effect of time of measurement (i.e., greater CORT after the acute exposure to the illuminated chamber, compared to baseline), F[1,42] = 137.05, p < 0.001. This effect, which is depicted in Fig. 5, was not affected by prior exposure to RS, neither in adolescent nor in adult rats.
Fig. 5. Baseline and acute-stress induced hormonal response in adolescent and adult rats.
Corticosterone response (ng/mL) in 38-day-old adolescent and 78-day-old adult Wistar rats before (i.e., baseline) and immediately after a 5-min exposure to inescapable acute stress (confinement in the white area of a light-dark box, illuminated with 1200 lux). Animals had experienced or not experienced chronic stress treatment during postnatal days 30–34 or 70–74 (5 days of restraint stress [120 min per day] or un-manipulated). The asterisk reflects a main effect of acute stress exposure (i.e., baseline vs. after stress). Vertical lines indicate SEM.
Experiment 3
Body weights
The ANOVA yielded significant main effects of age and day (F[1,80] = 1723.02, p < 0.001; F[5,400] = 437.77, p < 0.001), a significant stress × days interaction, (F[4,148] = 461.21), a significant stress × days interaction (F[5,400] = 38.22, p < 0.001), and a significant age × stress × days interaction (F[5,400] = 2.31, p < 0.05). To understand these interactions, follow-up ANOVAs (stress × days) were conducted for each age.
The ANOVA for adolescents yielded a significant main effect of days and a significant stress × days interaction, F[4,148] = 461.21, F[4,148] = 14.75; p < 0.001. Planned comparisons indicated that controls exhibit a gradual increase in body weight across days. Stressed adolescents, on the other hand, displayed significantly lower body weight than controls on the last stress day and on testing day. Adult animals exhibited only a significant increase in body weight across days, F[5,125] = 42.34, p < 0.001. Table 1 (bottom section) presents body weight (g) mean and SEM during stress exposure days. Body weight (g) prior to the loss of righting reflex test was as follows: 440.83 ± 7.91 and 419.5 ± 9.11, for control and stressed adults, and 165.58 ± 3.87 and 150.35 ± 2.76, for control and stressed adolescents.
Latency to loss of righting reflex and sleep time after ethanol dosing
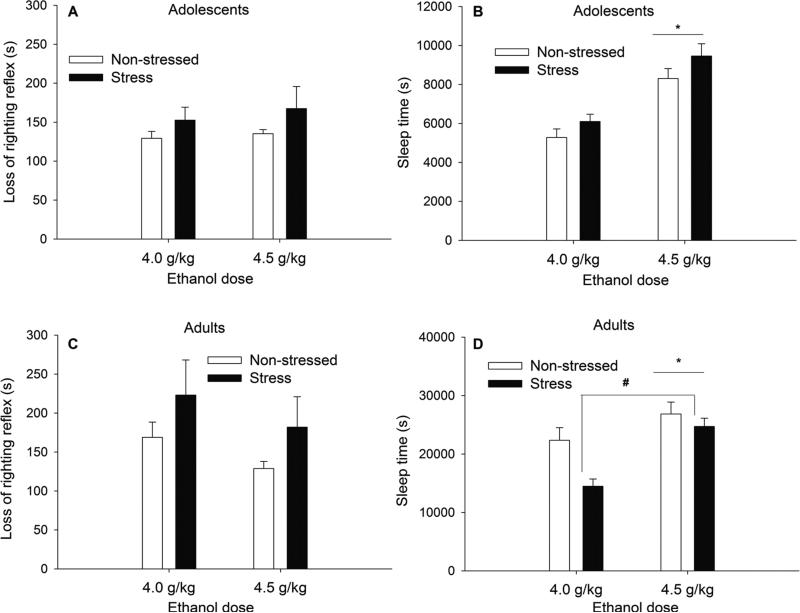
The ANOVA indicated a significant main effect of stress, F[1,76] = 4.84, p < 0.00. As depicted in Fig. 6, restraint induced a significant increase in the duration of loss of the righting reflex, which was statistically similar at both ages (Fig. 6, panels A and C).
Fig. 6. Sensitivity to the sedative and narcotic effects of ethanol in adolescent and adult rats.
