Abstract
Fear and anxiety-related disorders are remarkably common and debilitating, and are often characterized by dysregulated fear responses. Rodent models of fear learning and memory have taken great strides towards elucidating the specific neuronal circuitries underlying the learning of fear responses. The present review addresses recent research utilizing optogenetic approaches to parse circuitries underlying fear behaviors. It also highlights the powerful advances made when optogenetic techniques are utilized in a genetically defined, cell-type specific, manner. The application of next-generation genetic and sequencing approaches in a cell-type specific context will be essential for a mechanistic understanding of the neural circuitry underlying fear behavior and for the rational design of targeted, circuit specific, pharmacologic interventions for the treatment and prevention of fear-related disorders.
Keywords: Fear, Threat, Anxiety, conditioning, cell-type specific, optogenetics, TRAP, translating ribosome affinity purification, Amygdala
Introduction
Disorders whose major symptoms relate to the dysregulation of fear responses are usually characterized by over-generalization of fear and inability to extinguish fearful responses. Such dysregulation leads to a pathological expression of fear behaviors that can be quite debilitating, leading to a range of intrusive, hyperarousal, avoidance, cognitive, and depression symptoms. The treatment of fear-related disorders often involves cognitive-behavioral therapies, in particular exposure therapy, which mirrors behavioral extinction processes used in rodent models, relying on the repeated and non-reinforced presentation of cues previously associated with noxious stimulus.
Advances in cognitive-behavioral therapy approaches targeting traumatic memories have been made using cognitive enhancers, for example by targeting emotion-related synaptic plasticity via the NMDA, Dopamine, and Cannabinoid receptors1. Pharmacological interventions may be used to generally enhance plasticity within neural circuitry including that responsible for behavioral extinction. Across several fear- and anxiety-related disorders, the administration of cognitive enhancers, such as d-cycloserine, in conjunction with exposure-based psychotherapy has been shown to enhance the beneficial effects of behavioral therapy sessions in a rapid and long-lasting manner1,2. Despite these advances, insufficient knowledge of the underlying molecular and cellular mechanisms mediating fear acquisition, expression, and extinction continues to limit the specificity and effectiveness of further therapeutic breakthroughs. Therefore, a greater understanding of the neural circuitry mediating fear processing will catalyze further progress in the development of more selective treatments for fear-and anxiety-related disorders.
In this review, we will begin by discussing the understanding of the circuitry governing the acquisition and extinction of classically conditioned fear behaviors. We will continue by discussing the advent of optogenetic approaches and the contributions this technique has made to our knowledge of fear circuits. We will discuss the use of genetic techniques to determine which and how cell populations are recruited into memory traces. With a special focus on studies that involve behavioral manipulations, we will examine recent advances in the manipulation of identified cellular sub-populations housed within canonical fear and emotional learning related circuitries. Finally, we will provide a brief review of methods for cell-type specific isolation of RNA for sequencing.
As the basic neural circuitry governing fear behaviors continues to be elucidated at a rapid pace, it is necessary to act prospectively by applying these findings towards the discovery of applicable treatments for patients suffering from fear and anxiety related disorders. By uncovering cell-type specific markers for neural circuitry governing fear and anxiety behaviors in rodent models modern researchers have an opportunity to concurrently open avenues for more targeted pharmacological therapies in humans. Cell type specific markers may be conserved across species and targeting these convergences will maximize translational value of discoveries. This review is meant to highlight the need for further cell-type specific approaches in order to make rapid progress towards more selective and targetable pharmacological treatments of fear-related disorders in humans.
1. Background on Circuitry and Fear
Pavlovian conditioning
Pavlovian fear conditioning is a popular and powerful technique for studying learning and memory in animal models. This is primarily due to it being a rapidly acquired behavior with consistent and easily measured behavioral outputs that rely on a well-characterized core neural circuit. Fear conditioning, also discussed as threat conditioning3, occurs through the pairing of an initially innocuous conditioned stimulus (CS, e.g., an auditory tone during auditory fear conditioning or the context of training during contextual fear conditioning) with an aversive unconditioned stimulus (US, e.g., a mild foot shock). Following several CS-US pairings, the subject will exhibit fear response behaviors or conditioned responses (CRs) to presentations of the CS alone. The most common fear responses investigated are freezing (the cessation of all non-homeostatic movement) and fear potentiated startle (FPS, in which the amplitude of an animals' startle to a noise burst is potentiated upon combined presentation of the CS and noise burst)4,5.
In addition to measures of freezing and fear potentiated startle, there are a multitude of tests to parsimoniously examine an animal's motivational state. Briefly, in contrast to freezing or startle responses, tests demanding an active or passive avoidance response require an additional instrumental learning procedure to either perform or inhibit performance of an action such as shuttling in order to avoid a shock6-8. These learning paradigms utilize additional important circuitries and may provide further insights into the etiologies of fear related disorders9. The present review will focus primarily upon conditioned fear responses such as freezing and FPS following either the acquisition or extinction of fear; however, understanding the neural substrates governing additional motivated behaviors is likewise important for understanding the spectrum of fear-related processes.
Notably, fear responses are adaptive only when the CS clearly predicts the US. When these stimuli are no longer paired, such as during extinction (when the CS is repeatedly presented without any US reinforcement), a subject will learn that the CS is no longer predictive of the US, and CRs will decrease. Importantly, extinction is generally considered to be a new learning event that modulates rather than modifies the original learned fear association; for an excellent discussion of extinction see Myers and Davis, 200710. In this review, we refer to ‘fear conditioning’ or training as the period when CS – US pairings are presented; ‘fear extinction’ as a period when multiple or continuous CS presentations occur in the absence of the US, resulting in a decrement in CRs; ‘fear expression’ refers to eliciting CRs to a CS; and ‘extinction expression’ refers to the testing for suppression of CRs to a CS after extinction learning.
Fear learning: Basic circuitry and key players
The circuitry attributed to controlling elements of fear conditioning is ever expanding and we will discuss several additional areas in the course of this review; however, the core ‘canonical’ circuitry remains well understood and centers on the core amygdala nuclei. For recent in-depth reviews of the current understanding of the neural circuitries governing fear and anxiety see: 10-14. The core nuclei within the amygdala consist of the lateral (LA), basolateral (BA), and central (CeA) amygdala, which may be subdivided into the dorsolateral LA (LAdl), ventromedial LA (LAvm), ventrolateral LA (LAvl), anterior BA (BAa), posterior BA (BAp), central or capsular CeA(CeC), lateral CeA (CeL), and medial CeA(CeM). These nuclei may be even further subdivided. In the present review, the basolateral complex (BA + LA) will be abbreviated BLA.
Experimentally, dissections of CeC/CeL/CeM and LA/BA circuitries often fail to sufficiently discriminate between nuclei for a number of reasons, foremost due to their small sizes and close proximity. Specifically the CeC and the CeL tend to be conflated and the anterior aspect of the BAa is usually treated as representative of the whole BA or BLA. These, previously unavoidable, imprecisions may need to be corrected in time as more rigorous descriptions of micro-circuitries are performed. Furthermore, molecularly determined cell-type specific identification will lead to more powerful approaches to understanding microcircuit function in the future.
In the case of auditory fear conditioning (in which an auditory tone CS is paired with the US), salient information regarding the CS and US converge on the LA. Auditory information flows into the LA from the secondary auditory cortex (AuV) and auditory thalamus: medial geniculate nucleus/posterior intralaminar nucleus (MGn/PIN)15,16. Information regarding the US is communicated via the somatosensory cortex, somatosensory thalamus and periaqueductal grey (PAG)17,18. The LA integrates the information regarding both the tone and shock, and is a major site of learning related plasticity19. Projections from the LA can modulate CeA activity directly or indirectly through projections to the BA. Additional inhibitory controls come from the intercalated cell nuclei (ITC). The ITC are made up of islands of GABAergic neurons surrounding the BLA. ITC nuclei receive strong inputs from the LA and BA and may receive additional inputs from extrinsic regions such as the medial prefrontal cortex (mPFC)20,21. ITC nuclei act as regulators of information flow between the BLA and CeA by providing feed-forward inhibition to multiple nuclei of the CeA13,20,22-28. Interestingly, the dorsal ITC (ITCd) receive inputs from LA neurons and provide feed-forward inhibition of the CeL, while more ventral medial ITCs receive input from BA neurons and inhibit CeM populations29. The CeM is generally regarded as the main output station of the amygdala on account of its projections to the brain stem effector regions of fear behaviors such as the PAG, lateral hypothalamus and paraventricular nucleus of the thalamus (PVT)30-34.
