Abstract
Several psychosocial care interventions have been found effective in improving psychosocial outcomes in cancer patients. At present, there is increasingly being asked for information on the value for money of this type of intervention. This review therefore evaluates current evidence from studies investigating cost-effectiveness or cost-utility of psychosocial care in cancer patients. A systematic search was conducted in PubMed and Web of Science yielding 539 unique records, of which 11 studies were included in the study. Studies were mainly performed in breast cancer populations or mixed cancer populations. Studied interventions included collaborative care (four studies), group interventions (four studies), individual psychological support (two studies), and individual psycho-education (one study). Seven studies assessed the cost-utility of psychosocial care (based on quality-adjusted-life-years) while three studies investigated its cost-effectiveness (based on profile of mood states [mood], Revised Impact of Events Scale [distress], 12-Item Health Survey [mental health], or Fear of Progression Questionnaire [fear of cancer progression]). One study did both. Costs included were intervention costs (three studies), intervention and direct medical costs (five studies), or intervention, direct medical, and direct nonmedical costs (three studies). In general, results indicated that psychosocial care is likely to be cost-effective at different, potentially acceptable, willingness-to-pay thresholds. Further research should be performed to provide more clear information as to which psychosocial care interventions are most cost-effective and for whom. In addition, more research should be performed encompassing potential important cost drivers from a societal perspective, such as productivity losses or informal care costs, in the analyses.
Key words: Cancer patient, cost-effectiveness, cost-utility, economic evaluation, psychosocial care, supportive care

Introduction
Many cancer patients experience psychosocial problems during or after treatment, including depression, anxiety, fear of cancer progression, or problems with coping.[1,2,3] The prevalence of depression in cancer patients has been estimated at 8-24%[1] and the prevalence of anxiety at 18%.[2] Unmet care needs regarding these psychosocial problems have been reported in up to 89% of cancer patients.[4,5]
Several psychosocial care interventions have been developed in recent years aiming to target these problems and care needs in cancer patients, ranging from relatively low-intensive interventions (e.g., self-help or group interventions) to high-intensive interventions (e.g., individual cognitive behavioral therapy).[6] Further stepped care (i.e., an approach in which effective, yet least resource-intensive treatment is delivered first, followed by, when necessary, more resource-intensive treatments) and collaborative care interventions (i.e., a care model in which different healthcare disciplines closely collaborate to provide systematic treatment and follow-up) have been developed.[7,8,9] In general, psychosocial care interventions have been found effective in improving psychosocial outcomes, such as distress and quality of life, in cancer patients.[6,10]
Carlson and Bultz[11,12] hypothesized that providing psychosocial care to cancer patients may not only be effective in improving outcomes but also lead to cost savings in the long-term. Cancer patients benefitting from psychosocial care are hypothesized to make less use of other healthcare services (i.e., visits to the general practitioner or oncologist) called cost offset due to, for example, an increased ability to adhere to demanding treatments or lifestyle recommendations resulting in an improved overall health. In addition, productivity losses may be reduced due to an increased ability to work. Previous studies have indeed found such an association between better psychosocial outcomes and less healthcare utilization or costs[13,14,15,16] and higher rates of return to work.[17,18] However, other studies did not found such an association.[19,20]
Whether providing psychosocial care to cancer patients indeed is economically attractive can be assessed by performing economic evaluations, such as cost-effectiveness or cost-utility analyses.[21,22] The current health care system increasingly asks for this kind of evaluations[23,24] since the economic burden of cancer care is high[25] and choices have to be made regarding optimal resource allocation.
In cost-effectiveness and cost-utility analyses, the difference in total costs between different interventions or a new intervention and usual care are weighted against the differences in effectiveness, such as improvement in psychological distress or fear of cancer progression (called cost-effectiveness analyses), or differences in quality-adjusted-life-years (QALYs) (called cost-utility analyses).[21,22] This results in a ratio of the incremental costs for an incremental unit of effect, called incremental cost-effectiveness ratio (ICER). Cost-effectiveness and cost-utility analyses can be performed from different perspectives (e.g., a healthcare perspective or a societal perspective) which determine the cost categories taken into account in the analyses. In a healthcare perspective, costs of the healthcare system are taken into account while in a societal perspective, a broader spectrum of costs is measured including productivity losses and informal care costs.
Two systematic reviews[26,27] on the economic evaluation of psychosocial interventions have been published so far, one of which included studies up to 2013.[27] This last review revealed that psychosocial care interventions have the potential to be cost-effective.[27] However, studies combining exercise interventions and psychosocial support or on the most optimal follow-up strategy were also included[27] which hamper firm conclusions on the value for money of psychosocial care among cancer patients. Moreover, because new developments in psychosocial care are ongoing and studies on the cost-effectiveness and cost-utility of psychosocial care are increasingly being published in the past 2 years, a new search updating current evidence is warranted. The aim of this review was, therefore, to assess current evidence on the cost-effectiveness and cost-utility of psychosocial care interventions in cancer patients.
Methods
Literature search
A literature search was conducted in two electronic bibliographic databases namely PubMed (dates of coverage 1950-present) and Web of Science (1900-present) from inception to January 2016. Search terms included different terms for economic evaluations (e.g., cost-effectiveness or cost-utility analyses), cancer (e.g., neoplasm), psychosocial care (e.g., psychological care or supportive care), and psychosocial outcomes (e.g., depression or anxiety). Table 1 provides a detailed overview on the combinations of search terms used. In addition to this literature search, reference lists from eligible articles were manually searched and authors were asked for additional studies.
Table 1.