Loss of righting reflex (sec) and sleep time (sec) after 4.0 or 4.5 g/kg ethanol (i.p.) in adolescent (panels A and B) and adult (panels C and D) Sprague-Dawley rats as a function of stress treatment experienced during postnatal days 30–34 or 70–74 (5 days of restraint stress [120 min per day] or non-stressed). The asterisk indicates a significant main effect of ethanol dose on sleep time. The pound sign indicates a significant main effect of prior stress exposure on ethanol-induced sleep time in adults. Vertical lines indicate SEM.
The ANOVA for sleep time revealed significant main effects of age, stress, and dose (F[1,76] = 224.36, p < 0.001; F[1,76] = 4.13, p < 0.05, and F[1,76] = 28.49, p < 0.001; respectively) as well as significant age × stress, and age × dose interactions (F[1,76] = 9.19, p < 0.001; F[1,76] = 4.43, p < 0.05; respectively). The post hoc tests revealed that stress significantly diminished sleep time in adult, but not in adolescent, rats. The post hoc tests also revealed that sleep time was affected by dose in adult (i.e., greater hypnosis after 4.5 g/kg than after 4.0 g/kg), but not in adolescent, rats. Fig. 6 illustrates these results and reveals the overall greater ethanol-induced sleep time displayed by adults, when compared to adolescents (please note the differences in the y-axis scale of panels B and D).
Blood ethanol and corticosterone levels after ethanol dosing
Age-related differences were observed in CORT response (F[1,76] = 38.71, p < 0.001) and BECs at awakening time (F[1,76] = 52.23, p < 0.001). BEC and CORT levels at awakening were higher in adolescents than in adults and were not affected by stress exposure or ethanol dose. BECs (mg%) in restrained adolescents were 439.7 ± 10.3 and 449.5 ± 6.89 (4.0 and 4.5 g/kg groups, respectively), and 436.2 ± 10.9 and 457.5 ± 9.18 (4.0 and 4.5 g/kg groups, respectively) in control, non-stressed adolescents. BECs (mg%) in restrained adults were 375.31 ± 13.18 and 365.7 ± 13.2 (4.0 and 4.5 g/kg groups, respectively), and 371.8 ± 11.1 and 366.5 ± 26.5 (4.0 and 4.5 g/kg groups, respectively) in control, non-stressed adults. CORT values (ng/dL) in restrained adolescents were 537.0 ± 22.95 and 591.0 ± 31.77 (4.0 and 4.5 g/kg groups, respectively), and 532.3 ± 50.37 and 520.1 ± 30.42 (4.0 and 4.5 g/kg groups, respectively) in control, non-stressed adolescents. CORT scores (ng/dL) in restrained adults were 412.8 ± 17.9 and 392.8 ± 28.0 (4.0 and 4.5 g/kg groups, respectively), and 406.3 ± 24.7 and 430.5 ± 32.0 (4.0 and 4.5 g/kg groups, respectively) in control, non-stressed adults.
Discussion
The aim of this study was to explore age-related differences in the effects of RS on ethanol drinking and ethanol-induced hypnosis and sedation, and on basal and stress-induced anxiety and corticosterone response. The chronic RS procedure exacerbated free-choice ethanol drinking in adolescent rats but not in adult rats, although the effect was transient and subtle. Stress also altered anxiety response patterns and increased the duration of loss of the righting reflex after a high ethanol dose, yet these effects were similar at both ages. Adolescents, unlike adults, exhibited ethanol-induced motor stimulation. Ethanol-induced sleep time was much higher in adults than in adolescent rats, yet stress diminished ethanol-induced sleep time only in adults.
RS effects upon ethanol intake
Experiment 1 confirmed previous work conducted in male Wistar rats (Ploj et al., 2003), indicating that brief RS does not alter ethanol drinking in adult rats during post-stress, two-bottle choice tests, and extends these findings to a between-subjects (stressed vs. non-stressed controls) design from the within-subject (i.e., baseline stress/post stress) design used by Ploj et al. The current study also provided new information that RS facilitated subsequent ethanol ingestion in adolescent, but not in adult, rats (Experiment 1). There is little information on the consequences of restraint stress on ethanol acceptance on mice (for review and references, see Becker, Lopez, & Doremus-Fitzwater., 2011). Yet, the present results are consistent with previous studies in C57BL/6J mice, indicating that social isolation during adolescence, but not during adulthood, enhances subsequent ethanol consumption (Lopez et al., 2011). Taken together, these studies indicate that developmental timing of stress exposure is critical to determine the consequences of stress on ethanol intake. It is important to remark that, in the present study, the facilitative effect of stress on adolescent absolute and percent ethanol intake was transient and only found when stressed adolescents were given access to a relatively low ethanol concentration (i.e., 4% ethanol).