Outside of the core amygdalar nuclei lie many important regions; here we will discuss just a few: the hippocampus (HPC), medial prefrontal cortex (mPFC), nucleus accumbens (NAc), bed nucleus of the stria terminalis (BNST) and hypothalamus. Broadly speaking, the dorsal HPC (dHPC) is thought to be critical for encoding the contextual elements of fear conditioning while the ventral HPC (vHPC) is involved in encoding the valence of specific memories35,36. On this account, during the testing phase of auditory fear conditioning, freezing to the auditory CS is generally performed in a context separate from the conditioning context while in contextual fear conditioning, contextually evoked freezing is measured in the training context. The HPC connects to the BLA and the mPFC37, and post-training lesions of the HPC impair retrieval of contextual elements of fear38. Within the mPFC, the infralimbic (IL) and prelimbic (PL) cortices are intimately implicated in fear extinction and fear acquisition respectively21. The IL and PL send strong inputs to the amygdala and may gate inputs from the BLA into the CeA39,21,40. The NAc and BLA have robust reciprocal connections. These inputs have been strongly implicated in motivated cue responses, especially to appetitive cues41-43. The BNST, part of the ‘extended amygdala’, is a set of nuclei strongly implicated in the regulation of stress responses, which receives reciprocal connections from many regions including the amygdala, HPC and mPFC17,44,45. The ventromedial hypothalamus makes reciprocal connections with the CeA and makes up a key link in a parallel fear processing and defensive behavior network3,46,47.
2. Optogenetic tracing of fear circuitry
The dawn of modern genetic tools has allowed for remote control of genetically defined cellular sub-populations and has thus greatly enhanced the specificity of manipulations delineating the role of specific nuclei or connections between nuclei involved in fear responses.
Optogenetics is based upon the use of genetically encoded, optically responsive ion channels or pumps. Initially discovered by Negel and colleagues, and greatly expanded by Boyden, Deisseroth, Zhang and others, channelrhodopsin and subsequently other opsins were rapidly developed to become powerful tools for millisecond time-scale control of neural systems48-51. In the work described in the present review, most manipulations use optical stimulation with channelrhodopsin 2 (ChR2) or optical inhibition using halorhodopsin (NpHR) or archaerhodopsin (Arch). Although there are important differences between the many opsins available, we will generally broadly group them into either stimulatory or inhibitory function for the purpose of brevity. Several other strategies for genetically encoded control of neural circuits have been developed recently, most notably designer receptors exclusively activated by designer drugs (DREADDs), which are genetically encoded modified G protein coupled receptors (GPCRs) that may be activated by an otherwise inert ligand clozapine-N-oxide (CNO)52-54. DREADDs come in a variety of forms including those coupled to Gs, Gq, and Gi. While a full complement of tools is valuable for research in behavioral neuroscience, optogenetics has dominated the literature for the last five years.
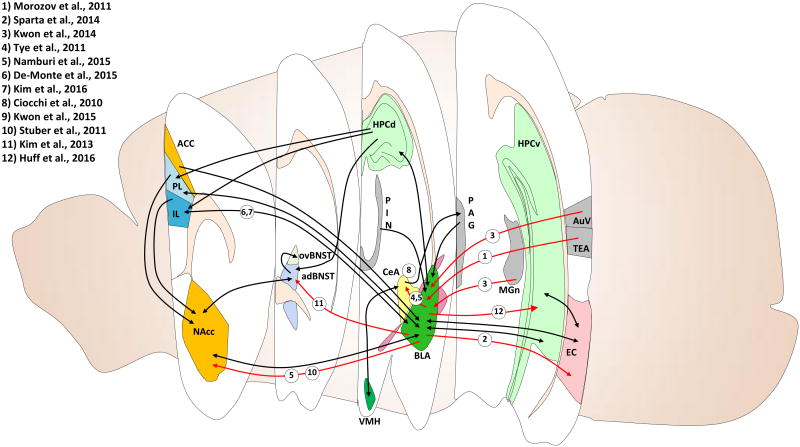
Below we will provide a review of some of the recent data using optogenetics to study the circuitry underlying fear behaviors and will focus on studies that provide data examining the behavioral consequences of optogenetic manipulations. We will discuss research in the context of the nuclei that were primarily interrogated for function in behavioral studies. For a summary of papers highlighted please see Table 1 and for a schematic of discussed projections see Figure 1.
Table 1.
Description of publications using optogenetics to query basic fear-related circuitries.
| Publication | Investigated Circuitry |
|---|---|
|
| |
| Morozov et al. (2011) | Inputs from TeA → LA receive feed forward inhibition from ITC while ACC → LA inputs do not. |
| Sparta et al. (2014) | BLA→ EC projections are necessary for the acquisition but not the expression of conditioned fear. |
| Kwon et al. (2014) | Activation of MGm→ BLA and AuV→ BLA projections is sufficient to act as a conditioned CS. |
| Tye et al. (2011) | Activation/inhibition of BLA→ CeA terminals is sufficient for anxiolysis/anxiogenesis, but activation of cell bodies is not. |
| Namburi et al. (2015) | Synaptic strengthening of BLA→ CeA projections after fear learning and of BLA→ NAc projections after appetitive training. |
| Do-Monte et a. (2015) | IL activity in rats is necessary for encoding but not retrieval of extinction memory. |
| Kim et al. (2016) | Inhibition/activation of IL activity in mice is sufficient for enhancement/blocking of extinction retrieval. |
| Ciocchi et al. (2010) | Activation of CeM is sufficient to produce spontaneous freezing. |
| Kwon et al. (2015) | Inputs from LAdl to ITCd generate feed-forward inhibition of CeL. ITCd receives additional GABAergic inputs to gate its activity during sub-threshold training |
| Stuber et al. (2011) | Activation of BLA→ NAc is sufficient to support ICSS. |
| Kim et al. (2013) | Activation/inhibition of BLA→ adBNST projections is anxiolytic/anxiogenic. |
Figure 1. Neural circuits involves in fear and anxiety-related behaviors in rodents.
Optogenetic, electrophysiological, and pharmacogenetic techniques have elucidated many specific circuitries underlying rodent fear and anxiety-related behaviors. Cross sectional views taken from different anterior-posterior positions within the rodent brain are marked with relevant brain regions and their distal projections. Projections highlighted in red are discussed in the present review; these highlighted circuits account for only a portion of identified circuitries, some of which are labeled with black arrows. ACC, anterior cingulate cortex; adBNST, anterodorsal nucleus of the BNST; AuV, secondary auditory cortex; BLA, basolateral amygdala; CeA, central amygdala; EC, entorhinal cortex; HPCd, dorsal hippocampus; HPCv, ventral hippocampus; IL, infralimbic division of the mPFC; MGn, medial geniculate nucleus; NAcc, nucleus accumbens; ov, oval nucleus of the BNST; PAG, periaqueductal gray; PIN, intralaminar thalamic nuclei; PL, prelimbic division of the mPFC; TEA, temporal association cortex; VMH, ventromedial hypothalamus.
Inputs to Lateral Amygdala
Morozov et al. (2011) found that projections from the temporal association cortex (TeA) to the LA receive feed-forward inhibition from GABAergic lateral ITC (ITCl) neurons in the external capsule, which was relieved by blockade of GABAergic transmission or removal of the external capsule. Anterior cingulate cortex (ACC) projections to the LA, however, received no such feed-forward inhibition55. This suggests that inputs from different regions receive heterogeneous inhibitory controls that might be differentially modulated during learning.
The hippocampus is necessary for encoding contextual elements of fear conditioning and some information flow is directed through the entorhinal cortex (EC)56. When interrogated optogenetically, strong glutamatergic projections from the BLA to the EC were confirmed. Interestingly, inhibition of these terminals during training was sufficient to block contextual fear learning even though this pathway is not necessary for the expression of contextual fear57. This confirms that unique combinations of activity are necessary for the encoding, expression and extinction of learned fear.
Examining the cortical regions involved in auditory processing of a CS, Nomura et al. (2015) demonstrated that unilateral optical inhibition of the auditory cortex is sufficient to act as a CS for both positive and negative valence training paradigms58. This study highlights the need to consider interoceptive stimuli as possible confounding variables in studies utilizing optogenetic activation and silencing manipulations. In another study, optogenetic activation of sensory inputs to the LA from the medial part of the medial geniculate nucleus (MGn) and secondary auditory cortex (AuV) paired with a foot shock was sufficient to act as a CS during fear conditioning. Additionally, optogenetic reactivation of these sensory inputs to the LA during testing sessions was sufficient to produce spontaneous freezing59. Direct activation of LA neurons is sufficient to act as a marginal US in the absence of any aversive stimulus when paired with a CS60, thus confirming that US induced activation of LA neurons is involved in associative fear learning, while also highlighting that non-specific activity is not sufficient to form strong associative memories.
Studies focused on Basolateral Amygdala
Limited work examining LA-BA-CeA connectivity using optogenetics has been completed as the close proximity of these nuclei makes exclusive targeting difficult. Tye et al. (2011) demonstrated that activation of BLA terminals in the CeA was sufficient for acute anxiolysis while inhibition was anxiogenic. Interestingly, these results were not recapitulated by activation of somata in the BLA29,61 This confirms the presence of direct projections from the BLA to the CeA without determining their function in the greater context of the circuit. In rats using an inhibitory avoidance task, optical stimulation or optical inhibition of the BLA for 15 minutes after training greatly enhanced or blunted the retention of that learning respectively62. These data confirm the BLA is involved in the consolidation of fear and anxiety-related emotional learning.
