Abstract
Objectives
To compare the institutional cost per person of screening and treatment between two groups of patients—those screened and those not screened before treatment for cervical cancer at Ocean Road Cancer Institute (ORCI) in Dar es Salaam, Tanzania—and to perform a cost-effectiveness analysis of the ORCI cervical cancer screening program.
Methods
The study included 721 screened and 333 unscreened patients treated at ORCI for cervical cancer from 2002 to 2011. We compared the cost of cervical cancer treatment per patient with life-years gained for patients screened at ORCI versus not screened.
Results
Patients with cancer were diagnosed at an earlier stage after participating in screening compared with nonparticipants. For example, 14.0% of stage I cancer patients had received screening by ORCI compared with 7.8% of unscreened cases. For stage IV cancer, these percentages were 1.4% and 6.9%, respectively. Average screening and treatment cost for patients receiving cancer screening ($2526) was higher than that for unscreened patients ($2482). However, we calculated an incremental cost-effectiveness ratio of $219 per life-year gained from receiving cervical cancer screening compared with not being screened, and thus the ORCI screening program was highly cost-effective. Furthermore, the screening program was associated with averting 1.3 deaths from cervical cancer each year resulting from earlier diagnoses of cancer cases, with the incremental cost-effectiveness ratio of $4597 per life saved.
Conclusions
Although Sub-Saharan Africa faces substantial challenges in population health management, our study highlights the potential benefits from expanding access to regular cervical cancer screening for women in this region.
Keywords: cancer screening, cancer treatment, cervical cancer, cost-effectiveness, institutional cost, Tanzania, sub-saharan Africa
Introduction
Each year, there are 500,000 cases and 230,000 deaths caused by cervical cancer worldwide [1]. Eighty-five percent of these cases are in developing countries [2]. Although cervical cancer is often detected early and treated successfully in the United States, the health care system in Tanzania faces substantial challenges in providing regular screening and treatment of cervical cancer [3]. In fact, Sub-Saharan Africa has the highest incidence of cervical cancer in the world, with an incidence rate of 50.9 cases per 100,000 women, and cervical cancer is the most common cause of death from cancer for women in Tanzania [3,4].
Ocean Road Cancer Institute (ORCI) in Dar es Salaam is the only specialized cancer treatment hospital in Tanzania. More than one-third of the cancer patients seen at ORCI are diagnosed with cervical cancer [3]. ORCI has a national network of clinics performing visual inspection with acetic acid (VIA) screenings by trained health care professionals [5,6]. VIA screenings have been found to be a lower resource alternative to effectively screen for cervical cancer, compared with cytology-based screening [6]. Unfortunately, access to screening services is limited, with only 12 of 21 regions in Tanzania having these screening clinics [7]. Furthermore, most screening clinics are in urban areas although 75% of the population lives in rural areas [2]. In Tanzania, only 1 in 20 women aged 30 to 50 years old ever receive cervical cancer screening, and consequently many women consult a health care provider only after developing late-stage cervical cancer [5,8-10].
Cancer screening, treatment, and care are provided at no cost to patients by the government of Tanzania [3]. However, the cost-effectiveness of providing cervical cancer screening is unclear. With limited resources being available in Tanzania, this is an important issue for continued funding and expansion of access to these services. Our study uses detailed cost and outcomes data on ORCI patients to examine the cost-effectiveness of cervical cancer screening services compared with status quo.
Methods
Patient Population
The study population included 721 screened patients (seen at the ORCI screening clinic) and 333 unscreened patients who visited the ORCI treatment clinic with no previous screenings at ORCI. Screened patients included those receiving at least one cervical cancer screening before being diagnosed with cancer. Both groups were treated for cervical cancer at ORCI during the period 2002 to 2011. Of the screened patients, 14.0% were clinically diagnosed as having stage I cancer, 48.4% as having stage II cancer, 36.2% as having stage III cancer, and 1.4% as having stage IV cancer. Of the previously unscreened cancer patients, 7.8% were having stage I cancer, 51.1% were having stage II cancer, 34.2% were having stage III cancer, and 6.9% were having stage IV cancer.