The levels of ethanol intake found across days in stressed adolescents are within the range that previous studies reported as reinforcing in immature (i.e., infants and adolescents) animals. For instance, 0.5 g/kg ethanol exerted appetitive and anxiolytic effects in both infant (Molina, Pautassi, Truxell, & Spear, 2007; Pautassi, Nizhnikov, Molina, Bohem, & Spear, 2007) and adolescent (Miranda-Morales & Pautassi, 2015; Pautassi et al., 2008) rats. In these studies, however, ethanol was given i.p. There are marked differences in the pharmacokinetics of ethanol following the oral and injection routes. Moreover, blood ethanol concentrations will be lower in animals that are consuming ethanol over 2 h, versus the administration of a dose of ethanol by gavage. It is, therefore, uncertain if ethanol drinking in the present study was driven by the pharmacological effects of ethanol or, instead, only by the sensory properties of the drug (i.e., flavor). There was also a subtle, yet significant, effect of acute ethanol administration upon later ethanol intake: adolescents treated with ethanol during the motor activity test drank more ethanol than adults that had also been treated with ethanol. This effect is consistent with previous studies (e.g., Fabio, Nizhnikov, Spear, & Pautassi, 2014) indicating a facilitative effect of ethanol exposure during adolescence on later ethanol acceptance.
Two important caveats of the present study are that BECs were not measured at the end of drinking sessions and that the intake procedure entailed substantial water deprivation. The intake test was a 2-bottle choice test, however, and the effect of stress on intake was specific to ethanol, with no effects of the prior stressor on total fluid intake.
The results of Experiment 1 are consistent with some, yet not all, of the findings of a recent meta-analytical study (Noori, Helinski, & Spanagel, 2014). The latter work indicated that Wistar rats may be highly sensitive to stress and stress-induced ethanol drinking, yet found adolescents to be less sensitive than adults to this effect. This difference may be due to the fact that the meta-analytical study indicated that RS was less likely to facilitate ethanol drinking than footshock, forced swim, or white noise exposure. This may suggest that under experimental conditions where RS has the ability to affect ethanol drinking, adolescents may be at greater risk for stress-induced drinking.
RS effects upon anxiety response; and behavioral and hormonal response to subsequent stress
Chronic RS altered exploration patterns within the LDB apparatus. When compared to their non-stressed peers, stressed rats spent significantly more time in the white area of the maze and made significantly more transfers between compartments, an effect that was statistically similar across the ages tested (Experiment 2). The brightly lit chamber of a LDB is an open, potentially dangerous area and, hence, greater time spent in that area is usually considered an index of a reduced anxiety response. This result seems to be at odds with previous reports of greater anxiety in the LDB after RS exposure in mice (e.g., Solomonow & Tasker, 2015), and with rat studies indicating either increased anxiety (Dagnino-Subiabre et al., 2005) or no effect of RS in the elevated plus-maze test (Doremus-Fitzwater, Varlinskaya, & Spear, 2009). Yet Cancela, Bregonzio, and Molina (1995) employed Wistar rats and reported, similar to Experiment 2, increased time spent in the bright area of the LDB after RS (2 h daily for 7 days). Similarly, a change from an RS-induced reduction to an RS-induced increase in open field activity was found in rats, as a function of time of test (close to or distal from RS exposure, respectively; Klenerová, Sída, Krejcí, Hlinák, & Hynie, 2007). A critical parameter seems to be the length of the stress procedure, with acute and brief RS resulting in heightened anxiety whereas chronic and protracted (i.e., five or more >60 min exposures) RS inducing anxiolysis, a result likely due to an opioid-dependent adaptation to stress (Cancela et al., 1995).
