Abstract
We generated a rhesus macaque induced pluripotent stem cell (riPSC) line, riPSC89, from rhesus embryonic fibroblasts (REFs). Fibroblasts were expanded from the skin of a rhesus macaque embryo at embryonic day 47. REFs and riPSCs had a normal male (42, XY) karyotype. The riPSC89 line was positive for markers of self-renewal including OCT4, NANOG, TRA-1-81 and SSEA4. Pluripotency was demonstrated through the generation of teratomas using transplantation into immunocompromised mice. The riPSC89 line may be a useful non-human primate resource to uncover developmental origins of disease, or used as a basic model to understand lineage specification in the primate embryo.
Resource details
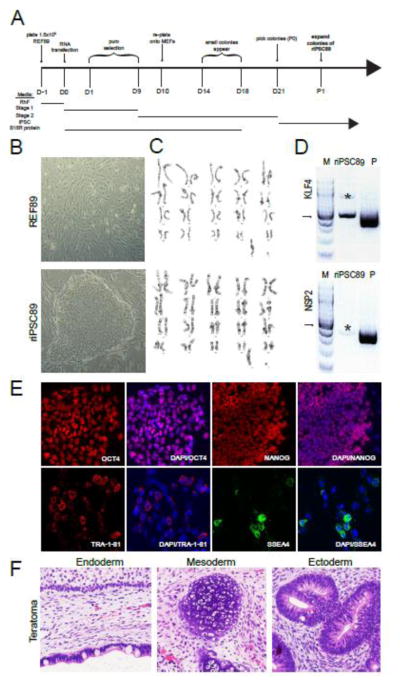
We generated an integration-free, virus-free rhesus macaque (Macacca mulata) induced pluripotent stem cell (riPSC) line from rhesus embryonic fibroblasts (REFs). REFs were expanded from embryonic skin, which was removed from the torso of an embryonic day 47 rhesus embryo in Carnegie stage 23 (embryo # 34989). The REFs in this study were called REF89. REF89 cells were reprogrammed using technology that takes advantage of the non-infectious, self-replicating Venezuelan equine encephalitis (VEE) virus, which replicates when the innate interferon immune response is chemically suppressed by recombinant B18R during the initial stages of induced reprogramming. The RNA replicon produces a synthetic RNA containing the reprograming factors OCT4, KLF4, SOX2, and GLIS1 (OKS-iG) as a polycistronic transcript. Using this technology, we reprogrammed REF89 cells with VEE-OKS-iG RNA and B18R RNA in the presence of human recombinant B18R protein for eighteen days (Fig. 1A). Pioneering riPSC colonies first appeared between day 14–18, and were picked on day 21 (Fig 1A). Picked colonies were expanded in iPSC media on mytomycin C inactivated mouse embryonic fibroblasts (MEFs) and split every 5–7 days. The established riPSC line which originated from a single colony was called riPSC89 to reflect its origin from REF89 (Fig 1B). riPSC89 and REF89 were both normal male 42, XY karyotype (Fig. 1C). Non-integration was confirmed by genomic PCR taking advantage of a size differential between endogenous KLF4 (519 bp) and the KLF4 transgene (416bp) and absence of the virus-specific nonstructural protein 2 (NSP2) (Fig. 1D). riPSC89 expressed markers of self-renewal, such as OCT4, NANOG, SSEA4 and TRA-1-81 (Fig. 1E). In order to assess pluripotency and differentiation, we performed a teratoma analysis by injecting undifferentiated colonies of riPSC89 into SCID beige mice. Using this assay, it was discovered that riPSC89 was competent to form teratomas, with evidence of ectoderm, mesoderm and endoderm by histology (Fig. 1F).
Fig 1. Characterization of riPSC89.
(A) Timeline of events during the reprogramming REF89 fibroblasts and subsequent establishment and characterization of the riPSC89 cell line from a single colony. (B) Morphology of the REF89 (Fibroblasts) and a colony of riPSC89 (iPSCs). (C) REF89 and riPSC89 were karyotypically normal male (42, XY). (D) Non-integration after reprogramming confirmed by PCR of riPSC89 genomic DNA (500ng). The PCR product originating from endogenous KLF4 = 514 bp. The PCR product originating from reprogramming plasmid KLF4 = 418 bp. NSP2 is only present in the plasmid DNA and not riPSC89 gDNA (M=100 base pair marker with arrow pointing to 500bp, P=plasmid DNA)
*denotes a non-specific PCR band of incorrect size. (E) riPSC89 cells express markers of self-renewal such as OCT4 and NANOG, as well as, the cell surface proteins SSEA4 and TRA-1-81. (F) Pluripotency of riPSC89 was confirmed using a teratoma assay, which contained cell types representing endoderm, mesoderm and ectoderm.