Cost Estimates
All costs collected were institutional costs provided by ORCI. Cervical cancer screening costs include the cost of supplies per patient for VIA and the salaries of nurses and physicians performing the screening. The cost of supplies included the cost of cleaning materials, forceps, and reagents including acetic acid, examination gloves, cotton swabs, and other supplies. Relevant supplies and their costs were identified on the basis of interviews with health professionals of ORCI and review of the 2013 Medical Stores Department Catalogue of ORCI. Cryotherapy cost was calculated by adding medical personnel cost (salaries and wages with length of staff time allocated to cryotherapy) and cost of supplies and equipment for cryotherapy. Costs of supplies and equipment were retrieved from the ORCI Medical Services Price List.
Treatment cost included labor, the supplies and services used for treatment, and patient hospital accommodation/admission. The services used for treatment were identified through 10 interviews with ORCI medical personnel including three physicians, one X-ray technician, one radiography technician, two nurses in the screening clinic, the hospital head nurse, and two laboratory technicians. The interviews were conducted in person in July 2014. They were semi-structured interviews, including six questions and observations of the health care workers with their patients. The typical questions were as follows: 1) How many cervical cancer patients do you see each day? 2) How long do you spend on average with each cervical cancer patient? 3) How much do each of the machines/medications/treatments cost that you use for cervical cancer patients? 4) Can you walk me through a typical session with a cervical cancer patient? 5) How do your sessions differ depending on the cancer stage of the cervical cancer patient? 6) Does the treatment plan set up differently depending on patients’ diagnosis information before screening? Depending on who was being interviewed, there were sometimes follow-up questions. If the interviewee was unaware of cost information, this question was taken to the accounting department. Each interview lasted approximately 1 hour. This information included treatment resources used to manage patients stratified by stage of cervical cancer diagnosis and included initial examination, cancer treatments, and follow-up care. The cost of these services was retrieved from the ORCI Medical Services Price List. Cost for supplies not found in the price list was obtained from the 2013 Medical Stores Department Catalogue.
Salary information for staff was provided by the ORCI accounting department. Consultation time per patient was determined in interviews with physicians and a review of 179 patient records from 2007 to 2011. Using these data, we calculated the average monetary cost of staff time allocated to treatment.
The model analyzed cost from the institutional perspective. Thus, only direct costs were included in the analysis, such as screening, treatment, and accommodation costs, which are funded by the government of Tanzania. Screening program costs were compiled in consultation with the head nurse of the cervical cancer screening center (Table 1). The cost of treatment for cervical cancer was derived from historical data, interviews, and patient records, as described above. The per-person cost was obtained by calculating the average of the cost of all patients treated from 2002 to 2011. Stage I and stage II patients received curative treatment and stage III and stage IV patients received palliative treatment. All cost was converted from Tanzanian shillings to US dollars (US $1 = 1706 Tanzanian shillings) using the currency exchange rate at the time of this study.
Table 1. Average screening variables per patient for cervical cancer at ORCI from 2002 to 2011.
| Variable | Value |
|---|---|
| Number of patients seen each day | 25 |
| Time to perform VIA screening | 10 min |
| Labor per person | |
| Daily salary for medical personnel | US $49.86 |
| Staff time per woman to perform cryotherapy procedure |
10 min |
| Cost of supplies | |
| Per patient cost of supplies for VIA | US $0.41 |
| Per patient cost of supplies and equipment for cryotherapy |
US $26.37 |
| Total screening cost | US $1.45 |
| Total cryotherapy cost | US $28.97 |
Note. US $1=1706 Tanzanian shillings.
ORCI, Ocean Road Cancer Institute; VIA, visual inspection with acetic acid.
Outcome Estimates
The probability distribution of cancer stage was found on the basis of previous research reporting the likelihood of cervical cancer diagnosis and staging for Tanzanian women in the period 2002 to 2011 [11]. Because cervical cancer survival probability has not yet been evaluated in Tanzania, we used estimates from a South African study that estimated average 5-year survival [12].
The primary health measure used in our study is the number of life-years gained. Undiscounted life-years were calculated as follows:
The expected number of remaining life-years was based on Tanzanian life expectancy of 62.6 years in 2014 for females [13]. Life-years were also discounted by 3% each year following previous literature. Discounted life-years were then aggregated to determine total life-years per person and total life-years for the 2013 participants with and without the screening program annually.