It is possible that the use of a chronic and protracted RS treatment favored, in conjunction with a test that occurred 72 h after termination of the last stressor, the emergence of an adaptation to stress in Experiment 2. We cannot dismiss the possibility, however, that the present LDB results reflect cognitive alterations (i.e., greater impulsivity or inadequate risk assessment) in the stressed rats rather than an anxiolytic-like effect. It has been observed that adolescent rats intermittently exposed to ethanol via vapor inhalation (Desikan, Wills, & Ehlers, 2014) or via their drinking water (Hughes, 2011) exhibited greater time spent in the white area of the LDB than controls, a result considered an index of increased impulsivity. The possibility that repeated RS induces cognitive alterations is just a hypothesis and more work is needed to test it. Behavioral response to acute stress (Experiment 2), on the other hand, was modulated by prior RS only in adults. Specifically, acute stress reduced locomotor activity in adult subjects that had been exposed to chronic RS. These behavioral differences were not associated with alterations in CORT levels. The acute stressor induced a four- to five-fold increase in CORT, an increase that was similar in adolescents and adults, and in rats exposed or not exposed to RS. Possibly, these lack of age- and RS-related differences could be attributable to a ceiling effect in hormone release.
RS effects upon ethanol-induced motor activity, loss of righting reflex, and sleep time
RS did not affect sensitivity to ethanol-induced behavioral stimulation in the open field (Experiment 1). Adolescents, but not adults, exhibited a significant increase in motor activity after ethanol, an effect that was not affected by previous stress exposure. In Experiment 1, animals were tested in a novel open field and were given ethanol via i.g. intubation, which favored the expression of stimulant effects of ethanol. These results are consistent with studies conducted in Swiss mice (Quoilin, Didone, Tirelli, & Quertemont, 2012) and Sprague-Dawley rats (Acevedo et al., 2013), which considered the motor stimulant effects of ethanol as a proxy for ethanol's positive reinforcing effects.
In agreement with previous studies (Silveri & Spear, 1998), Experiment 3 found shorter ethanol-induced sleep times and higher waking blood ethanol levels in adolescents than adults (i.e., approximately 2.5 vs. 7 h in adolescent and adult rats given 4.5 g/kg, respectively). CORT waking levels were also higher in adolescents, probably as a consequence of their higher BECs, and were not affected by prior stress exposure in either adolescents or adults. The marked age difference in duration of sleep was observed even when latency to loss of righting reflex was similar across ages, indicating dissociation between these indices. Moreover, although adult sleep time was significantly greater than that observed in adolescents across groups, this difference was attenuated by previous, chronic RS. Despite this behavioral difference, there was no difference in the BEC at awakening time between stressed and non-stressed adults. Based on the BEC data, therefore, a cautious conclusion is that that stress did not alter sensitivity to the hypnotic effect of ethanol.
Experiment 1 and 3 used different strains of rats. These experiments yielded strain- and age-related differences in the effects of stress on body weight. Chronic RS decreased body weight in adult, but not in adolescent, Wistar rats; whereas adolescent, but not adult, SD rats exhibited RS-induced body weight alterations. It has been suggested that the Wistar strain may be more sensitive to stress-induced drinking than the Sprague-Dawley strain (Noori et al., 2014). It should be noted, however, that previous studies indicated that SD rats are sensitive to the anxiogenic consequences of RS, an effect that was reversed by ethanol only in adolescents (Varlinskaya & Spear, 2012).
Limitations and concluding comments
Important limitations of the present set of experiments are the use of different strains of rats and different routes of ethanol administration, which may have added confounding variables to the study. Despite these limitations, the experiments demonstrated that adolescent rats were more sensitive to stress-induced ethanol drinking than their adult counterparts, although this effect was transient and quite modest. Ethanol induced much greater sedation in adults than in adolescents, and only adolescents exhibited ethanol-induced motor stimulation. The results add to a growing number of studies indicating the presence of adolescent-specific sensitivities to ethanol that may facilitate initiation and escalation of ethanol intake (Spear & Swartzwelder, 2014).
Research Highlights.
Adolescent, but not adult, rats exhibited greater ethanol drinking after stress.
Ethanol induced motor activation in adolescents and motor depression in adults.
Chronic stress altered anxiety response in adolescents, but not in adults.
Chronic stress diminished ethanol-induced sleep time in adults only.