A study from Namburi et al. (2015) attempted to more clearly define the role of different projections from the BLA in valence specific behaviors. Retrograde transported fluorescent beads (retrobeads) were infused into the CeA or nucleus accumbens (NAc) of mice trained to associate a tone with an aversive foot shock or a rewarding sucrose delivery. Using whole-cell patch clamping, the authors found that NAc projecting BLA neurons exhibited synaptic strengthening following training to a rewarding cue and synaptic weakening in response to aversive cue training. Conversely, CeA projecting BLA neurons experienced synaptic strengthening after an aversive training and weakening after reward training. Using a similar approach with a rabies virus to retrogradely express ChR2 in NAc or CeA projectors, the authors found that stimulation of NAc projecting cell bodies was sufficient to support appetitive optical intracranial self-stimulation. Conversely, optical activation of CeA projecting cell bodies supports aversive real time place aversion. Additionally, optically inhibiting CeA projecting BLA neurons mildly blunted fear acquisition and supported reward conditioning63.
In this same study by Namburi et al. (2015), following the functional dissection of CeA vs. NAc projecting BLA neurons, cell bodies were then manually dissociated and collected based upon their projection specific uptake of retrobeads. RNA from these cells was sequenced and several genes specifically upregulated in CeA projectors vs. NAc projectors were uncovered63,64. This publication is an excellent example interrogation of cell populations in a projection specific manner.
Additional evidence that target specific projections from the BLA may play a role in the consolidation of select types of memory comes from Huff et al. (2016). The authors activated or inhibited projections from the BLA to the vHPC during a modified contextual freezing conditioning task so as to determine whether these projections are necessary for encoding context or foot-shock memory. In this task animals were placed in conditioning context A on day 1 then on day 2 placed in context A immediately foot shocked and removed. This training paradigm appears to separate consolidation of contextual memory on day 1 from foot-shock memory on day 2. Interestingly, activation of these projections following contextual training had no effect upon fear memory; however, activation following foot-shock enhanced fear learning. This suggests that afferents from BLA to vHPC may be primarily involved in encoding aversive, but not contextual elements of fear conditioning65.
Studies focused on medial Prefrontal Cortex
A number of groups have used optogenetics to confirm the differential roles of the reciprocal projections from the PL and IL of the mPFC to the amygdala in fear expression and fear extinction, respectively66. The PL is involved in the expression of fear following conditioning while the IL is involved in the expression of extinction to a specific cue67-72. In a foundational piece of work using precise, limited infusions of GABAA agonist muscimol Sierra-Mercado et al.(2011) demonstrated that inactivation of the PL during fear extinction blocked fear expression; however, fear extinction, as measured 24-hours later was not affected21. Conversely, when the IL was temporarily inactivated during fear extinction no effects were observed on fear expression; however, the next day there was significant deficit in extinction learning observed. Taken together this data demonstrate that the PL is necessary for fear expression while the IL is necessary for fear extinction.
In rats and mice, optical activation of glutamatergic neurons in the IL during fear extinction was found to blunt fear expression and enhance extinction; conversely inhibition of the IL blocked fear extinction67,73. In rats, optical inhibition of excitatory neurons in the IL during extinction retrieval or extinction expression had no effect on freezing, suggesting that consolidated extinction memories are stored elsewhere and the IL may not be necessary for their expression67. Opposing this result is work in mice demonstrating that unilateral inhibition of all neurons in the IL is sufficient to blunt extinction recall while activation of excitatory neurons is sufficient to enhance extinction expression68. There may be some species differences in the specific projections between the mPFC and amygdalar nuclei to account for these differences; however, taken together these studies confirm the important role of the IL in extinction and highlight the need for its continued study72,74.
The Central Nucleus of the Amygdala
Ciocchi et al. (2010) demonstrated that optical activation of the CeM is sufficient to drive spontaneous freezing while inactivation of the CeL was likewise sufficient to drive unconditioned freezing75. This confirms the role of the CeM as a main output nucleus in the fear pathway under the inhibitory control of CeL. Activation of BLA inputs to the CeA is sufficient to acutely suppress anxietylike behavior as measured in the open-field test, while inhibition increases those behaviors. Activation of BLA projections to the CeA increases activity in CeL neurons and causes feed-forward inhibition of CeM neurons61. These studies confirm the known circuitry for BLA to CeL to CeM and suggest that more complex control mechanisms maybe in place based on evidence that the direct activation of BLA somata did not elicit the changes in anxiety-like behaviors that stimulation of projections alone did.
The Intercalated Cell Masses
Although excellent work examining activity and plasticity in ITC with fear learning has confirmed their role as dynamic regulators of information flow between nuclei, optogenetic characterization of the ITC has proven difficult on account of their small size and distribution27. Kwon et al. (2015) recently performed an in-depth characterization of the dorsal ITC (ITCd), which receive strong inputs from the LAdl. Performing either weak or strong fear conditioning, the authors found learning-related strengthening of GABAergic inputs onto ITCd only after weak fear conditioning, suggesting that the ITCd is involved in gating sub-threshold behavioral learning. This plasticity is dependent upon dopamine receptor 4 (D4) and blockade of D4 or knock-down with shRNA is sufficient to transform previously subthreshold training into supra-threshold trainings, greatly enhancing fear expression. Interestingly, treatment of animals with corticosterone precipitates PTSD-like enhancements in fear learning and blocks ITCd plasticity, suggesting that during stress, previously subthreshold learning is not gated by ITCd, thus allowing for its consolidation and enhancement of fear responses76.
The ITC represents an intriguing target for cell type specific manipulations. Expressing the mu opioid receptor (MOR), dopamine receptor 1 (D1), and forkhead box protein 2 (FoxP2), these islands have a wealth of targets for transgenic approaches77. Work by Likhtik et al. (2008) in rats used dermorphin, a peptide that is a high affinity agonist of MOR, conjugated to a toxin, saporin, to selectively ablate medial ITCs (mITC). Medial ITC's provide feed-forward inhibition to the CeA and are located at the BLA-CeA border. Behaviorally, rats were fear conditioned and extinguished followed by ablation of mITC. When tested for extinction retention a week later, peptide-toxin infused rats exhibited significant deficits in extinction expression when compared to scrambled controls. This suggests that the mITC are necessary for the retention and/or expression of fear extinction24. The success of this cell-type specific manipulation suggests that with additional tools selective, non-ablative manipulation of the ITCs is possible.
Bed Nucleus of the Stria Terminalis
The BNST, a core element of the ‘extended amygdala’ has been noted for its crucial role in sustained fear and anxiety-like behavior; in fact it may act as a back-up for producing many of the same behavioral outputs often attributed to the amygdala44. Limited optical analysis of direct connections between amygdala and BNST has been done to date. Kim et al. (2013) found that optically stimulating glutamatergic BLA inputs to the anterior dorsal BNST (adBNST) elicited strong anxiolytic-like behavior. Conversely, optical inhibition of these populations is anxiogenic as measured with the elevated plus maze task. Anxiolytic behaviors are likely induced by activation of feed-forward inhibition from adBNST to oval BNST78. This study hints at a potentially complex interplay between core and extended amygdala function that may come to light with future study.
3. Search for the memory engram
While the studies described above confirm the basic circuitries involved in fear responses and fear learning, many fundamental questions about these processes remain. As it appears select ensembles of neurons, not entire nuclei, are involved in the encoding of distinct memories; one major area of investigation has been to discover how these ensembles are recruited and whether they are static over time. This line of research, when combined with next cell-type specific techniques, may prove to be a more efficient avenue to discover behaviorally relevant subpopulations than the candidate gene approach now utilized.
Building on foundational research demonstrating that distinct ensembles of neurons encode memory traces of unique contexts more recent work has focused on labeling neurons during different experiential epochs79. Reijmers (2007) et al. introduced a transgenic line known as the Tet-tag mouse that allows for the activity dependent tagging of neuron populations. The Tet-tag mouse system utilizes tetracycline transactivator (tTA) protein expression driven under the c-fos promoter and tetracycline response element (TRE) control of lacZ to permanently mark neurons active during a specific time period. The labeling period is determined by when the experimenter removes doxycycline from the mouse's diet. Doxycycline blocks binding of tTA to the TRE so, removal of doxycycline allows binding of tTA to the TRE. The labeling period is then closed by returning the mouse to doxycycline chow, which inhibits the function of tTA. Using this system, Reijmers et al., confirmed that BLA neurons active during fear conditioning are subsequently reactivated during fear recall80. This result has been confirmed in many areas using both appetitive and aversive training paradigms81. These data suggest that stable networks of neurons within previously described nuclei are consistently recruited for the encoding and expression of a learned fear behavior.