Search strategy
| PubMed (MedLine) | Web of Science |
|---|---|
| Neoplasms[MeSH] OR neoplasm[ti] OR Cancer[ti] OR “chronic cancer patients”[ti] OR “cancer survivors”[ti]) AND (((cost* OR economic[ti]) AND (analysis OR analyses OR effectiveness OR utility OR evaluation OR benefit)) OR (cost-analysis OR cost-analyses OR cost-effectiveness OR cost-utility OR cost-benefit OR cost-evaluation OR cost-effective*)) AND (“supportive care”[ti] OR “psychosocial care”[ti] OR “psychological care”[ti] OR “after care”[ti] OR anxiety[ti] OR depression[ti] OR social[ti] OR psychosocial[ti] OR cognitive[ti] OR stress[ti] OR mood[ti] OR pain[ti] | TITLE: (neoplasm OR Cancer OR chronic cancer patients OR cancer survivors) AND TITLE: (supportive care OR psychosocial care OR psychological care OR after care OR anxiety OR depression OR social OR psychosocial OR cognitive OR stress OR mood OR pain) AND TITLE: (cost* OR economic) AND TITLE: (analysis OR analyses OR effectiveness OR utility OR evaluation OR benefit OR cost-analysis OR cost-analyses OR cost-effectiveness OR cost-utility OR cost-benefit OR cost-evaluation OR cost-effective*) |
MeSH: Medical subject heading; ti: Title
Study inclusion and exclusion criteria
Research articles were included if they:
Presented results on the cost-effectiveness or cost-utility of psychosocial care interventions;
Used QALYs or a psychosocial outcome measure as outcome;
Included adult cancer patients only; and
Full-text was available in English or Dutch.
Research articles were excluded if they:
Assessed the cost-effectiveness or cost-utility of an exercise intervention;
Were not yet published as full-text; or
Were reviews (although reference lists were checked). No limits were set for year of publication.
Selection procedure and data extraction
Screening of the databases for relevant articles was performed by two of the authors (FJ and VvZ). First, title and abstract of all identified records were screened for potential relevance. Consequently, full-text of potentially relevant articles was assessed for eligibility based on the inclusion and exclusion criteria. Differences in study selection between the two authors were solved by discussion and when needed, a third person (IVdL) was consulted.
All studies found eligible for inclusion in this review were thoroughly read and relevant data were extracted. Data extracted included general information (i.e., name of the author, year of publication, country in which the study was conducted), study design, study population (i.e., cancer diagnosis, important eligibility criteria, and number of patients), intervention and control treatment (i.e., type of treatment and treatment duration), follow-up period, outcome measures, study perspective (e.g., healthcare or societal perspective), included cost categories (i.e., intervention, direct medical, direct nonmedical, indirect medical, or indirect nonmedical costs), and study results. All costs identified were converted to dollar-prices using the exchange rate of the index year reported in the article. In case the index year was not reported, the assumed index year was used.
Main findings of the included studies regarding the cost-effectiveness or cost-utility of psychosocial care interventions were summarized in a permutation matrix with nine possible cost-effectiveness/cost-utility outcomes.[28] All studies were allocated to one of the nine possibilities based on main evidence for incremental costs (lower, equal, or higher costs) and incremental effects (lower, equal, or higher effects).
Quality assessment
The quality of the included studies was assessed using the 10-item checklist of Drummond et al.[21,22] One author (FJ) conducted the quality assessment. When an article referred to previous publications (e.g., design paper or study on effectiveness) for additional information, this study was retrieved as well for quality assessment. A total score per study was calculated by counting the numbers of items scored positively (+1) or partly positive (+0.5), resulting in a score ranging from 0 to 10. In addition, the percentage of studies that met a particular criterion was calculated.
Results
Identification and selection of the literature
In total, 539 records were screened for eligibility based on title and abstract, of which 25 were selected for full-text review [Figure 1]. In addition, two articles were added based on reference checking or authors knowledge. After full-text review, 11 studies were included in the study.
Figure 1.
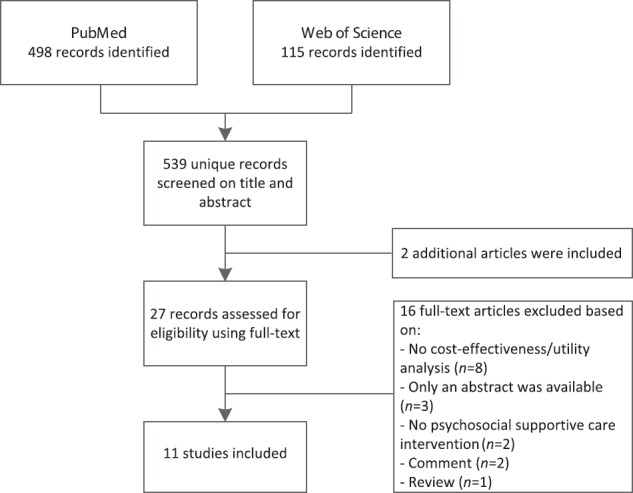
Flow diagram
Table 2 provides an overview of the selected studies. Studies were published between 2006 and 2015, of which seven recently (i.e., 2014 or 2015).[29,30,31,32,33,34,35] Most studies were conducted in the United Kingdom[34,35,36] and the USA[31,32,37] (both three studies), followed by Canada,[38] Germany,[39] Sweden,[29] The Netherlands,[33] and Australia[30] (all one study). Nine studies were cost-effectiveness or cost-utility studies conducted alongside a randomized controlled trial (RCT) on effectiveness of psychosocial care[29,30,31,32,35,36,37,38,39] while two studies used a decision analytic model, in which the cost-utility was estimated based on multiple sources of data.[33,34]
Table 2.