We also estimated differences in the number of cancer-related deaths with and without the program to predict the number of lives saved associated with the program.
Cost-Effectiveness Analysis
An incremental cost-effectiveness ratio (ICER) was calculated by dividing the difference in total costs by the difference in life-years gained and the number of cancer-related deaths averted between the screened and unscreened groups. Differences in total costs include the screening costs associated with the program plus the difference in treatment costs between the screening participants and nonparticipants. Differences in treatment costs are based on differences in the probability distributions of cancer stages and the average costs of treatment for each stage between participants and nonparticipants. Finally, gross domestic product (GDP) per capita in 2013 for Tanzania was used to determine the cost-effectiveness of the screening program [14]. The program is determined to be cost-effective relative to status quo if the ICER is less than three times the GDP per capita. This criterion is consistent with the cost-effectiveness threshold used by the World Health Organization [15].
Results
Table 1 itemizes screening costs. These costs include the monetized staff time used to screen patients and costs of supplies. In the screening clinic at ORCI in Dar es Salaam, Tanzania, the average screening cost per patient is US $1.45 and the average cryotherapy cost per patient is US $28.97.
The proportions of screened and unscreened patients by stage of cancer used for this analysis are reported in Table 2. There are a significantly higher number of screened patients who were reported as having stage 1 cancer than unscreened patients. In contrast, there were significantly more unscreened patients who were reported as having stage 4 cancer than screened patients. There was no significant difference between screened and unscreened patients at stages 2 and 3.
Table 2. Proportion of screened and unscreened patients by stage.
| Stage | Screened patients* | Unscreened patients† |
|---|---|---|
| Stage 1 | 101 (14.01%)‡ | 26 (7.81%)‡ |
| Stage 2 | 349 (48.40%) | 170 (51.05%) |
| Stage 3 | 261 (36.20%) | 114 (34.23%) |
| Stage 4 | 10 (1.39%)‡ | 23 (6.91%)‡ |
| Total | 721 (100.00%) | 333 (100.00%) |
Note. A χ2 test was conducted to compare the proportion of patients at each stage between the screened and unscreened groups.
Screened patients refer to those patients screened for cervical cancer and then treated.
Unscreened patients refer to those patients who were not screened for cervical cancer and came to Ocean Road Cancer Institute for treatment.
Significant differences between screened and unscreened patients.
Average treatment costs are outlined in Table 3. The average cost of treating an early stage (stages 1 and 2) patient is nearly $3000 compared with about $1740 for patients with late-stage (stages 3 and 4) cancer. Early stage patients receive curative radiotherapy and chemotherapy costing $1547.48 and $316.53, respectively, whereas late-stage patients only receive palliative radiotherapy at $773.52.
Table 3. Average treatment cost per patient by cancer stage at ORCI from 2002 to 2011.
| Variable | Stage 1 (US $) | Stage 2 (US $) | Stage 3 (US $) | Stage 4 (US $) |
|---|---|---|---|---|
| Average labor costs | ||||
| Physician | 46.04 | 56.48 | 46.64 | 43.72 |
| X-Ray & ultrasound techs | 1.55 | 1.55 | 1.55 | 1.55 |
| Lab techs | 4.49 | 4.49 | 4.49 | 4.49 |
| Radiation techs | 7.98 | 7.98 | 5.59 | 5.59 |
| Team planning and initial consultation | 46.89 | 46.89 | 46.89 | 46.89 |
| Supplies and equipment | ||||
| X-Ray & ultrasound | 52.74 | 52.74 | 52.74 | 52.74 |
| Lab work | 144.74 | 144.74 | 144.74 | 144.74 |
| Curative radiotherapy | 1547.48 | 1547.48 | - | - |
| Chemotherapy | 316.53 | 316.53 | - | - |
| Palliative radiotherapy | - | - | 773.52 | 773.52 |
| Inpatient accommodation costs including rooms and meals | 820.40 | 820.40 | 668.23 | 668.23 |
| Total | 2988.84 | 2999.28 | 1744.39 | 1741.47 |
Note.US $1=1706 Tanzanian shillings.