It is auspicious to use this work as a springboard for understanding many of the current efforts in the study of learning and memory to determine which cell populations are recruited for select elements of fear behaviors. Efforts to illuminate distinct cell populations that regulate select fear behaviors must consider not only the different genetically defined populations within nuclei, but also the internal determinants within a neuron that promote its recruitment to a memory trace. Furthermore, these factors likely differ between brain regions.
Within the hippocampus, much progress has been made towards labeling individual place memory ‘engrams’ (or physical manifestations of stored memory trace) using the Tet-tag system. This system may be used to produce ChR2 (or any transgene) in neural populations active during a certain training period. These populations may then be reactivated or silenced in an alternate context or any number of other experimental conditions. In a series of papers (Ramirez et al. 2013, Redondo et al. 2014, Ryan et al. 2015), the Tonegawa group performed an in-depth analysis of engrams formed in the HPC and the BLA during either negatively and positively valenced activities such as contextual fear conditioning and mating. Together these studies demonstrated that labeling a portion of the neurons in the dentate gyrus (DG) or BLA that are active during contextual fear conditioning with ChR2, and subsequently reactivating them later, results in light-induced freezing in a naïve context. Conversely activating the engram of a neutral context in an aversively trained context interferes with context-elicited freezing, thus suggesting that the simultaneous activation of multiple place engrams causes mixed behavioral responses. Similar patterns were found when looking at engrams generated during appetitive tasks such that reactivation of appetitive engrams caused place preference in a neutral context. Interestingly, when engrams encoded in contexts paired with an aversive or appetitive task are reactivated during retraining with tasks of the opposite valence, DG engrams could be recoded to be associated with a new valence while BLA engrams continued to code for behavioral outputs consistent with the valence of the original conditioning. Finally, memories that were formed during contextual fear conditioning may be blocked by inhibiting protein synthesis with the drug anisomycin directly after training or reconsolidation; however, the reactivation of engrams formed during that training session still elicited freezing. This distinction suggests that the content of an engram may be represented in its pattern of projections while the encoding and retrieval of a memory requires molecular processes underlying memory consolidation82-85.
Trouche et al. (2016) used a similar system to express Arch, an inhibitory opsin, in a hippocampal place engram and observed several interesting phenomena. In an experimental context (A), neurons originally labeled during encoding of that place engram increased their firing in response to re-exposure to context A, while another population exhibited firing suppression. When tagged neurons were silenced in context A, an alternative population was found to compensate and increased firing to context A; behaviorally, mice with silenced engrams acted as if they were in a new context. Over six days of trials the alternative ensemble created a second engram to that first elicited by context A. Importantly, if context A was initially paired with cocaine this remapping protocol abolished cocaine conditioned place preference, thus blocking the recall of the initial association between context A and cocaine administration. These observations contain important suggestions that HPC engrams are not fixed and that previously associated place memories may be altered to subsequently rid the subject of previously acquired associations86.
Complementing these findings, work from Josselyn and colleagues has demonstrated that memory traces are not necessarily allocated to pre-determined ensembles of neurons within a nucleus. Allocation is based upon naturally oscillating expression levels of CREB, which bias neural ensembles towards being recruited to an engram in an excitability dependent manner. Neurons that have high levels of CREB at the time of training are more likely to be recruited to a memory engram87,88. CREB increases neuronal excitability and many of the molecular processes underlying synaptic plasticity and memory consolidation. By experimentally increasing levels of CREB or neuronal excitability using optogenetics or DREADDs in a sub-population of neurons of the LA, Yiu et al., (2014) were able to increase targeted neuronal recruitment into a memory trace. Both optogenetic and chemogenetic manipulations also increased the strength of the memory as measured by the ability of a context to elicit conditioned freezing during a fear expression session88.
4. Cell Type Specific Targeting of Behavioral Processes
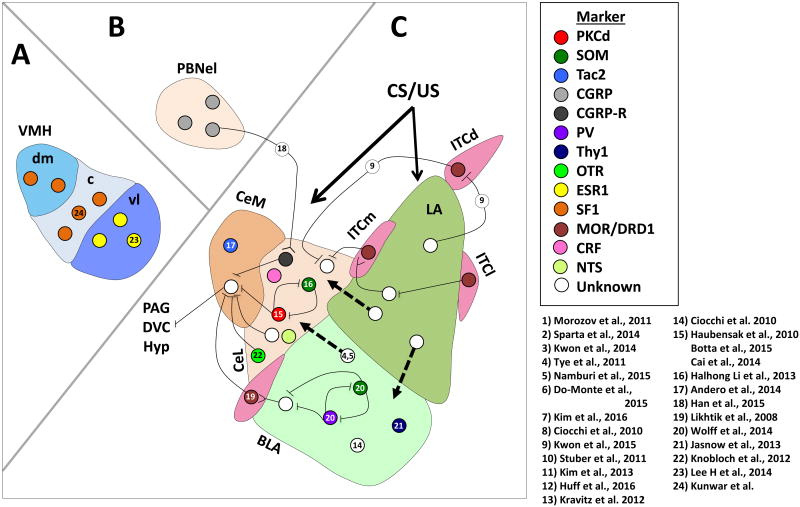
An understanding of the neural circuits underlying behavior is clearly valuable for the study of the biology of learning and memory as highlighted in the above sections. However, without translationally tractable strategies for identifying targets to modulate fear responses and learning in humans, the value of further dissection of this circuitry will remain somewhat esoteric. One promising strategy is the manipulation of genetically defined neuronal populations whose global modulation may have beneficial results in the regulation of specific behavior or learning processes. Here we will review a number of papers that utilize cell-type specific techniques to interrogate neural cicruits underlying behavior; for a summary of papers highlighted please see Table 2 and for a schematic of described populations and projections see Figure 2.
Table 2.
Description of publications using cell-type specific methodologies to query fear related circuitry.
| Publication | Investigated Circuitry |
|---|---|
|
| |
| Kravitz et al. (2012) | Optical activation of D1 direct/D2 indirect pathway supports place preference/place avoidance. |
| Ciocchi et al. (2010) and Haubensak et al. (2010) | Identified PKCd + population as decreasing firing during fear conditioning, relieving inhibition of PAG projecting CeM population, supporting fear expression. |
| Botta et al. (2015) | Activity in CeL PKCd population supports fear generalization and tonic activity in these neurons is dynamically regulated by extrasyaptic α5-GABAAR. |
| Cai et al. (2014) | Activation of CeL PKCd neurons is acutely anxiolytic. |
| Halhong Li et al. (2013) | SOM+ neurons of CeL represent opposing population to PKCd population; increasing activity with fear learning. Activity in these neurons is sufficient to support spontaneous freezing. |
| Andero et al. (2014) | CeA Tac2 neurons are necessary for fear acquisition. Antagonism of Tac2 receptor is sufficient to block fear consolidation. |
| Han et al. (2015) | PBN→ CeA transmits US information. Inhibition of PBN CGRP neurons blocks FC while activation is sufficient for generation of fear responses. |
| Likhtik et al. (2008) | Ablation of ITCm is sufficient to impair expression of extinction. |
| Wolff et al. (2014) | PV and SOM neurons in the BLA create disinhibitory circuit gating cortical and thalamic inputs to principal neurons. |
| Jasnow et al. (2013) | Activation of BLA Thy1-ChR2 population is sufficient to block fear acquisition and enhance fear extinction. |
| Knobloch et al. (2012) | Activation of hypothalamic OT fibers in CeL is sufficient to increase feed-forward inhibition of CeM in an OT dependent manner. |
| Lee H et al. (2014) | ESR1 neurons in the VMHvl generate investigative/mounting/attack behaviors in an intensity/recruitment dependent manner. |
| Kunwar et al. (2015) | SF1 neurons of the VMHdm/c generate freezing/escape behaviors in an intensity/recruitment dependent manner. |
| Huff et al. (2016) | Activation of BLA→ vHPC projections is sufficient to support aversive learning, but not contextual learning. |
Figure 2. Microcircuits and specific neuronal populations in the amygdala, ventromedial hypothalamus (VMH) and parabrachial nucleus (PBN) involved in fear and anxiety-related behaviors.
A) Microcircuits and cell populations in the ventromedial hypothalamus. B) PBN projections to the CEA. C) Amygdala microcircuits and subnuclei. Known microcircuits discussed in the present review are noted; dashed black arrows denote projections between amygdala subnuclei. Forked lines indicate glutamatergic projections whereas crossed lines indicate GABAergic projections. BLA, basolateral amygdala; c, central division of the ventromedial hypothalamus; CEAm, medial subdivision of the central amygdala; CEAl, lateral subdivision of the central amygdala; CGRP, calcitonin gene-related peptide; CGRP-R, calcitonin gene-related peptide receptor; dm, dorsal medial division of the ventromedial hypothalamus; DVC, dorsal vagal complex; ESR1, estrogen receptor; Hyp, hypothalamus; ITC, intercalated cell nuclei; ITCd, dorsal intercalated cell nuclei; ITCl, lateral intercalated cell nuclei; ITCm, medial intercalated cell nuclei; MOR, mu opioid receptor; OT, oxytocin; PAG, periaqueductal gray; PBNel, external lateral subdivision of the PBN; PKCd, protein kinase C delta; PV, parvalbumin; SF1, steroidogenic factor 1; SOM, somatostatin; Tac2, tachykinin 2; vl, ventrolateral division of the ventromedial hypothalamus; VMH, ventromedial hypothalamus.