Characteristics of the included studies
| Characteristics | Design | Study population | Treatment | Follow-up | Outcome(s)a | Perspective | Results |
|---|---|---|---|---|---|---|---|
| Lemieux et al.[38] Canada | RCT | Women with metastatic breast cancer (n=125) | Weekly supportive-expressive psychosocial group therapy plus standard care. Patients were asked to attend group sessions for at least 1 year Control: Usual care, which comprised educational materials and psychosocial treatment when deemed necessary |
2-year (mean) | Mood (POMS) | Healthcare perspective, including intervention costs and direct medical costs | Intervention costs were on average $1394 per patient Psychosocial group therapy was more costly ($+3526, NS) and more effective (POMS effect size of 0.32, significant) than usual care ICER was $5550 for an effect size of 0.5 in mood |
| Mandelblatt et al.[37] USA | RCT | Women treated with surgery for invasive breast cancer four to 6 weeks ago (n=389) | An educational video addressing re-entry challenges in physical health, emotional well-being, interpersonal relations and life perspectives plus the control booklet Individual psycho-educational counseling (one face-to-face and one telephone session) plus the educational video and control-booklet Control: A booklet-control condition |
6-month | Distress (IES-R) | Societal perspective, including intervention costs (which includes patient opportunity costs) and direct medical costs Only intervention costs were included in the CEA analyses |
Intervention costs were $11 (control), $26 (video) and $134 (video plus counseling) per participant Individual counselling was most costly, while equally effective as the educational video condition and therefore dominated. The educational video condition was more costly ($+15) and more effective (IES-R incremental effect −0.002, NS) than a booklet control condition ICER was $7275 per unit improvement in IES-R |
| Strong et al.[36] United Kingdom | RCT | Mixed cancer patients with a prognosis >6 months and screened for major depressive disorder (HADS ≥15 and major depressive disorder assessed in a Structured Clinical Interview) (n=200) | Nurse-delivered DCPC comprising education about depression and its treatment (including antidepressant medication), problem-solving treatment, and communication with each patient’s oncologist and general practitioner. A maximum of 10 individual sessions of 45 min were provided over 3 months followed by additional sessions when necessary Control: Usual care. Each patient’s general practitioner was informed about the major depression diagnosis and was provided with advice on antidepressant drug if requested |
6-month | QALYs (EQ-5D) | Healthcare perspective, including intervention costs and direct medical costs | Intervention costs were on average $487 (£262) per patient DCPC was more costly ($+623 (£335), significant) and more effective (incremental QALYs +0.063, significant) than usual care ICER was $9,818 (£5,278) per QALY gained |
| Sabariego et al.[39] Germany | RCT | Mixed cancer patients with increased fear of cancer progression and treated with a 3-week inpatient rehabilitation program (n=174) | Four sessions of 90 min of CBT in addition to the standard rehabilitation program Control: Four sessions of 90 min of SET in addition to the standard rehabilitation program |
1-year | Fear of progression (FoP-Q); Mental health (SF-12 mental) | Societal perspective, including intervention costs, direct medical costs, direct nonmedical and indirect nonmedical costs Indirect nonmedical costs were not included in the CEA analyses | Incremental intervention costs were on average $57 (€47) per patient (or $345 (€282) per group) CBT was less costly ($−2889 (€−2362) or $−3,322 (€−2716) depending on analyses, both NS), while almost equal in effectiveness (FoP-Q incremental effect +0.03, NS and SF-12 incremental effect +0.16, both NS) compared to SET ICER was $−96,309 (€−78,742) per unit improvement in FoP-Q. ICER was $−20,763 (€−16,976) per unit improvement in SF-12 |
| Arving et al.[29]Sweden | RCT | Breast cancer patients about to start adjuvant treatment (n=168) | Individual psychological support from a nurse trained in psychological techniques (INS) Individual psychological support from a psychologist (IPS) No maximum number of sessions were set Control: Usual care including referral to a psychiatrist or social worker when needed |
2-year | QALYs (EORTC QLQ-C30 mapped into EQ-5D) | Healthcare perspective, including intervention costs and direct medical costs | Intervention costs were per patient on average $690 (€560) for the INS group and $805 (€653) for the IPS group INS as well as IPS were less costly ($−8786 (€−7130) and $−6630 (€−5381), both significant) and more effective (incremental QALYs +0.09, NS and +0.16, both NS) compared to usual care INS and IPS were dominant compared to usual care |
| Choi Yoo et al.[31] USA | RCT | Mixed cancer patients with clinical significant depression (PHQ-9 ≥10 and endorsement of depressed mood and/or anhedonia) or pain (definitely or possibly cancer-related and BPI worst pain score ≥6) (n=405) | Centralized telecare management for pain and depression coupled with automated home-based symptom monitoring Control: Usual care, which comprised informing patients on their depressive and pain symptoms and providing screening results to the oncologist |
1-year | QALYs (disease free days; SF-12 converted to SF-6D; modified EQ-5D and a VAS scale) | Healthcare perspective, including intervention costs | Intervention costs were on average $953 (all patients) or $1189 (depressed patients only) per patient Centralized telecare management was more costly ($+953) and more effective (incremental QALYs +0.088, significant (EQ-5D) or +0.013 (SF-12)) than usual care ICER was $10,826 or $73,287 per QALY gained In depressed patients (n=309) the ICER ranged from $1972 to $2695 per disease-free day gained or from $18,018 to $49,549 per QALY gained |
| Walker et al.[34] United Kingdom | Decision analytic model | Hypothetical patient diagnosed with cancer (female 63-years) attending specialist cancer outpatients services (base-case) | Systematic identification for major depressive disorder (HADS ≥15 and major depressive disorder assessed in a Structured Clinical Interview), followed by a nurse-delivered DCPC. DCPC comprised education about depression and its treatment (including antidepressant medication), problem-solving treatment, and communication with each patient’s oncologist and general practitioner, in addition to usual care. A maximum of 10 individual sessions of 45 min was provided over 4 months, followed by additional sessions when necessary Control: Usual care, consisting of identification and treatment of major depression by patient’s general practitioner |
5-year | QALYs | Healthcare perspective, including intervention costs | Intervention costs were per patient $676 (£464) for the intervention group and $532 (£365) for the control group DCPC was more costly ($+144 (£99)) and more effective (incremental QALYs +0.009) than usual care ICER was $17,132 (£11,765) per QALY gained |