Acknowledgments
This work, a cooperative project between INIMEC-CONICET-UNC, IFEC-CONICET, LACE and SUNY Binghamton, was supported by grants PICT 2012 and PIP2012 to RMP and MBV, R01 AA018026 P50 AA017823 to LPS, and by a student fellowship awarded by CIN to MSF. The authors would like to express their gratitude to Beatriz Haymal, Lynda Ruffini, Jessica Sweatt, Maria Belen Acevedo, and Sarah Sanders for their technical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo MB, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM. High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence. Developmental Psychobiology. 2010;52:424–440. doi: 10.1002/dev.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo MB, Nizhnikov ME, Spear NE, Molina JC, Pautassi RM. Ethanol-induced locomotor activity in adolescent rats and the relationship with ethanol-induced conditioned place preference and conditioned taste aversion. Developmental Psychobiology. 2013;55:429–442. doi: 10.1002/dev.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaszczuk V, Bender C, Pereno GL, Beltramino CA. Alcohol-induced neuronal death in central extended amygdala and pyriform cortex during the postnatal period of the rat. International Journal of Developmental Neuroscience. 2011;29:733–742. doi: 10.1016/j.ijdevneu.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, Henderson AN, Badia-Elder NE, Stewart RB. Neuropeptide Y (NPY)-induced reductions in alcohol intake during continuous access and following alcohol deprivation are not altered by restraint stress in alcohol-preferring (P) rats. Pharmacology, Biochemistry, and Behavior. 2011;97:453–461. doi: 10.1016/j.pbb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Cancela LM, Bregonzio C, Molina VA. Anxiolytic-like effect induced by chronic stress is reversed by naloxone pretreatment. Brain Research Bulletin. 1995;36:209–213. doi: 10.1016/0361-9230(94)00185-4. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcoholism: Clinical and Experimental Research. 2004;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Chester JA, de Paula Barrenha G, DeMaria A, Finegan A. Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference. Alcohol and Alcoholism. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- Dagnino-Subiabre A, Terreros G, Carmona-Fontaine C, Zepeda R, Orellana JA, Díaz- Véliz G, et al. Chronic stress impairs acoustic conditioning more than visual conditioning in rats: morphological and behavioural evidence. Neuroscience. 2005;135:1067–1074. doi: 10.1016/j.neuroscience.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Desikan A, Wills DN, Ehlers CL. Ontogeny and adolescent alcohol exposure in Wistar rats: open field conflict, light/dark box and forced swim test. Pharmacology, Biochemistry, and Behavior. 2014;122:279–285. doi: 10.1016/j.pbb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson SD, Kashawny SK, Thiebes KP, Charles DY. Decreased sensitivity to ethanol reward in adolescent mice as measured by conditioned place preference. Alcoholism: Clinical and Experimental Research. 2009;33:1246–1251. doi: 10.1111/j.1530-0277.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism: Clinical and Experimental Research. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology & Behavior. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabio MC, March SM, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM. Prenatal ethanol exposure increases ethanol intake and reduces c-Fos expression in infralimbic cortex of adolescent rats. Pharmacology, Biochemistry, and Behavior. 2013;103:842–852. doi: 10.1016/j.pbb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabio MC, Nizhnikov ME, Spear NE, Pautassi RM. Binge ethanol intoxication heightens subsequent ethanol intake in adolescent, but not adult, rats. Developmental Psychobiology. 2014;56:574–583. doi: 10.1002/dev.21101. [DOI] [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcoholism: Clinical and Experimental Research. 2011;35:1842–1851. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. Adult anxiety-related behavior of rats following consumption during late adolescence of alcohol alone and in combination with caffeine. Alcohol. 2011;45:365–372. doi: 10.1016/j.alcohol.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Jones BC, Connell JM, Erwin VG. Isolate housing alters ethanol sensitivity in long-sleep and short-sleep mice. Pharmacology, Biochemistry, and Behavior. 1990;35:469–472. doi: 10.1016/0091-3057(90)90187-m. [DOI] [PubMed] [Google Scholar]
- Klenerová V, Sída P, Krejcí I, Hlinák Z, Hynie S. Effects of two types of restraint stress on spontaneous behavior of Sprague-Dawley and Lewis rats. Journal of Physiology and Pharmacology. 2007;58:83–94. [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–364. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME. The effects of restraint stress on voluntary ethanol consumption in rats. Experimental and Clinical Psychopharmacology. 1999;7:318–323. doi: 10.1037//1064-1297.7.4.318. [DOI] [PubMed] [Google Scholar]