The majority of studies mentioned thus far have focused on differences between ‘genetically defined’ glutamatergic or GABAergic sub-populations between nuclei; however, it has become obvious that not all excitatory and inhibitory neurons are created equal. In work by Herry et al. (2008), multiple excitatory cell populations in the BA that differentially respond to fear expression vs. fear extinction were found in actively behaving mice. One population was found to increase its firing rate in response to the presentation of the CS directly after auditory fear conditioning and then to decrease firing as the CS-US association was extinguished; these identified neurons were functionally labeled as “Fear ON” neurons, whose activity supports fear expression. Another distinct population was found to have little activity in response to presentation of the CS just after FC but instead increased activity as the CS-US association was extinguished; these were accordingly labeled “Fear OFF” or “Fear Extinction” neurons, whose activity supports the suppression of fear behaviors. Interestingly, “Fear ON” neurons were found to receive inputs from the vHPC and project to the mPFC while “Fear OFF” neurons had only reciprocal connections with the mPFC. Finally, the selective inactivation of the BA with muscimol prevented both fear extinction and fear renewal, suggesting that the BA is necessary for signaling behavioral transitions rather than the storage of fear memories themselves89.
This study set firm ground-work by demonstrating that within previously identified nuclei, such as the primarily glutamatergic BA, there are sub-populations of neurons that have divergent roles in behavior and learning. Unfortunately, without knowing the genetic identities of these neuron populations, it is impossible to selectively manipulate them during behavior. In order to uncover more specific, targetable populations, it will be necessary to identify additional, less globally expressed, sub-population markers, specifically genes or proteins that are differentially expressed in the population of interest compared to other neurons.
A retrospective example of this type of strategy may be observed in the modulation of the direct and indirect pathways of the striatum. The striatum is well known for its role in informational integration and motor control. This system relies upon global modulation by dopamine; direct pathway neurons express dopamine receptor 1 (D1), a Gs-coupled GPCR, while indirect pathway neurons express dopamine receptor 2 (D2), a Gi-coupled GPCR90. In the case of Parkinson's disease, rebalancing this striatal system by increasing global dopamine with L-DOPA administration is a palliative approach. The differential expression patterns within these two pathways has allowed for these circuitries to be directly manipulated using optogenetics as demonstrated by Kravitz et al. (2012). Using different promoter-cre mouse lines to virally express ChR2 specifically in either the direct or indirect pathway neurons, the authors demonstrated that activation of the direct pathway is reinforcing while activation of the indirect pathway is punishing as measured with place preference or place avoidance tasks91. Taken together these studies demonstrate the feasibility of identifying genetically-defined cell populations that differentially support aversive and appetitive behavior. In the present section, we will examine genetically identified cell populations within the amygdala (both core and extended regions) and related areas that have confirmed roles in fear behaviors.
Differential Molecular Markers of Central Amygdala Cell Types: PKCd, Sst, and Tac2
Recently, a growing number of inhibitory microcircuits have been reported. These circuits often function through mutual inhibition where the inhibition of one inhibitory population by another leads to the disinhibition of a third ‘output’ population that reads out the signaling tone of the circuit. These types of circuits are especially fruitful as several cell-type specific markers for sub-populations of inhibitory neurons have been described.
To interrogate the micro-circuitries of the CeA, Ciocchi et al. (2010) and Haubensak et al. (2010) used single unit recordings to interrogate population firing in the CeL of awake behaving mice. The authors identified two populations of neurons whose activity changed after fear conditioning; one that increased firing in response to the CS (CeLON, ∼ 30%) and another that decreased firing during the same period (CeLOFF, ∼ 25%). These populations were further found to be mutually inhibitory. The CeLOFF population was found to project to and inhibit a CeM population projecting to the PAG, a region associated with the behavioral freezing responses during fear expression. Importantly this CeLOFF population expressed a relatively cell-type specific serine- and threonine-kinase gene, protein kinase C delta (PKCd), thus allowing for genetic targeting and manipulation of this population, which lead to confirmation of its role within the CeM fear controlling circuitry underlying fear conditioning behavior75,92.
Pursuing the observation that increases in tonic activity in PKCd-expressing (PKCd+) neurons strongly correlate with fear generalization, Botta et al. (2015) examined the contributions of PKCd+ neurons to acute fear responses and anxiety-like behaviors. Following a discriminative training protocol where the US is paired with one CS (CS+), but not another CS (CS-), PKCd+ neurons were activated using optogenetics during alternate CS+/CS- presentations. Optical stimulation drove fear generalization as measured by an increase in the ratio of freezing to CS- / CS+ stimuli. Optical stimulation of PKCd+ neurons was also accompanied by increased anxiety-like behaviors as measured by decreased time spent in the open arm of an elevated-plus maze (EPM) and decreased time spent in the center of an open field. These behavioral changes were attributed to excitability changes driven by α5 subunit containing GABAA receptors located on the extra-synaptic dendritic region. Increased tonic activity of PKCd+ neurons caused by a reduction in extrasynaptic inhibition after fear conditioning was associated with decreased α5-GABAAR mediated conductance, and furthermore this change was significantly correlated with anxiety-like behaviors in the EPM. Finally, cell-type specific knock-down of α5-GABAAR with a shRNA was sufficient to increase anxiety-like behavior and fear generalization75,92-94. These results suggest overlap between the circuits mediating anxiety-like behaviors and the generalization of cued fear behaviors.
An important clue as to the identity of the observed PKCd-, CeLON population, comes from Halhong Li et al. (2013). Somatostatin (SOM+) neurons located within the CeL are largely non-overlapping with PKCd+ neurons (∼13% overlap). At basal conditions, excitatory input from the LA onto SOM+ neurons is comparatively weak compared to SOM- populations; however, after fear conditioning this relationship switches; consistent with enhanced excitatory drive after learning. Interestingly, selectively silencing of SOM+ neurons with a Gi-DREADD during fear conditioning abolished this switch and blunted fear acquisition, thus suggesting that post-synaptic activity is required for the observed synaptic strengthening and that this switch is necessary for fear learning. Mutual inhibition between the SOM+ and SOM- (partially PKCd+) populations was uncovered. Finally, optical activation of SOM+ neurons was sufficient for the generation of spontaneous freezing in naïve animals while optical inhibition was sufficient to block freezing during a fear expression test95. This study identifies SOM+ neurons of the CeL as containing a complementary population to the PKCd+ population in the CeL disinhibitory circuit controlling CeM output. SOM+ neurons inhibit PKCd+ neurons during fear conditioning, allowing for increased activity in the CeM and the expression of fear behaviors.
The tachykinin 2 (Tac2)-expressing cell population, appears to be found in both the CeL and CeM, depending upon anterior-posterior position of reference. At more posterior locations within the CeL, Tac2 mRNA expression partially overlaps with that of both somatostatin (Sst or SOM) and corticotrophin releasing factor (Crf), but not Prkcd (PKCd); however, more anteriorly, the large CeM Tac2 population is expressed in an independent population (unpublished data). Andero et al. (2014) recently identified Tac2 as a dynamically regulated gene whose expression rapidly rises after fear conditioning, and returns to baseline by 2 hours post training. After fear conditioning, the protein product of Tac2, neurokinin B (NkB), is strongly upregulated. Notably, intra-amygdala application of an NkB receptor (Nk3R) antagonist, osanetant, blunts fear consolidation when given directly following fear conditioning. Over-expression of the Tac2 gene is sufficient to enhance fear learning, and this manipulated enhancement can be blocked with the Nk3R antagonist. Finally, silencing Tac2-expressing neurons in the CeA during fear conditioning using Gi-DREADD is sufficient to blunt fear acquisition. This study identified the Tac2 and Nk3R expressing populations as excellent targets for cell-type specific manipulation of fear learning and behaviors, which may be particularly interesting in their role in the output nuclei of the CeA96.