| Mewes et al.[33] The Netherlands | Decision analytic model | Hypothetical cohort of 1000 breast cancer patients with matched clinical characteristics as in the RCT | A 6-week CBT program of 90 min each A 12-week home-based exercise program, individually tailored during an intake with a physiotherapistb Control: A usual care, waiting-list control group |
5-year | QALYs (SF-36 converted to EQ-5D) | Healthcare perspective, including intervention costs and direct medical costs | Intervention costs were $247 (€190) per patient CBT was more costly ($239 (€+184)) and more effective (incremental QALYs +0.008) than the weight-list control group. ICER was $29,266 (€22,502) per QALY gained |
| Lengacher et al.[32]USA | RCT | Breast cancer patients who completed treatment within 2 years prior to study enrollment (n=104) | A 6-week mindfulness stress reduction program, which consisted of 2-hour group sessions once a week Control: A usual care, waiting-list control group. Usual care comprised standard posttreatment clinic visits |
12-week | QALYs (SF-12) | Societal perspective, including intervention costs and direct nonmedical costs (i.e., patient opportunity costs) | Intervention costs were $666 per patients The mindfulness program was more costly ($+666 (intervention costs) and $+592 (patient opportunity costs)) and more effective (incremental QALYs +0.03, significant) than usual care ICER was $22,200/QALY for the direct costs and $19,733/QALY for the patient opportunity costs |
| Chatterton et al.[30] Australia | RCT | Mixed cancer patients with elevated levels of distress (score ≥4 on the distress thermometer) (n=336) | Psychologist-led, individual cognitive behavioral intervention (PI) (maximum 5 sessions) Control: Nurse-led, single-session self-management intervention (NI) |
12-month | QALYs (AQOL-8D) | Healthcare perspective, including intervention costs, direct medical costs and direct nonmedical costs (e.g., costs of support services) | Intervention costs were on average $60 (NI) and $181 or $202 (PI) per patient In patients with low distress (BSI <63) the psychologist-led intervention was more costly (+$335, NS) and more effective (incremental QALYs +0.016, NS) than the nurse-led intervention In patients with high levels of distress (BSI >63) the psychologist-led intervention was less costly (−$332, NS) and more effective (incremental QALYs +0.037, NS) than the nurse led intervention |
| Duarte et al.[35] United Kingdom | RCT | Mixed cancer patients with a prognosis >12 months and screened for major depressive disorder (HADS ≥15 and major depressive disorder assessed in a Structured Clinical Interview) (n=500) | Nurse-delivered DCPC comprising education and its treatment (including antidepressant medication), problem-solving treatment, and communication with each patient’s oncologist and general practitioner, in addition to usual care. A maximum of 10 individual sessions of 45 min were provided over a 4 months period, followed by some additional sessions when necessary Control: Usual care, patient’s general practitioner and oncologist were informed about the major depression diagnosis and ask to treat their patients as they normally would |
48-week | QALYs (EQ-5D) | Healthcare perspective, including intervention costs and direct medical costs | Intervention costs were on average $935 (£642) per patient Including only depression-related healthcare costs, DCPC was more costly ($+919 (£631), sig) and more effective (incremental QALYs +0.066, significant) than usual care ICER was $13,905 (£9,549) per QALY gained |
aOnly those outcomes (i.e., psychosocial outcomes or quality adjusted life-years) that were used in this systematic review are presented, bOnly results of the cognitive behavioral therapy group are presented (i.e., results regarding the exercise program are not presented). RCT: Randomized controlled trial; POMS: Profile of mood states; NS: Not significant; ICER: Incremental cost-effectiveness ratio; IES-R: Revised Impact of Events Scale; CEA: Cost-effectiveness analyses; HADS: Hospital Anxiety and Depression Scale; DCPC: Depression care for people with cancer; QALYs: Quality-adjusted life-years; EQ-5D: EuroQol 5-dimensions; CBT: Cognitive behavioral therapy; SET: Supportive-experimental therapy; FoP-Q: Fear of Progression Questionnaire; SF-12: 12-item Health Survey; INS: Individual psychosocial support from a trained nurse; IPS: Individual psychosocial support from a psychologist; EORTC QLQ-C30: The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30-questions; PHQ: Patient Health Questionnaire; BPI: Brief Pain Inventory; SF-6D: Short-Form 6-dimensions; VAS: Visual analog scale; SF-36: Medical Outcomes Study 36-Item Short-Form Health Survey; PI: Psychologist-led, individual cognitive behavioral intervention; NI: Nurse-led, single-session self-management intervention; AQOL-8D: Quality of life - eight dimension; BSI: Brief Symptom Index
Study populations and psychosocial care interventions
Of all nine studies that were performed alongside an RCT, four studies were conducted in breast cancer patients[29,32,37,38] and five studies were conducted in a mixed cancer population[30,31,35,36,39] which also consisted mainly of breast cancer patients. The two model studies used a hypothetical cohort of 1000 breast cancer patients[33] or one hypothetical female cancer patient.[34] In six studies, all patients were included regardless of baseline scores on psychosocial outcomes;[29,32,33,34,37,38] however, in five studies, selection criteria for psychosocial outcomes were set.[30,31,35,36,39] In the studies by Strong et al. and Duarte et al.,[35,36] patients were included when they had a diagnosis of major depressive disorder (based on screening followed by a structured clinical interview). In a study by Choi Yoo et al.,[31] patients screened with clinical significant depression or pain were included. Sabariego et al.[39] included patients screened with increased fear of cancer progression. Finally, Chatterton et al.[30] included patients with elevated levels of distress measured using the distress thermometer.
Studies were heterogeneous regarding the psychosocial care intervention investigated. Four studies investigated a collaborative care intervention,[31,34,35,36] of which three studies investigated the intervention called “depression care for people with cancer,” consisting of a nurse-delivered intervention comprising depression education and its treatment, problem-solving treatment, and communication with each patient's oncologist and general practitioner.[34,35,36] The other study investigated a centralized telecare management intervention for pain and depression coupled with automated home-based symptom monitoring.[31] Four studies investigated a group intervention such as cognitive behavioral group therapy,[33,39] supportive-expressive psychosocial group therapy,[38] and a mindfulness program in groups.[32] Mandelblatt et al.[37] investigated a psycho-education intervention (an educational video addressing re-entry challenges) or a psycho-education intervention combined with individual psycho-educational counseling. Finally, in the studies by Arving et al. and Chatterton et al.,[29,30] the cost-utility of individual psychological support incorporating cognitive behavioral therapy was studied.