Lab, laboratory; ORCI, Ocean Road Cancer Institute; techs, technicians.
The average cost to both screen and treat patients is provided in Table 4. Weighted average costs are calculated using total average treatment costs by stage from Table 3 and the distribution of screened and unscreened patients across cancer stages. Thus, the weighted average cost of treating participants in the ORCI screening program is $2526.06 compared with $2482.00 for unscreened participants (Table 4).
Table 4. Average treatment cost of screened and unscreened patients from 2002 to 2011.
| Stage | Total costs (US $) |
Screened patients, n (%) |
Unscreened patients, n (%) |
Average treatment cost for screened patients (US $) |
Average treatment cost for unscreened patients (US $) |
|---|---|---|---|---|---|
| Stage 1 | 2988.84 | 101 (14.01) | 26 (7.81) | 418.73 | 233.43 |
| Stage 2 | 2999.28 | 349 (48.40) | 170 (51.05) | 1451.65 | 1531.13 |
| Stage 3 | 1744.39 | 261 (36.20) | 114 (34.23) | 631.47 | 597.10 |
| Stage 4 | 1741.47 | 10 (1.39) | 23 (6.91) | 24.21 | 120.34 |
| All | 721 (100) | 333 (100) | 2526.06 | 2482.00 |
Note.US $1=1706 Tanzanian shillings.
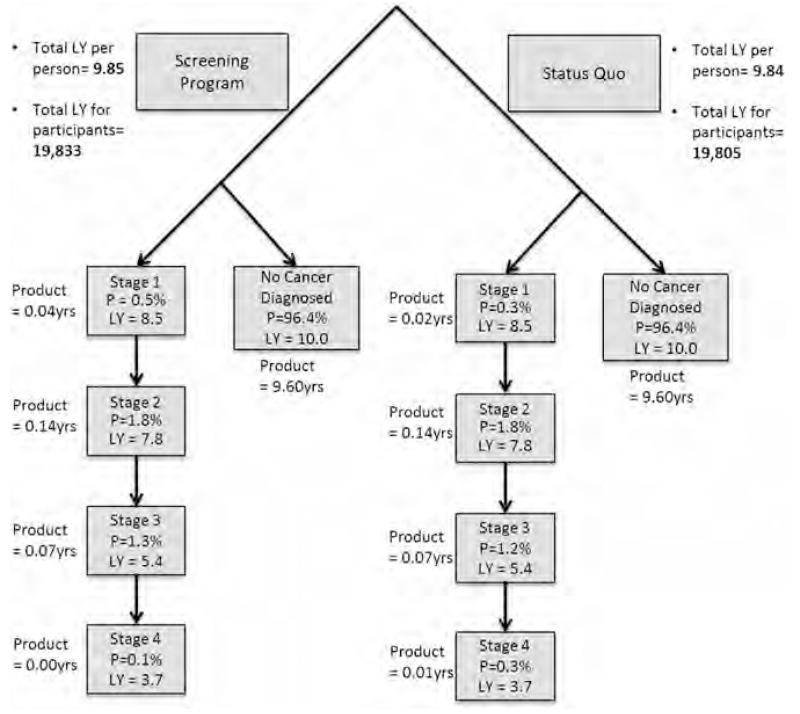
Figure 1 illustrates probability distribution of cancer stage and associated discounted life-years remaining. A decision tree showed that the probability of getting early stage cancer was slightly higher for participants in the screening program than for those in status quo. Implementation of the screening program for cervical cancer for the 2,013 female participants increased the total number of discounted life-years by 27.9 years from 19,805 to 19,833 discounted life-years.
Fig. 1.
Probabilities and discounted life-years for each cancer stage for women with and without screening program. LY, expected life-years remaining for each stage of cancer, discounted by 3% each year following; P, probability distribution of each cancer stage; total LYs for participants, overall weighted LYs for entire study participants with screening and without screening program, calculated by total LYs per person × total number of participants; total LYs per person, overall weighted LYs per person, calculated by aggregating the product of each stage (P × LY).