The Parabrachial Nucleus and Calcitonin Gene-Related Peptide
So far we have exclusively discussed thalamic inputs to the LA as the major contributors of US information to the CeA. Recently, Han et al. (2015) examined an alternative US input pathway to the CeA; a circuit from parabrachial nucleus (PBN) to the CeL was found to also transmit information regarding the US. Han et al. found that the external lateral subdivision of the PBN (PBel) expressed high levels of Calca, the gene encoding for calcitonin gene-related peptide (CGRP), which regulates pain transmission and can directly produce unconditioned freezing when infused in the CeA. Using cre-dependent tetanus toxin expression to silence synaptic transmission in PBel CGRP neurons throughout contextual fear conditioning and subsequent expression tests, the authors demonstrated that silencing these neurons in the PBel was sufficient to decrease freezing in all phases of contextual fear conditioning and expression, suggesting that these inputs to the CeL are necessary for learning in response to painful stimuli. Mice in which PBel CGRP neurons were silenced had normal withdrawal responses from nociceptive stimuli; however, escape behaviors and freezing were reduced suggesting that nociception was normal, but behavioral responding to painful stimuli was blocked. Optogenetic activation of PBel CGRP neurons was also sufficient to drive both context and auditory-cued fear conditioning when used as a US during training. Finally, targeting the CGRP receptor (CGRPR) expressing population of the CeL, the authors demonstrated that activation of these neurons was sufficient to create generalized fear responding when used as the US in contextual and cued fear conditioning97. This work highlights the observation that the canonical thalamic route for US information to the CeA must be updated to include information flow from the PBN. Furthermore, both the CGRP and CGRPR cell populations may be amenable to cell-type specific modulation, an interesting avenue for further investigation.
BLA inhibitory neuronal sub-populations: PV and SOM
Within the basolateral amygdala, several cell-type specific targets have been discovered. Wolff et al. (2014) identified a partial inhibitory micro-circuit within the BLA demonstrating some similarities to inhibitory circuits in the CeA. In this study, the selective activation or inhibition of the parvalbumin expressing (PV+) population specifically during the US presentation of fear conditioning blocked or enhanced fear learning to a CS, respectively. Combined with work demonstrating that inhibition of PV+ neurons leads to enhanced excitability in principal neurons, these data suggest that the selective modulation of the PV+ neuronal population may be necessary for fear learning. In awake behaving mice, the authors further observed spike suppression of PV+ neurons during US presentation confirming the physiological relevance of optogenetic manipulations. Interestingly, when looking at CS-induced activity, the authors observed the opposite pattern of activity wherein PV+ neurons increased their responding to the CS. Furthermore, optogenetic activation of PV+ neurons during the CS, but not US, actually enhanced fear learning. This prompted the discovery of a polysynaptic disinhibitory circuit including somatostatin positive (SOM+) populations whereby during CS presentation, PV+ neurons increase activity, inhibiting SOM+ neurons, thus leading to disinhibition of principle neurons receiving cortical or thalamic auditory inputs93. These data align well with an additional disinhibitory circuit found in the auditory cortex also involving PV+ neurons98. Notably, these types of disinhibitory circuits have been discovered in many areas of the brain suggesting that disinhibition may in fact be a major mechanism of associative learning and memory99. It is possible that globally manipulating the tone of such inhibitory circuits may provide a possible therapeutic method for many associative learning disorders; however much remains to be understood about GABAergic regulation, oscillatory networks, and different interneuron populations for such approaches to be feasible in a reliable and predictive manner.
Thy1-population of pyramidal BA neurons
Given the great success with targeting inhibitory populations in the amygdala, equal success might be expected from excitatory populations; however, to date comparatively few of these have been uncovered. Jasnow et al. (2013) described a BA population marked by the Thy1.2 promoter cassette derived lines: Thy1-ChR2 line 18 and Thy1-eYFP line H. These lines mark a common developmental population originating from the pallial zones of the telencephalon100. From an evolutionary perspective, populations with common developmental origins are likely to have complementary roles especially those generating neocortical circuits often implicated in top-down regulation of older striatal-like populations such as the CeA101. Using these transgenic lines the authors demonstrated that this BA Thy1 population was entirely glutamatergic and, within the temporal lobe, localized almost exclusively within the anterior BAa. Optical activation of this population during presentation of the US blocks the consolidation of fear learning. Likewise optical activation of the Thy1 population during presentation of the CS during extinction dramatically enhanced extinction consolidation. Finally the authors found that activation of BA Thy1-ChR2 neurons generated polysynaptic feed-forward inhibition of evoked excitatory potentials in the CeM generated by electrical stimulation of the LA102. Taken together these data confirm the presence of functionally segregated glutamatergic populations within the BA, that putatively may align with the functionally defined FearExtinction population defined (and discussed above) by Herry et al 89. These data further highlight the need for the generation of additional cell type specific markers in this area.
Hypothalamic sub-populations: OT, ESR1, SF1
Originating in the hypothalamus, oxytocin (OT) expressing neuronal inputs projecting into the CeA have been shown to play important roles in modulating distinct elements of fear behaviors103,104. Knobloch et al. (2012) demonstrated in rats that activation of glutamatergic fibers from OT expressing hypothalamic nuclei elicit co-release of oxytocin onto CeL neurons and also increase inhibition of CeM populations in an OT dependent manner. Importantly, activation of OT fibers was sufficient to block context dependent freezing in previously contextually fear conditioned rats57,105. This study highlights the importance of extra-amygdala populations in fear behaviors and encourages a broadening of our view of possible cell type specific targets.
Another possible target for cell-type specific modulation is the estrogen receptor 1 expressing (ESR1+) population of neurons that is enriched in the ventrolateral division of the ventromedial hypothalamus (VMHvl), medial amygdala (MeA) and BAp. Lee H et al. (2014) recently identified the ESR1+ population in the VMHvl as being active during aggressive behaviors between male mice. Cell-type specific strong optical activation of this ESR1+ population or ESR1- population elicited either attack or no behavioral change, respectively, in males in the resident intruder task. Optical inhibition of the ESR1+ population was sufficient to rapidly block or stop an aggressive encounter. The authors observed that low intensity stimulation or low viral infection efficiencies were sufficient to provoke mounting or close inspection of both male and female intruders by male mice and that by increasing the intensity of photostimulation or number of neurons infected, these behaviors could be transitioned to attack behaviors. Together these experiments suggest that ESR1+ neurons of the VMHvl control a range of social interaction behaviors in a recruitment-dependent manner47. This study begins to demonstrate the wealth of extra-amygdalar targets for modulation of a variety of defensive behaviors. Furthermore, it suggests the importance of understanding the role of the BAp ESR1+ cell populations. As fear-related disorders in humans encompass a wide variety of perturbed and dysregulated behaviors, these targets may be of great translational value, and may be an important target in understanding sex differences in emotion-related behaviors.
Another genetically identified subpopulation found to be intimately involved in social behaviors was found by Kunwar et al. (2015). The steroidogenic factor 1 (SF1+) population of the dorsal medial and central ventromedial hypothalamus (VMHdm/c) is non-overlapping with the previously discussed ESR1+ population. Optical stimulation of SF1+ neurons causes freezing behaviors and occasional activity bursts similar to those observed in escape behaviors. These behaviors had a similar dependency on stimulation intensity as the ESR1+ populations; higher intensity stimulation, higher frequency stimulation or increased numbers of virally infected neurons more often generated activity bursts. Interestingly, very low intensity stimulation was found to be aversive and precipitated conditioned place avoidance. Additionally, SF1+ stimulation produced persistent defensive behaviors, anxiety-like behaviors and elevations of serum corticosterone. Finally, genetically targeted ablation of SF1 neurons blunted predator avoidance and anxiety-like behaviors46. This study demonstrates that the SF1+ is intimately involved in aversive and anxiety-like behaviors and represents a tractable target for cell-type specific modulation of fear and anxiety-related behaviors.
Alternative targets
In addition to the populations discussed above, several other promising gene targets, which to this point have remained out of reach or incompletely characterized, may now be accessible for future pursuit. Many neuropeptides have extensive literatures associating them with behavioral learning106. The corticotrophin releasing factor (CRF) population of the CeL has yielded several clues to its role in behavior suggesting that activity in this population may support fear learning107. Neuropeptide S (NPS) appears to exert strong anxiolytic influences on the amygdala and supports fear extinction through its receptor (NPSR1). NPSR1 has strong expression specificity in the medial aspect of the BAa and the LAdl108. Interestingly, in humans, polymorphisms in the NPSR1 and 5HTTLPR genes epistatically confer risk of enhanced startle responses in anxiety-promoting contexts109. An analogous NPSR1 SNP to that found in humans was also recently found in mice and rats bred for high anxiety traits; this SNP increases GR responsiveness of gene transcription110. These are just a few of the large number of identified pathways that participate in behavioral modulation that are ripe for analysis with cell-type specific tools.
Connections between the BA and the NAc have long been implicated in supporting reward learning and responding to previously reward-paired cues; however, much less attention has been paid to this connection in the context of fear learning43. Stuber et al. (2011) directly investigated this connection via viral infection of BLA cell bodies followed by optical manipulations of terminals in the NAc. Optical stimulation of BLA terminals in the NAc was sufficient to support intracranial self-stimulation (ICSS) and ICSS was prevented with blockade of D1 receptors, suggesting that BLA afferents synapse selectively on D1 expressing neuronal populations41,43. These results suggest a variety of roles for the BLA across motivated behaviors. Although these projections have mostly been studied in light of appetitive tasks, they may play a crucial role in fear extinction by rebalancing the valence assigned to a previously learned association.