Most studies compared the intervention groups with usual care[29,31,32,33,34,35,36,38] which comprised informing the patient's general practitioner on major depressive disorder diagnosis,[35,36] identification and treatment of major depressive disorder diagnosis by patient's general practitioner,[34] referral to a psychiatrist or social worker when needed,[29] provision of educational materials and psychosocial treatment when deemed necessary,[38] informing patients on their depressive and pain symptoms and providing screening results to the oncologist,[31] or standard posttreatment clinic visits.[32] In one study, it was not entirely clear what usual care encompassed.[33] Three studies compared the intervention groups with a booklet control condition,[37] supportive-experiential group therapy,[39] or a nurse-led self-management intervention.[30]
Methods of the cost-effectiveness and cost-utility studies
Seven studies performed cost-utility analyses[29,30,32,33,34,35,36] using the EuroQol five-dimension (EQ-5D),[35,36] the 12-Item Health Survey (SF-12),[32] assessment of quality of life — Eight dimensions (AQOL-8D),[30] mapping of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), or the medical outcomes study 36-Item Short-Form Health Survey (SF-36) into EQ-5D scores[29,33] or using estimates based on previous studies.[34] Three studies performed cost-effectiveness analyses using profile of mood states (mood),[38] Revised Impact of Events Scale (distress),[37] 12-Item Health Survey (mental health), or Fear of Progression Questionnaire (fear of cancer progression)[39] as outcome measures. One study performed both cost-utility and cost-effectiveness analyses with depression-free days gained (calculated using the 20-item Hopkins symptoms checklist) as the outcome in the cost-effectiveness analyses.[31]
Follow-up period for measurement of effects and costs was mostly 6 to 12 months after the intervention.[30,31,35,36,37,38,39] One study had a follow-up period of 12 weeks,[32] one study of 2 years,[29] and the two model studies had a follow-up period of 5 years.[33,34]
The majority of studies used the healthcare perspective for measuring costs[29,30,31,33,34,35,36,38] while three studies used a societal perspective[32,37,39] although cost inputs were not always consistent with the perspective taken. In the actual cost-effectiveness analyses, three studies included intervention costs only,[31,34,37] five studies included intervention and direct medical costs (e.g., hospitalization or visit to the general practitioner),[29,33,35,36,38] and three studies included intervention, direct medical, and direct nonmedical costs (e.g., cost for support services).[30,32,39] One study measured indirect nonmedical costs (e.g., productivity losses); however, these costs were not included in the actual analyses.[39]
Cost-effectiveness of the included psychosocial care interventions
Information on the cost-effectiveness or cost-utility of the different psychosocial care interventions is presented in Table 2. In Table 3, these findings are summarized using a permutation matrix. Two studies found evidence that costs were lower while the intervention was more effective, indicating dominance of psychosocial care.[29,30] Arving et al.[29] found that individual psychological support provided by a nurse or psychologist was significantly less costly ($8786 or $6630, respectively) and more effective in gaining QALYs (nonsignificant incremental QALYs of +0.09 and +0.16, respectively) compared to usual care. Chatterton et al.[30] found that in highly-distressed cancer patients treated with cognitive behavioral group therapy, total costs were on average $332 nonsignificantly lower, and more QALYs were gained (nonsignificant incremental QALYs of +0.037) compared to a nurse-led self-management intervention. However, in less-distressed patients, less strong evidence in favor of cognitive behavioral group therapy compared to the self-management intervention was found (i.e., costs were $335 higher and incremental QALYs were +0.016).
Table 3.
Permutation matrix
| Incremental effectiveness | ||||
|---|---|---|---|---|
| Incremental costs | More effective | Equal effective | Less effective | |
| More costly | Lemieux et al.[38] (supportive expressive psychosocial group therapy) Mandelblatt et al.[37] (educational video or educational video combined with psycho-educational counseling) Strong et al.[36] (nurse-delivered collaborative care) Choi Yoo et al.[31] (centralized telecare management) Walker et al.[34] (nurse-delivered collaborative care) Mewes et al.[33] (cognitive behavioral group therapy) Lengacher et al.[32](mindfulness stress reduction program) Duarte et al.[35] (nurse-delivered collaborative care) |
|||
| Equal in costs | ||||
| Less costly | Arving et al.[29] (individual psychological support from a nurse or psychologist) Chatterton et al.[30] (psychologist-led, individual cognitive behavioral intervention) |
Sabariego et al.[39] (cognitive behavioral group therapy) | ||
One study showed lower costs in the psychosocial intervention group compared to the control group while effectiveness was almost equal.[39] This study by Sabariego et al.[39] found on average $2889-$3322 nonsignificantly lower costs in the cognitive behavioral group therapy group compared to the supportive-experiential group therapy. No major difference in effects was found on fear of progression or mental health. The probability that cognitive behavioral therapy was more cost-effective compared to supportive-experimental group therapy without additional costs was 92%, indicating that cognitive behavioral group therapy is likely to be cost-effective.
All of the eight other studies found evidence that psychosocial care is more effective albeit at higher costs.[31,32,33,34,35,36,37,38] Whether the psychosocial care interventions investigated in these studies can be seen as cost-effective depends on the willingness-to-pay for an incremental unit of effect. Of the eight studies, four studies investigated a collaborative care intervention compared to usual care.[31,34,35,36] These studies found that incremental costs were $144-$953 higher while incremental QALYs were 0.009-0.088 higher. The corresponding incremental costs for an incremental QALY gained (i.e., ICER) were $9818/QALY,[36] $13,905/QALY,[35] $17,132/QALY,[34] or ranged from $10,826/QALY to $73,287/QALY, depending on the method used to measure QALYs.[31]
Three of the other four studies that found higher effects and higher costs investigated the cost-effectiveness of psychosocial group interventions.[32,33,38] Lemieux et al.[38] found that supportive-expressive psychosocial group therapy was significantly more effective in improving mood than usual care. However, total costs were higher ($+3526), resulting in incremental costs of $5550 for an effect size of 0.5 mood. Mewes et al.,[33] who investigated the cost-effectiveness of cognitive behavioral group therapy, found $239 higher costs and an incremental QALY gain of 0.008 in the intervention group compared to the waiting-list usual care group. The ICER was $29,266/QALY. In addition, Lengacher et al.[32] found that a mindfulness program in groups was more costly ($+666) while significantly more effective in gaining QALYs (incremental QALY gain of +0.03) than a waiting-list usual care group. This resulted in an ICER of $22,200/QALY.