More results for cost-effectiveness are presented in Table 5. Using these findings and the results from Tables 1 and 4, we calculated an ICER of $219 per life-year gained associated with the ORCI screening program compared with status quo. With a GDP per capita of $1750 [14], and using the World Health Organization standard of less than three times the GDP per capita as the threshold value for determining cost-effectiveness, we determined that Tanzania’s cervical cancer screening program was highly cost-effective in increasing life-years [15]. Furthermore, we predict that the screening program was associated with averting 1.3 deaths from cervical cancer each year resulting from earlier diagnoses of cancer cases. In this case, the ICER was only $4597 per life saved from having the screening program versus status quo.
Table 5. Cost-effectiveness results for the screening program vs. no screening program for cervical cancer in increasing discounted life-years and averting cancer-related deaths.
| Categories | Outcome | Cost | Incremental effectiveness | Incremental cost | ICER | Dominance |
|---|---|---|---|---|---|---|
| Discounted life-years | ||||||
| Intervention | 19,833.3 | $185,980 | ||||
| Status quo | 19,805.4 | $179,873 | 27.9 | $6107 | $219 | Dominated |
| Number of cancer-related deaths | ||||||
| Intervention | 32.7 | $185,980 | ||||
| Status quo | 34.0 | $179,873 | 1.3 | $6107 | $4597 | Dominated |
Note.US $1=1706 Tanzanian shillings.
ICER, incremental cost-effectiveness ratio [(Screening program cost plus difference in treatment costs)/difference in outcomes].
Discussion
The results from our cost-effectiveness analysis show that the screening program in Tanzania improves life-years gained. The impact of the screening program in diagnosing individuals at an earlier stage of cancer, resulting in increased life expectancy, is consistent with previous studies [16-19]. Women who are regularly screened can often be diagnosed when they are asymptomatic stage 1. These women have an excellent chance of survival and rarely have to go through extensive treatment [2]. Stage 2 patients require a combination of chemotherapy and radiation therapy and sometimes surgery. As each stage progresses, more treatment and follow-up are needed. The chance of survival greatly decreases as each stage progresses [1]. For example, stage 1 patients have 93% chance of 5-year survival, whereas stage 4 patients have 15% chance of 5-year survival in the United States [20]. The likelihood of survival for late-stage patients may be even lower in Tanzania because of substantially more limited health care resources compared with the United States.
The screening program at ORCI is inexpensive to operate. ORCI provides a single-visit screening service in which women can be screened through VIA, and if an abnormality is found, cryotherapy can be performed in the same visit. Cryotherapy is used if there are abnormal precancerous lesions detected in the cervix. Screening and cryotherapy procedures could end up saving the government a significant amount of money in catching the lesions before they turn malignant and cancer treatment becomes necessary. These screening costs are lower than the costs found in a similar study that looked at the costs of cervical cancer screening in other Sub-Saharan African countries [21]. The lower cost in Tanzania may be because Tanzania already has an established screening program and because ORCI offers a single-visit cryotherapy service. Also, the previous study assumed that screening costs across all Sub-Saharan African countries were the same, whereas our study focused exclusively on Tanzania [21].
One unexpected result of this study is the significantly higher cost of treating early stage compared with late-stage cancer cases. In Tanzania, late-stage cases are not treated with the intention of curing the cancer. These patients receive palliative care to help reduce pain, and then are sent home from the hospital. The high doses of radiotherapy and chemotherapy and palliative care needed for treatment and palliation of advanced-stage patients is beyond the financial ability of the Tanzanian government or the patients. Thus, women diagnosed at late stages are generally not treated. An increase in cervical cancer screening may initially cost the government more money because of the increase in early stage patients. However, because of the prevented loss of life of late-stage patients, this extra cost will eventually be offset in the long-term. In fact, our results show that even with the added costs from screening and treating early stage cancer patients compared with late-stage cancer patients, the cervical cancer screening program in Tanzania is cost-effective because of the increase in life-years associated with screening.
Our data show that the average number of women who were screened each year at ORCI from 2002 to 2011 was 2013 women. Our analysis suggests that this screening program resulted in an additional 28 discounted life-years among these women. With an expansion of the screening program to 10,000 women per year, the screening program could save approximately 139 discounted life-years, or avert more than six deaths from cervical cancer annually. The impact of the increased screening is a substantial shift of women toward early stage diagnoses, resulting in a greater chance of survival.