6. Cell type specific transcriptome sequencing
In the case of several cell-type specific markers mentioned above, direct manipulation of the protein product of the identifier gene is possible; however, in most cases this is either impossible or translationally impractical. In these cases it is necessary to identify additional pharmacologically tractable targets for remote control of these populations in a closed system. To efficiently molecularly phenotype these populations the most expedient route is cell-type specific RNA sequencing.
Guez-Baber et al. (2011) reported a strategy (see 111 for protocol) for the isolation of striatal neurons expressing c-fos after cocaine exposure in rats. Through this process, neurons are rapidly dissociated, fixed and sorted using fluorescence activated cell sorting (FACS). Collection and sequencing of high quality RNA from sorted samples allows for either activity dependent or cell type specific interrogation of neuronal RNA content112. This protocol has since been adapted for cell-type specific RNA interrogation to great success. This method has the advantage that it allows for the comparison of the cell population of interest compared to all other neurons, as well as for the rapid collection of large numbers of cells. Other methods of cell-type specific RNA isolation do not allow for the collection of control RNA specifically from marker-negative neurons112. Additionally, FACS is a valuable tool when combined with mouse lines expressing transgenes under activity dependent promoters (ex. the Tet tag mouse described in earlier sections). In the case of the Tet tag mouse, neurons active during the dox-off period will express Beta-galactosidase; alternatively neurons labeled acutely by cfos-shEGFP may be collected within a few hours. Both of these labels may be targeted and used as fluorescent markers for FACS113. Alternatives to FACS to achieve similar ends include manual cell-sorting63,114, laser-capture microdissection115,116, and single cell expression analysis117.
Another technology that allows cell-type specific RNA interrogation is translating ribosome affinity pull-down (TRAP). This technique utilizes transgenic expression of a modified ribosomal subunit appended to GFP (L10a-GFP) to selectively pull down ribosomes and the RNAs being translating at the time of collection118. This method yields very high quality RNA and is methodologically less intensive than previously mentioned techniques such as FACS. When a conditional TRAP expressing line (e.g. Rosa26-f-s-TRAP119) is crossed with any cell-type specific promoter-cre line, the resulting double transgenic mouse will express L10a-EGFP in the population of interest. This technique may also be used in a similar activity-dependent manner to FACS sorting (Cell-type specific activity dependent interrogation necessitates a novel line or combination of previously available lines)120. However, cell-type specific RNA pull-down is not possible without the ability to genetically target populations, thus limiting its usefulness to the selection of established cre-drivers that are currently available.
In cases where genetic markers for functionally specified cell populations are not available, it is possible to interrogate gene changes in a projection-specific manner. We previously discussed Namburi et al. (2015) where the authors parsed the RNA content of CeA vs. NAc projecting BLA neurons63. To interrogate gene changes in specifically LA projecting thalamic and cortical populations Katz et al.(2015) retrogradely labeled these projecting neurons and performed laser micro-dissection of cell bodies. RNA content of these neurons was analyzed either at baseline or after fear conditioning, and the authors found projection-specific differences in gene changes121. This type of projection-specific RNA sequencing might easily be combined with FACS using retrobeads for sorting, or with TRAP by infusing a trans-synaptic transported cre virus (AAV-EF1a-mCherry-IRES-WGA-Cre, available through UNC viral vector core) into the f-s-TRAP mouse.
Summary
Cell-type specific interrogation of the behavioral and molecular profiles of select neuronal populations within the brain is likely the most expedient avenue towards the identification of selective compounds that modulate distinct circuitries involved in fear and anxiety related behaviors and associated disorders. In rodent models, optogenetics has rapidly confirmed and expanded the known neural circuitries underlying fear related behaviors. By identifying and manipulating genetically marked subpopulations of previously described nuclei, recent progress has been made towards circuit specific control of fear. In order to fully elucidate the molecular profiles of previously identified sub-populations, cell-type specific isolation may be employed to generate RNA expression profiles for these neurons. Taking this combinatorial approach, additional targets for pharmacological manipulation of fear-related populations may subsequently be more rapidly generated. Novel, cell-type specific, cognitive enhancers may provide unique avenues for the treatment of fear- and anxiety-related disorders.
Highlights.
Cell-type specific analysis of fear circuitry is necessary for translational progress.
Cell-type specific optogenetic and chemogenetic tracing of fear circuitry.
Updates on cell-type specific molecular interrogation.
Acknowledgments
Support was provided by NIH (R01MH096764) and by an NIH/NCRR base grant (P51RR000165) to Yerkes National Primate Research Center.
Footnotes
Disclosures: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacology & therapeutics. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues H, et al. Does D-cycloserine enhance exposure therapy for anxiety disorders in humans? A meta-analysis. PloS one. 2014;9:e93519. doi: 10.1371/journal.pone.0093519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeDoux JE. Coming to terms with fear. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2871–2878. doi: 10.1073/pnas.1400335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanselow MS. Conditioned and unconditional components of post-shock freezing. The Pavlovian journal of biological science. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of comparative and physiological psychology. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 6.Sousa N, Almeida OF, Wotjak CT. A hitchhiker's guide to behavioral analysis in laboratory rodents. Genes, brain, and behavior. 2006;5 Suppl 2:5–24. doi: 10.1111/j.1601-183X.2006.00228.x. [DOI] [PubMed] [Google Scholar]
- 7.Methods of Behavior Analysis in Neuroscience. 2nd. CRC Press/Taylor & Francis; 2009. [PubMed] [Google Scholar]
- 8.Picciotto MR, Wickman K. Using knockout and transgenic mice to study neurophysiology and behavior. Physiological reviews. 1998;78:1131–1163. doi: 10.1152/physrev.1998.78.4.1131. [DOI] [PubMed] [Google Scholar]
- 9.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiology of learning and memory. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 10.Myers KM, Davis M. Mechanisms of fear extinction. Molecular psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 11.Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. Journal of neurophysiology. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 12.Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrlich I, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological reviews. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. The Journal of comparative neurology. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- 16.Linke R, Braune G, Schwegler H. Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Experimental brain research. 2000;134:520–532. doi: 10.1007/s002210000475. [DOI] [PubMed] [Google Scholar]
- 17.McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 18.LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behavioral neuroscience. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- 20.Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Frontiers in behavioral neuroscience. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brigman JL, et al. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:4590–4600. doi: 10.1523/jneurosci.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millhouse OE. The intercalated cells of the amygdala. The Journal of comparative neurology. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- 24.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palomares-Castillo E, et al. The intercalated paracapsular islands as a module for integration of signals regulating anxiety in the amygdala. Brain research. 2012;1476:211–234. doi: 10.1016/j.brainres.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 26.Blaesse P, et al. mu-Opioid Receptor-Mediated Inhibition of Intercalated Neurons and Effect on Synaptic Transmission to the Central Amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:7317–7325. doi: 10.1523/JNEUROSCI.0204-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busti D, et al. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5131–5144. doi: 10.1523/jneurosci.6100-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcellino D, et al. Intercalated and paracapsular cell islands of the adult rat amygdala: a combined rapid-Golgi, ultrastructural, and immunohistochemical account. Neuroscience. 2012;226:324–347. doi: 10.1016/j.neuroscience.2012.08.067. [DOI] [PubMed] [Google Scholar]
- 29.Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Current opinion in neurobiology. 2012;22:717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Repa JC, et al. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nature neuroscience. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- 32.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in neurosciences. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 33.Gentile CG, Jarrell TW, Teich A, McCabe PM, Schneiderman N. The role of amygdaloid central nucleus in the retention of differential pavlovian conditioning of bradycardia in rabbits. Behavioural brain research. 1986;20:263–273. doi: 10.1016/0166-4328(86)90226-3. [DOI] [PubMed] [Google Scholar]
- 34.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. The Journal of comparative neurology. 1999;403:229–260. [PubMed] [Google Scholar]
- 36.McDonald AJ, Mott DD. Functional neuroanatomy of amygdalohippocampal interconnections and their role in learning and memory. Journal of neuroscience research. 2016 doi: 10.1002/jnr.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lesting J, et al. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PloS one. 2011;6:e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanselow 1998 HPC lesion.pdf.