The last study that reported higher effects although at higher costs was a study by Mandelblatt et al.[37] This study only included intervention costs in the actual cost-effectiveness analyses. They reported that a psycho-education intervention (which consisted of an educational video addressing re-entry challenges) was more costly ($+15) while marginally more effective (nonsignificant incremental effect in distress of −0.002) compared to a booklet control condition. A psycho-education intervention combined with individual psycho-educational counseling was not more effective than the booklet control condition or psycho-education alone while total costs were higher. Psycho-education combined with individual psycho-educational counseling can therefore be seen as dominated. In additional analyses, direct medical costs between the three groups were compared which showed no significant differences.
Quality of the Included Studies
The quality of the included studies was in general moderate; total score ranged from 5 to 9 [Table 4]. Lemieux et al.[38] scored the lowest while Arving et al., Walker et al., and Duarte et al.[29,34,35] scored the highest. It was remarkable that in four studies, the cost-effectiveness or cost-utility of a psychosocial care intervention was investigated while the effectiveness was not yet properly established.[30,32,37,38] Another major concern was the inclusion of all relevant costs and consequences; three studies only included intervention costs,[31,34,37] hampering the measurement of a potential cost offset. In addition, only two studies measured informal care costs[30,39] and only one study measured productivity losses.[39] Another concern was the measurement of costs and consequences; three studies did not provide clear information regarding the source of data,[32,36,38] and two studies omitted costs from the actual cost-effectiveness analyses without giving clear arguments.[37,39] Furthermore, four studies did not give sufficient information on the valuation of costs and consequences, lacking for instance information on index year.[31,32,33,36] A positive point was that the studies, except for one,[39] performed sensitivity analyses. In addition, all of the studies provided information on incremental costs and incremental effects.
Table 4.
Quality assessment of the included studies
| Items | Lemieux et al.[38] | Mandelblatt et al.[37] | Strong et al.[36] | Sabariego et al.[39] | Arving et al.[29] | Choi Yoo et al.[31] | Walker et al.[34] | Mewes et al.[33] | Lengacher et al.[32] | Chatterton et al.[30] | Duarte et al.[35] | % yes or NA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Was a well-defined question posed? | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 73 |
| Was a description of the alternatives given? And were all relevant alternatives omitted? | Yes | Partly | Yes | Partly | Yes | Yes | Yes | Yes | Yes | Partly | Yes | 73 |
| Was the effectiveness established? | Partly | Partly | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Partly | Yes | 64 |
| Were all relevant and important costs and consequences identified for each alternative? | No | No | No | Yes | No | No | No | No | No | No | No | 9 |
| Were costs and consequences measured accurately in appropriate units? | Partly | No | Partly | No | Yes | Yes | Yes | Yes | Partly | Yes | Yes | 55 |
| Costs and consequences valued credibly? | Yes | Yes | Partly | Yes | Yes | Partly | Yes | No | Partly | Yes | Yes | 64 |
| Were costs and consequences adjusted for differential timing? | No | NA | NA | NA | NA | NA | Yes | Yes | NA | NA | NA | 91 |
| Was an incremental analysis of costs and consequences of alternatives performed? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100 |
| Was allowance made for uncertainty for the estimates of costs and consequences? | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 91 |
| Did the presentation and discussion of study results include all relevant issues? | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 73 |
| Total | 5 | 6 | 7 | 7, 5 | 9 | 7, 5 | 9 | 8 | 6 | 8 | 9 |
NA: Not applicable
Discussion
In this study, we aimed to assess current evidence on the cost-effectiveness and cost-utility of psychosocial care interventions in cancer patients. Eleven studies were included in this review, of which seven in recent years (2014 or 2015). Two of the included studies, both on individual psychological support found lower costs and higher effects compared to the control group[29,30] while one study on cognitive behavioral group therapy found lower costs and equal effects compared to the control group.[39] These findings support the hypothesis of Carlson and Bultz[11,12] that psychosocial care not only can improve outcomes but also can lead to cost savings. However, eight other studies on collaborative care, group interventions and psycho-education, found higher effects and higher costs compared to the control group,[31,32,33,34,35,36,37,38] indicating that psychosocial care is likely to be effective although at additional costs.
Whether these additional costs are acceptabledepends on the willingness-to-pay for an incremental unit of effect. Several willingness-to-pay thresholds have been suggested in the literature, with higher thresholds for more serious diseases.[40] An often used threshold is the National Institute for Health and Clinical Excellence threshold of about $28,992-$43,488/QALY (£20,000-£30,000/QALY).[41,42] Based on these thresholds, six of the eight studies in the present study that found higher costs and higher effects are likely to be cost-effective (ICER ranged from $9818 to $29,266/QALY, with one outlier at $73,287/QALY).[31,32,33,34,35,36] The other two studies found incremental costs of $5550 for an effect size of 0.5 in mood[38] or marginal higher costs ($+15) for a marginal incremental effect in distress of –0.002.[37] No clear willingness-to-pay thresholds exist for these outcome measures although the incremental costs for an effect size 0.5 in mood may be judged as acceptable.[38]
In summary, findings thus showed that psychosocial care is likely to be cost-effective at potentially acceptable willingness-to-pay thresholds, with three interventions[29,30,39] even cost-effective at a willingness-to-pay threshold of zero. It was remarkable that of these three studies,[29,30,39] two studies investigated individual psychological support.[29,30] However, no clear conclusions can be drawn regarding the dominance of individual psychological support compared to other psychosocial care interventions since there was considerable heterogeneity among studies. Studies differed regarding psychosocial care intervention investigated, care provided in the control group, study population targeted, used outcome measure, and included cost categories, which hampers comparability of the results. Further research is therefore called for.