Similar to our study, another study of cost-effectiveness in developing countries also found that cervical cancer screening was cost-effective [22]. This study used data from India, Thailand, Kenya, Peru, and South Africa to assess the cost-effectiveness of screening strategies, and found that the reduction in the lifetime risk of invasive cervical cancer with a single screening ranged from 25% to 31%. The study concluded that the most effective strategy was a single-visit VIA test [22], which is the case at ORCI.
Cancer screening also provides other benefits not included in our study data. Previous data show that 80% of women die within 5 years of diagnosis of cervical cancer in Africa [3], and this substantially increases financial burdens as well as emotional stress on the families. This early loss of life prevents future income that the women could earn and results in economic costs related to other family members having to raise the children. Also, many women may be screened and diagnosed with invasive cancer, but never seek treatment because of the costs of travel [10]. This is where increased screening and the use of cryotherapy could play an important role. The low cost of screening and cryotherapy combined with benefits to women of not needing to go through the long process of treatment is beneficial to all. Cervical cancer is 100% preventable when the lesions are treated through screening before they become cancerous, and the increase in screening could save women these high travel costs to be treated at ORCI [23].
One strength of our study is its origination from a detailed inventory of clinical and demographic data of all cervical cancer patients seen at ORCI during the period 2002 to 2011 [11]. Another strength is the consistency of staffing at ORCI. Most ORCI employees remain in their jobs for long periods and perform the same screening and treatment regimen. Our access to detailed cost and management information at ORCI from different sources was also an additional strength of the study.
The study also has limitations. The only screening site considered in this study was ORCI. There are a few other screening sites around the country that were not included in the costs. However, those sites do not include treatment facilities for rapid treatment of cervical cancer patients. Also, our study may not have captured all treatment costs such as those for surgical resection. However, the proportion of early stage cervical cancer patients for whom surgery would be indicated is very limited. Palliative care costs beyond the palliative radiation are not included in this study. The institution does not provide any additional palliative care and patients self-pay for palliative medications after being discharged from the hospital. This information would likely increase the financial burden for late-stage patients. Because of data limitation, demographic information of screened and unscreened population, such as education and age, was not available in this study. Age and education status of the screened population between cancer and noncancer patients was illustrated in another study [11]. A final limitation is that indirect costs for cervical cancer are not included in this study. The inclusion of these costs would likely increase the cost-effectiveness of screening because of the increase in cost savings from decreasing cervical cancer mortality.
In summary, the cost-effectiveness of screening shown in this study warrants the expansion of the screening program of ORCI to regional sites outside of the capital city. Currently, there are only 12 screening sites in Tanzania and most of them are in urban areas [7]. Supply of the treatment programs, including cryotherapies and trained health care professionals, and access to regional health care facilities should be followed. Other Sub-Saharan African countries without current screening could use this cost model as an example to start screening programs in their countries. Future studies could refine the cost-effectiveness measurements by including detailed information on the cost of palliative care and indirect cost of disease-related morbidity and mortality.
Supplementary Material
Acknowledgments
Source of financial support: This work was supported by the Cancer Epidemiology Education in Special Populations (CEESP) Program of the University of Nebraska Medical Center (grant no. R25CA112383).
Footnotes
Conflict of interest: The authors have indicated that they have no conflicts of interest with regard to the content of this article.
Supplemental material accompanying this article can be found in the online version as a hyperlink at http://dx.doi.org/10.1016/j.vhri.2016.03.002 or, if a hard copy of article, at www.valueinhealthjournal.com/issues (select volume, issue, and article).