- 39.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song C, Ehlers VL, Moyer JR., Jr Trace Fear Conditioning Differentially Modulates Intrinsic Excitability of Medial Prefrontal Cortex-Basolateral Complex of Amygdala Projection Neurons in Infralimbic and Prelimbic Cortices. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:13511–13524. doi: 10.1523/JNEUROSCI.2329-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7167–7173. doi: 10.1523/jneurosci.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain research Brain research reviews. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 46.Kunwar PS, et al. Ventromedial hypothalamic neurons control a defensive emotion state. eLife. 2015;4 doi: 10.7554/eLife.06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H, et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagel G, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Sciences. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyden ES. A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 biology reports. 2011;3:11. doi: 10.3410/B3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 51.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nature methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 52.Alexander GM, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. The Journal of clinical investigation. 2011;121:1424–1428. doi: 10.1172/jci46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacological reviews. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morozov A, Sukato D, Ito W. Selective suppression of plasticity in amygdala inputs from temporal association cortex by the external capsule. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:339–345. doi: 10.1523/JNEUROSCI.5537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitamura T, et al. Entorhinal Cortical Ocean Cells Encode Specific Contexts and Drive Context-Specific Fear Memory. Neuron. 87:1317–1331. doi: 10.1016/j.neuron.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sparta DR, et al. Inhibition of projections from the basolateral amygdala to the entorhinal cortex disrupts the acquisition of contextual fear. Frontiers in behavioral neuroscience. 2014;8:129. doi: 10.3389/fnbeh.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nomura H, et al. Memory formation and retrieval of neuronal silencing in the auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9740–9744. doi: 10.1073/pnas.1500869112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwon JT, et al. Optogenetic activation of presynaptic inputs in lateral amygdala forms associative fear memory. Learning & memory. 2014;21:627–633. doi: 10.1101/lm.035816.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johansen JP, et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tye KM, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huff ML, Miller RL, Deisseroth K, Moorman DE, LaLumiere RT. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3597–3602. doi: 10.1073/pnas.1219593110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Namburi P, et al. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520:675–678. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nieh EH, Kim SY, Namburi P, Tye KM. Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain research. 2013;1511:73–92. doi: 10.1016/j.brainres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huff ML, Emmons EB, Narayanan NS, LaLumiere RT. Basolateral amygdala projections to ventral hippocampus modulate the consolidation of footshock, but not contextual, learning in rats. Learning & memory. 2016;23:51–60. doi: 10.1101/lm.039909.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arruda-Carvalho M, Clem RL. Prefrontal-amygdala fear networks come into focus. Frontiers in systems neuroscience. 2015;9:145. doi: 10.3389/fnsys.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Do-Monte FH, Manzano-Nieves G, Quinones-Laracuente K, Ramos-Medina L, Quirk GJ. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:3607–3615. doi: 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HS, Cho HY, Augustine GJ, Han JH. Selective Control of Fear Expression by Optogenetic Manipulation of Infralimbic Cortex after Extinction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:1261–1273. doi: 10.1038/npp.2015.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arruda-Carvalho M, Clem RL. Pathway-selective adjustment of prefrontal-amygdala transmission during fear encoding. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:15601–15609. doi: 10.1523/jneurosci.2664-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Senn V, et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Cho JH, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron. 2013;80:1491–1507. doi: 10.1016/j.neuron.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riga D, et al. Optogenetic dissection of medial prefrontal cortex circuitry. Frontiers in systems neuroscience. 2014;8:230. doi: 10.3389/fnsys.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. Journal of neurophysiology. 2011;105:3054–3066. doi: 10.1152/jn.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciocchi S, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 76.Kwon OB, et al. Dopamine Regulation of Amygdala Inhibitory Circuits for Expression of Learned Fear. Neuron. 2015;88:378–389. doi: 10.1016/j.neuron.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 77.Soleiman MT. Opioid Inhibition of Intercalated Input to the Central Amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:13272–13274. doi: 10.1523/JNEUROSCI.2578-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim SY, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature neuroscience. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 80.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science (New York, N Y) 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 81.Tonegawa S, Liu X, Ramirez S, Redondo R. Memory Engram Cells Have Come of Age. Neuron. 2015;87:918–931. doi: 10.1016/j.neuron.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Liu X, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramirez S, et al. Creating a false memory in the hippocampus. Science (New York, N Y) 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 84.Ramirez S, et al. Activating positive memory engrams suppresses depression-like behaviour. Nature. 2015;522:335–339. doi: 10.1038/nature14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Memory. Engram cells retain memory under retrograde amnesia. Science (New York, N Y) 2015;348:1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trouche S, et al. Recoding a cocaine-place memory engram to a neutral engram in the hippocampus. Nature neuroscience. 2016 doi: 10.1038/nn.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han JH, et al. Neuronal competition and selection during memory formation. Science (New York, N Y) 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 88.Yiu AP, et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 89.Herry C, et al. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 90.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 91.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature neuroscience. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haubensak W, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolff SB, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- 94.Botta P, et al. Regulating anxiety with extrasynaptic inhibition. Nat Neurosci. 2015;18:1493–1500. doi: 10.1038/nn.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li H, et al. Experience-dependent modification of a central amygdala fear circuit. Nature neuroscience. 2013;16:332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andero R, Dias BG, Ressler KJA. Role for Tac2, NkB, and Nk3 Receptor in Normal and Dysregulated Fear Memory Consolidation. Neuron. 2014 doi: 10.1016/j.neuron.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell. 2015;162:363–374. doi: 10.1016/j.cell.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Letzkus JJ, et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- 99.Letzkus JJ, Wolff SB, Luthi A. Disinhibition, a Circuit Mechanism for Associative Learning and Memory. Neuron. 2015;88:264–276. doi: 10.1016/j.neuron.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 100.Porrero C, Rubio-Garrido P, Avendano C, Clasca F. Mapping of fluorescent protein-expressing neurons and axon pathways in adult and developing Thy1-eYFP-H transgenic mice. Brain research. 2010;1345:59–72. doi: 10.1016/j.brainres.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 101.Swanson LW. The Amygdala and Its Place in the Cerebral Hemisphere. Annals of the New York Academy of Sciences. 2003;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]
- 102.Jasnow AM, et al. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:10396–10404. doi: 10.1523/JNEUROSCI.5539-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- 104.Viviani D, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science (New York, N Y) 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 105.Knobloch HS, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 106.Bowers ME, Choi DC, Ressler KJ. Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiology & behavior. 2012;107:699–710. doi: 10.1016/j.physbeh.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gafford GM, Ressler KJGABA. NMDA receptors in CRF neurons have opposing effects in fear acquisition and anxiety in central amygdala vs. bed nucleus of the stria terminalis. Hormones and behavior. 2015;76:136–142. doi: 10.1016/j.yhbeh.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jungling K, et al. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Glotzbach-Schoon E, et al. Contextual fear conditioning in virtual reality is affected by 5HTTLPR and NPSR1 polymorphisms: effects on fear-potentiated startle. Frontiers in behavioral neuroscience. 2013;7:31. doi: 10.3389/fnbeh.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Slattery DA, et al. Selective breeding for high anxiety introduces a synonymous SNP that increases neuropeptide S receptor activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:4599–4613. doi: 10.1523/JNEUROSCI.4764-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guez-Barber D, et al. FACS purification of immunolabeled cell types from adult rat brain. Journal of neuroscience methods. 2012;203:10–18. doi: 10.1016/j.jneumeth.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guez-Barber D, et al. FACS identifies unique cocaine-induced gene regulation in selectively activated adult striatal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:4251–4259. doi: 10.1523/JNEUROSCI.6195-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cruz FC, et al. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nature reviews Neuroscience. 2013;14:743–754. doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hempel CM, Sugino K, Nelson SB. A manual method for the purification of fluorescently labeled neurons from the mammalian brain. Nature protocols. 2007;2:2924–2929. doi: 10.1038/nprot.2007.416. [DOI] [PubMed] [Google Scholar]
- 115.Luo L, et al. Gene expression profiles of laser-captured adjacent neuronal subtypes. Nature medicine. 1999;5:117–122. doi: 10.1038/4806. [DOI] [PubMed] [Google Scholar]
- 116.Yao F, et al. Microarray analysis of fluoro-gold labeled rat dopamine neurons harvested by laser capture microdissection. Journal of neuroscience methods. 2005;143:95–106. doi: 10.1016/j.jneumeth.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 117.Toledo-Rodriguez M, et al. Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cerebral cortex. 2004;14:1310–1327. doi: 10.1093/cercor/bhh092. [DOI] [PubMed] [Google Scholar]
- 118.Heiman M, Kulicke R, Fenster RJ, Greengard P, Heintz N. Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP) Nature protocols. 2014;9:1282–1291. doi: 10.1038/nprot.2014.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou P, et al. Interrogating translational efficiency and lineage-specific transcriptomes using ribosome affinity purification. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15395–15400. doi: 10.1073/pnas.1304124110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Drane L, Ainsley JA, Mayford MR, Reijmers LG. A transgenic mouse line for collecting ribosome-bound mRNA using the tetracycline transactivator system. Frontiers in molecular neuroscience. 2014;7:82. doi: 10.3389/fnmol.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katz IK, Lamprecht R. Fear conditioning leads to alteration in specific genes expression in cortical and thalamic neurons that project to the lateral amygdala. Journal of neurochemistry. 2015;132:313–326. doi: 10.1111/jnc.12983. [DOI] [PubMed] [Google Scholar]