Materials and methods
Rhesus Embryonic Fibroblast Derivation
The rhesus macaque embryo (embryo # 34989) was obtained from the time mated breeding (TMB) program at the Oregon National Primate Research Center (ONPRC) following Institutional Animal Care and Use Committee Approval. The experimental female and the stud male were housed together for a total of four days from day 11-14 of the menstrual cycle. Ovulation was monitored in the female using serum estradiol to monitor the time of the the estradiol peak (ovulation), which occurred on day 12. The male was removed 48 hours later. The day of fertilization (embryonic day 1) was estimated to occur 48 hours after the serum estradiol peak. Pregnancy was confirmed by measuring serum progesterone and also by ultrasound. On embryonic day 47 (49 days after the estradiol peak), the embryo was removed by cesarean section, and Carnegie staging was performed according to Gribnau and Geijsberts (1981). Skin samples were removed from the torso and back of the embryo to generate rhesus embryonic fibroblasts (REFs). The freshly isolated skin samples were digested with 2mg/ml Collagenase IV (Life Technologies) at 37°C, 5.0% CO2 for 2 hours. Digested samples were centrifuged at 1200 rpm and washed in DMEM/F12 (Life Technologies) media prior to plating on 0.1% gelatin (Sigma) coated plates in Rhesus Fibroblast (RhF) media consisting of DMEM/F12 (Life Technologies), 15% Fetal bovine serum (GE Healthcare), Non-Essential Amino Acids (Invitrogen), Glutamax™ (Gibco), Penicillin-Streptomycin-Glutamine (Gibco), and Primocin (Invivogen) at 37°C, 5.0% CO2. Outgrowths were monitored for up to 2 weeks (passage 0), with media changes every 2–3 days. REFs were passaged using 0.25% Trypsin (Gibco) and were re-plated at a density of 1.5 × 105 cells/well of a 6-well plate (Corning) on 0.1% gelatin (Sigma). The resulting REF cells were called REF89.
Generating the riPSC89 line
Reprogramming was performed using the Simplicon™ Reprogramming Kit (EMD Millipore) on REF89 cells at passage 3. One day before reprogramming, 1.5 × 104 REF89 cells were plated in one well of a 6-well plate (Corning) coated with 0.1% gelatin (Sigma) in RhF media. On the day of reprogramming, REF89 cells were pre-treated with the Human Recombinant B18R protein. Cells were then transfected once with both B18R RNA and VEE-OKS-iG RNA using the RiboJuice™ mRNA Transfection Kit (EMD Millipore) and allowed to recover overnight in Stage 1 media (Advanced DMEM (Gibco), 10% FBS (GE Healthcare), 2mM L-glutamine (Life Technologies) supplemented with 200ng/mL human recombinant B18R protein (EMD Millipore). From day 1 until day 9 post transfection, 0.1ug/mL Puromycin (Invivogen) was added to the Stage 1 media, with daily media changes. Ten days after transfection, REF89 cells were detached using Accumax™ (Sigma) and 1.0 × 104 transfected cells were plated in one well of a 6-well plate (Corning) on inactivated MEFs in Stage 2 media which consists of MEF conditioned medium (R & D Systems) supplemented with 10ng/mL bFGF (R&D system), TGF-β RI Kinase Inhibitor IV, Sodium Butyrate and PS48 (all from Simplicon) and 200 ng/mL B18R protein (EMD Millipore). Stage 2 media was changed every other day. Small colonies appeared on day 14 after transfection and by day 18, B18R protein was removed from the media. Putative riPSC colonies were manually picked on day 21 and re-plated on inactivated MEFs in regular iPSC media composed of DMEM/F-12 (Life Technologies), 20% KSR (Life Technologies), 10ng/mL bFGF (R & D Systems), 1% nonessential amino acids (Life Technologies), 2mM L-Glutamine (Life Technologies), Primocin™ (Invivogen), and 0.1mM β-mercaptoethanol (Sigma) on mytomicin C treated MEFs (passage 0). Seven days later, riPSC colonies were manually picked and expanded in iPSC media (passage 1). The riPSC89 line was expanded from a single colony starting at passage 2.