REFERENCES
- [1].Schink JC. Oncology: An Evidence-Based Approach. Springer Science and Business Media, Inc.; New York, NY, USA: 2006. Cervix, vulva, and vagina. [Google Scholar]
- [2].The United Republic of Tanzania . Tanzania Service Delivery Guidelines for Cervical Cancer Prevention and Control. Ministry of Health and Social Welfare; Dar es Salaam, Tanzania: 2011. [Google Scholar]
- [3].The United Republic of Tanzania . National Cervical Cancer Prevention and Control Strategic Plan 2011-2015. Ministry of Health and Social Welfare; Dar es Salaam, Tanzania: 2011. [Google Scholar]
- [4].Denny LA, Sankaranarayanan R, De Vuyst H, et al. Recommendations for cervical cancer prevention in sub-Saharan Africa. Vaccine. 2013;31(Suppl. 5):F73–4. doi: 10.1016/j.vaccine.2012.11.077. [DOI] [PubMed] [Google Scholar]
- [5].Mwaiselage J. Outreach of Cervical Cancer Prevention Program in Tanzania: Achievements and Challenges; Presented at the Center for Global Health International Symposium; Cairo, Egypt. 2010. [Google Scholar]
- [6].Sankaranarayanan R, Gaffikin L, Jacob M, et al. Elsevier Ireland Ltd A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstetr. 2005;89:S4–12. doi: 10.1016/j.ijgo.2005.01.009. [DOI] [PubMed] [Google Scholar]
- [7].Andrews L. Standard Operating Procedures for Implementation of Cervical Cancer Screening and Early Treatment Program. 2012. [Google Scholar]
- [8].Kazaura MR, Kombe D, Yuma S, et al. Health seeking behavior among cancer patients attending Ocean Road Cancer Institute, Tanzania. East Africa J Public Health. 2007;4:19–22. [PubMed] [Google Scholar]
- [9].Mosha D, Mahande M, Ahaz J, et al. Factors associated with management of cervical cancer patients at KCMC hospital, Tanzania: a retrospective cross-sectional study. Tanzan J Health Res. 2009;11:70–4. [Google Scholar]
- [10].Peters LM, Soliman AS, Bukori P, et al. Evidence for the need of educational programs for cervical screening in rural Tanzania. J Cancer Educ. 2010;25:153–9. doi: 10.1007/s13187-009-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gard A, Soliman A, Ngoma T, et al. Most women diagnosed with cervical cancer by a visual screening program in Tanzania completed treatment: evidence from a retrospective cohort study. BMC Public Health. 2014;14:910. doi: 10.1186/1471-2458-14-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sinanovic E, Moodley J, Barone M, et al. The potential cost-effectiveness of adding a human papillomavirus vaccine to the cervical cancer screening programme in South Africa. Vaccine. 2009;27:6196–202. doi: 10.1016/j.vaccine.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [13].Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- [14].World Health Organization [Accessed: December 18, 2014];United Republic of Tanzania. Retrieved from: http://www.who.int/countries/tza/en/
- [15].WHO-CHOICE [Accessed: February 8, 2015];Cost-effectiveness thresholds. Retrieved from: http://www.who.int/choice/costs/CER_thresholds/en/
- [16].Denny L, Kuhn L, De Souza M, et al. Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. J Am Med Assoc. 2005;294:2173–81. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- [17].Teguete I, Muwonge R, Traore CB, et al. Can visual cervical screening be sustained in routine health services? Experience from Mali, Africa. BJOG. 2012;119:220–6. doi: 10.1111/j.1471-0528.2011.03122.x. [DOI] [PubMed] [Google Scholar]
- [18].Elovainio L, Nieminen P, Miller A. Impact of cancer screening on women’s health. Int J Gynecol Obstetr. 1997;58:137–47. doi: 10.1016/s0020-7292(97)02859-2. [DOI] [PubMed] [Google Scholar]
- [19].Sankaranarayanan R, Esmy PO, Rajkumar R, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomized trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- [20].American Cancer Society [Accessed: December 8, 2014];Survival rates for cervical cancer by stage. 2014 Retrieved from: http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-survival.
- [21].Mvundura M, Tsu V. Estimating the costs of cervical cancer screening in high-burden Sub-Saharan African countries. Int J Gynaecol Obstetr. 2014;126:151–5. doi: 10.1016/j.ijgo.2014.02.012. [DOI] [PubMed] [Google Scholar]
- [22].Goldie S, Gaffikan L, Goldhaber-Fiebert J, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158–68. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- [23].National Comprehensive Cancer Network . Clinical Practical Guidelines in Oncology: Cervical Cancer. Vol. 1. National Comprehensive Cancer Network; Fort, Washington: 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.