Several recommendations can be formulated for these further studies. At first, more studies should be performed to investigate which psychosocial care interventions are most likely to be cost-effective and for whom these psychosocial care interventions are most likely to be cost-effective. It may be assumed that in line with findings on effectiveness,[6] psychosocial care interventions are especially cost-effective in preselected patients who suffer from psychosocial problems. Five of the 11 studies included in this review preselected patients based on psychosocial outcomes. However, no clear conclusion can be drawn as to whether these studies were more cost-effective than studies that did not preselect patients since studies that did and did not preselect patients differed regarding the type of intervention provided.
In addition, further studies should focus on the cost-effectiveness or cost-utility of psychosocial care from a societal perspective as recommended in several guidelines.[22,43,44] In this review, no study included productivity losses in the actual analyses (although one study measured productivity losses),[39] and only two studies[30,39] measured informal care costs. Productivity losses and informal care costs have been shown to provide an important contribution to the overall economic burden of cancer.[25] Since it can be hypothesized that the provision of psychosocial care can reduce both productivity losses and costs of providing informal care,[11,12,17,18] further studies should take these costs into account, especially when healthcare is being paid for by the society.
Moreover, additional research should be performed using the QALY as outcome measure as also recommended in pertinent guidelines,[42,43] which will enhance comparability of results among different psychosocial interventions as well as enhance comparability to cost-effectiveness or cost-utility of other (supportive) care interventions. Although the more recent studies included in this review already used the QALY as outcome measure, the strategies to calculate QALYs widely differed. Different measurement instruments were used to calculate QALYs such as the EQ-5D, SF-6D, and the AQOL-8D. In addition, different strategies were used for mapping outcomes of other instruments, such as the EORTC QLQ-C30 or SF-36, into EQ-5D scores. A more uniform approach is recommended to enhance comparability.
Some limitations of this review are evident. At first, included studies were in general of moderate quality. Several studies lacked sufficient information on the effectiveness of the studied intervention, the source of data, the reasons for data omission, the valuation of costs and consequences or did not include all relevant costs and consequences which may limit validity of findings. In addition, studies showed considerable heterogeneity in studied psychosocial care interventions and study methods, hampering the formulation of clear conclusions. Furthermore, most studies were conducted among breast cancer patients and may therefore not be representative for other patient groups. Finally, all studies were conducted in Western countries, hampering generalizability to other non-Western countries. A clear strength of this review is that it encompassed an up to date literature search which included seven studies published in 2014 or 2015, which were not yet included in the most recent review.[27] This reflects the fast-growing number of studies that are conducted on the cost-effectiveness or cost-utility of psychosocial care. In addition, several protocol papers of currently ongoing studies were identified,[9,45,46,47,48,49,50] which will provide new evidence on the cost-effectiveness or cost-utility of psychosocial care in the coming years.
Conclusion
Results of this review revealed that psychosocial care is likely to be cost-effective at different, potentially acceptable, willingness-to-pay thresholds. Heterogeneity of studies, however, hampered the comparison of findings and consequently the formulation of clear conclusions regarding the most cost-effective psychosocial care interventions. New studies providing insight on which psychosocial care interventions are most likely to be cost-effective and for whom are therefore called for. In these new studies, potential important cost drivers from a societal perspective, such as productivity losses or informal care costs, should be taken into account.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Krebber AM, Buffart LM, Kleijn G, Riepma IC, de Bree R, Leemans CR, et al. Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23:121–30. doi: 10.1002/pon.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncol. 2013;14:721–32. doi: 10.1016/S1470-2045(13)70244-4. [DOI] [PubMed] [Google Scholar]
- 3.Simard S, Thewes B, Humphris G, Dixon M, Hayden C, Mireskandari S, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300–22. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]
- 4.Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer?. A systematic review. Support Care Cancer. 2009;17:1117–28. doi: 10.1007/s00520-009-0615-5. [DOI] [PubMed] [Google Scholar]
- 5.Jansen F, van Uden-Kraan CF, van Zwieten V, Witte BI, Verdonck-de Leeuw IM. Cancer survivors’ perceived need for supportive care and their attitude towards self-management and eHealth. Support Care Cancer. 2015;23:1679–88. doi: 10.1007/s00520-014-2514-7. [DOI] [PubMed] [Google Scholar]
- 6.Faller H, Schuler M, Richard M, Heckl U, Weis J, Küffner R. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: Systematic review and meta-analysis. J Clin Oncol. 2013;31:782–93. doi: 10.1200/JCO.2011.40.8922. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe M, Walker J, Holm Hansen C, Martin P, Symeonides S, Gourley C, et al. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): A multicentre randomised controlled effectiveness trial. Lancet. 2014;384:1099–108. doi: 10.1016/S0140-6736(14)61231-9. [DOI] [PubMed] [Google Scholar]
- 8.Walker J, Hansen CH, Martin P, Symeonides S, Gourley C, Wall L, et al. Integrated collaborative care for major depression comorbid with a poor prognosis cancer (SMaRT Oncology-3): A multicentre randomised controlled trial in patients with lung cancer. Lancet Oncol. 2014;15:1168–76. doi: 10.1016/S1470-2045(14)70343-2. [DOI] [PubMed] [Google Scholar]