Karyotyping Analysis
REF89 and riPSC89 were karyotyped using metaphase spreads and G-banding by Cell Line Genetics (Madison, WI).
Teratoma Formation Assay
RiPSC89 was digested with 1mg/mL collagenase IV (Life Technologies), and the resulting colonies were re-suspended in cold Matrigel (Corning). riPSC89 colonies in matrigel were injected into the left and right testis of n=2 CB17/Icr.Cg-Prkdcscid Lystbg/Cr (scid beige) mice using survival surgery (n=4 testes total). Each testis was injected with 40uL of matrigel containing ~2.5 × 106 riPSC89 cells as small clumps. Two months after the transplant, the mice were euthanized and the testes containing tumors were excised and fixed in 4% Paraformaldehyde (PFA) in phosphate buffered saline (PBS). The tumors were processed to paraffin for hematoxylin and Eosin (H&E) staining. Diagnosis of teratoma was made using an Olympus BX-61 light microscope. All animal work was first approved by the UCLA Office of Animal Research Oversight.
Immunofluorescence staining
riPSC89 cells were fixed in 4% PFA, permeabilized with PBS plus 0.5% Triton™X-100 (Sigma) then washed in PBST (PBS with 0.1% Tween-20 (Sigma)) and blocked in 10% donkey serum (Jackson Immunoresearch) for 30 minutes at room temperature. After washing in PBST, cells were incubated overnight at 4°C with primary antibodies diluted in PBS containing 2.5% donkey serum. The primary antibody concentrations were, goat-antihuman OCT4 (1:100, Santa Cruz), goat-anti-human NANOG (1:100, R&D Systems), mouse-anti-human SSEA-4 (1:100, Developmental Studies Hybridoma Bank), and mouse-anti-human TRA-1-81 (1:100, eBiosciences). After washing in PBST, the cells were incubated for 1 hour at room temperature with secondary antibodies in PBS containing 2.5% donkey serum. For sections stained with primary antibodies raised in goat, AF594-conjugated donkey anti-goat (1:100, Life Technologies) was used. For detecting primary antibodies raised in mice, AF488- or AF594-conjugated donkey-anti-mouse (1:100, Life Technologies) were used. Cells were then washed in PBST prior to mounting under coverslips with Prolong Gold Antifade mountant with DAPI (Life Technologies). Images of cells were acquired with a Zeiss LSM 880 confocal laser-scanning microscope. Images were analysed using ImageJ version 1.51d (NIH).
PCR of genomic DNA to confirm non-integration
Genomic DNA was isolated from riPSC89 using Quick-gDNA™ miniprep kit (Zymogen). T7-VEE-OKS-iG plasmid DNA (Addgene), was isolated from bacteria using the plasmid DNA miniprep kit (Qiagen). At least 500ng of gDNA was used to confirm non-integration of VEE-OKS-iG RNA. Primers sets and PCR conditions used for the amplification of the genes NSP2 and KLF4 have been previously reported (Yoshioka et al., 2013).
Acknowledgments
We would like to thank Dr. Saran Karumbayaram for providing guidance in the culture and expansion of the primate fibroblasts. We would also like to thank Steven Dowdy for depositing the T7-VEE-OKS-iG plasmid (Addgene). This project was funded by NIH grant P01HD075795. Non human primate time mated breeding was supported by NIH OD P51OD011092 (JDH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Yoshioka N, Gros E, Li H, Kumar S, Deacon DC, Maron C, Muotri AR, Chi NC, Fu X, Yu BD, Dowdy SF. Efficient Generation of Human iPSCs by a Synthetic Self-Replicative RNA. Cell Stem Cell. 2013;13(2):246–254. doi: 10.1016/j.stem.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribnau A, Geijsberts L. Developmental Stages in the Rhesus Monkey (Macaca mulatta) Vol. 68. Berlin Heidelberg New York: Springer-Verlag; 1981. [DOI] [PubMed] [Google Scholar]