- Miranda-Morales RS, Pautassi RM. Pharmacological characterization of the nociceptin/orphanin FQ receptor on ethanol-mediated motivational effects in infant and adolescent rats. Behavioural Brain Research. 2015;298(Pt A):88–96. doi: 10.1016/j.bbr.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Edition. National Academies Press; Washington, DC: 2011. [Google Scholar]
- Ng Cheong Ton MJ, Brown Z, Michalakeas A, Amit Z. Stress induced suppression of maintenance but not of acquisition of ethanol consumption in rats. Pharmacology, Biochemistry, and Behavior. 1983;18:141–144. doi: 10.1016/0091-3057(83)90264-2. [DOI] [PubMed] [Google Scholar]
- Noori HR, Helinski S, Spanagel R. Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addiction Biology. 2014;19:225–232. doi: 10.1111/adb.12125. [DOI] [PubMed] [Google Scholar]
- Parker CC, Ponicsan H, Spencer RL, Holmes A, Johnson TE. Restraint stress and exogenous corticosterone differentially alter sensitivity to the sedative-hypnotic effects of ethanol in inbred long-sleep and inbred short-sleep mice. Alcohol. 2008;42:477–485. doi: 10.1016/j.alcohol.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcoholism: Clinical and Experimental Research. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov M, Molina JC, Boehm SL, 2nd, Spear N. Differential effects of ethanol and midazolam upon the devaluation of an aversive memory in infant rats. Alcohol. 2007;41:421–431. doi: 10.1016/j.alcohol.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Abate P, Spear NE, Molina JC. Heightened ethanol intake in infant and adolescent rats after nursing experiences with an ethanol-intoxicated dam. Alcoholism: Clinical and Experimental Research. 2004;28:895–905. doi: 10.1097/01.alc.0000128223.95184.c9. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of short and long periods of maternal separation on brain opioid peptide levels in male Wistar rats. Neuropeptides. 2003;37:149–156. doi: 10.1016/s0143-4179(03)00043-x. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Nursing from an ethanol- intoxicated dam induces short- and long-term disruptions in motor performance and enhances later self-administration of the drug. Alcoholism: Clinical & Experimental Research. 2004;28:1039–1050. doi: 10.1097/01.alc.0000131298.32045.96. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Ethanol-mediated operant learning in the infant rat leads to increased ethanol intake during adolescence. Pharmacology, Biochemistry, and Behavior. 2008;90:640–650. doi: 10.1016/j.pbb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E, Quertemont E. Developmental differences in ethanol-induced sensitization using postweanling, adolescent, and adult Swiss mice. Psychopharmacology (Berl) 2012;219:1165–1177. doi: 10.1007/s00213-011-2453-7. [DOI] [PubMed] [Google Scholar]
- Rockman GE, Hall A, Hong J, Glavin GB. Unpredictable cold-immobilization stress effects on voluntary ethanol consumption in rats. Life Sciences. 1987;40:1245–1251. doi: 10.1016/0024-3205(87)90580-7. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol. 2004;33:31–39. doi: 10.1016/j.alcohol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gorman K, Amit Z. Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol. 1990;7:321–326. doi: 10.1016/0741-8329(90)90090-y. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcoholism: Clinical and Experimental Research. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism: Clinical and Experimental Research. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Solomonow J, Tasker JG. Anxiety Behavior Induced in Mice by Acute Stress. Tulane Undergraduate Research Journal. 2015;2:14–19. [Google Scholar]
- Song M, Wang XY, Zhao M, Wang XY, Zhai HF, Lu L. Role of stress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcoholism: Clinical and Experimental Research. 2007;31:2001–2005. doi: 10.1111/j.1530-0277.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neuroscience and Biobehavioral Reviews. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Developmental Psychobiology. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiology & Behavior. 1997;63:143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcoholism: Clinical and Experimental Research. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacology, Biochemistry, and Behavior. 2010;96:228–235. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacology, Biochemistry, and Behavior. 2012;100:440–450. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol and Alcoholism. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer B, Brown DR, Michels KM. Statistical principles in experimental design. 3rd. ed. McGraw Hill; New York: 1991. [Google Scholar]