- 9.Krebber AM, Leemans CR, de Bree R, van Straten A, Smit F, Smit EF, et al. Stepped care targeting psychological distress in head and neck and lung cancer patients: A randomized clinical trial. BMC Cancer. 2012;12:173. doi: 10.1186/1471-2407-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart SL, Hoyt MA, Diefenbach M, Anderson DR, Kilbourn KM, Craft LL, et al. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J Natl Cancer Inst. 2012;104:990–1004. doi: 10.1093/jnci/djs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson LE, Bultz BD. Efficacy and medical cost offset of psychosocial interventions in cancer care: Making the case for economic analyses. Psychooncology. 2004;13:837–49. doi: 10.1002/pon.832. [DOI] [PubMed] [Google Scholar]
- 12.Carlson LE, Bultz BD. Benefits of psychosocial oncology care: Improved quality of life and medical cost offset. Health Qual Life Outcomes. 2003;1:8. doi: 10.1186/1477-7525-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han X, Lin CC, Li C, de Moor JS, Rodriguez JL, Kent EE, et al. Association between serious psychological distress and health care use and expenditures by cancer history. Cancer. 2015;121:614–622. doi: 10.1002/cncr.29102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebel S, Tomei C, Feldstain A, Beattie S, McCallum M. Does fear of cancer recurrence predict cancer survivors’ health care use? Support Care Cancer. 2013;21:901–6. doi: 10.1007/s00520-012-1685-3. [DOI] [PubMed] [Google Scholar]
- 15.Thewes B, Butow P, Bell ML, Beith J, Stuart-Harris R, Grossi M, et al. Fear of cancer recurrence in young women with a history of early-stage breast cancer: A cross-sectional study of prevalence and association with health behaviours. Support Care Cancer. 2012;20:2651–9. doi: 10.1007/s00520-011-1371-x. [DOI] [PubMed] [Google Scholar]
- 16.Pan X, Sambamoorthi U. Health care expenditures associated with depression in adults with cancer. J Community Support Oncol. 2015;13:240–7. doi: 10.12788/jcso.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verdonck-de Leeuw IM, van Bleek WJ, Leemans CR, de Bree R. Employment and return to work in head and neck cancer survivors. Oral Oncol. 2010;46:56–60. doi: 10.1016/j.oraloncology.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Tevaarwerk AJ, Lee JW, Sesto ME, Buhr KA, Cleeland CS, Manola J, et al. Employment outcomes among survivors of common cancers: The Symptom Outcomes and Practice Patterns (SOAPP) study. J Cancer Surviv. 2013;7:191–202. doi: 10.1007/s11764-012-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkar S, Sautier L, Schilling G, Bokemeyer C, Koch U, Mehnert A, et al. Anxiety and fear of cancer recurrence and its association with supportive care needs and health-care service utilization in cancer patients. J Cancer Surviv. 2015;9:567–75. doi: 10.1007/s11764-015-0434-2. [DOI] [PubMed] [Google Scholar]
- 20.Azuero C, Allen RS, Kvale E, Azuero A, Parmelee P. Determinants of psychology service utilization in a palliative care outpatient population. Psychooncology. 2014;23:650–7. doi: 10.1002/pon.3454. [DOI] [PubMed] [Google Scholar]
- 21.Drummond MF, Aguiar-Ibanez R, Nixon J. Economic evaluation. Singapore Med J. 2006;47:456–61. [PubMed] [Google Scholar]
- 22.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. 3rd ed. Oxford: Oxford University Press; 2005. Methods for the Economic Evaluation of Health Care Programmes. [Google Scholar]
- 23.Aaronson NK, Mattioli V, Minton O, Weis J, Johansen C, Dalton SO, et al. Beyond treatment — Psychosocial and behavioural issues in cancer survivorship research and practice. EJC Suppl. 2014;12:54–64. doi: 10.1016/j.ejcsup.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12:933–80. doi: 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 25.Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: A population-based cost analysis. Lancet Oncol. 2013;14:1165–74. doi: 10.1016/S1470-2045(13)70442-X. [DOI] [PubMed] [Google Scholar]
- 26.Gordon LG, Beesley VL, Scuffham PA. Evidence on the economic value of psychosocial interventions to alleviate anxiety and depression among cancer survivors: A systematic review. Asia Pac J Clin Oncol. 2011;7:96–105. doi: 10.1111/j.1743-7563.2011.01395.x. [DOI] [PubMed] [Google Scholar]
- 27.Dieng M, Cust AE, Kasparian NA, Mann GJ, Morton RL. Economic evaluations of psychosocial interventions in cancer: A systematic review. Psychooncology. 2016 Jan 26; doi: 10.1002/pon.4075. doi: 10.1002/pon.4075. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Nixon J, Khan KS, Kleijnen J. Summarising economic evaluations in systematic reviews: A new approach. BMJ. 2001;322:1596–8. doi: 10.1136/bmj.322.7302.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arving C, Brandberg Y, Feldman I, Johansson B, Glimelius B. Cost-utility analysis of individual psychosocial support interventions for breast cancer patients in a randomized controlled study. Psychooncology. 2014;23:251–8. doi: 10.1002/pon.3411. [DOI] [PubMed] [Google Scholar]
- 30.Chatterton ML, Chambers S, Occhipinti S, Girgis A, Dunn J, Carter R, et al. Economic evaluation of a psychological intervention for high distress cancer patients and carers: Costs and quality-adjusted life years. Psychooncology. 2015 Nov 3; doi: 10.1002/pon.4020. doi: 10.1002/pon.4020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Choi Yoo SJ, Nyman JA, Cheville AL, Kroenke K. Cost effectiveness of telecare management for pain and depression in patients with cancer: Results from a randomized trial. Gen Hosp Psychiatry. 2014;36:599–606. doi: 10.1016/j.genhosppsych.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lengacher CA, Kip KE, Reich RR, Craig BM, Mogos M, Ramesar S, et al. A cost-effective mindfulness stress reduction program: A randomized control trial for breast cancer survivors. Nurs Econ. 2015;33:210–8, 232. [PubMed] [Google Scholar]
- 33.Mewes JC, Steuten LM, Duijts SF, Oldenburg HS, van Beurden M, Stuiver MM, et al. Cost-effectiveness of cognitive behavioral therapy and physical exercise for alleviating treatment-induced menopausal symptoms in breast cancer patients. J Cancer Surviv. 2015;9:126–35. doi: 10.1007/s11764-014-0396-9. [DOI] [PubMed] [Google Scholar]
- 34.Walker S, Walker J, Richardson G, Palmer S, Wu Q, Gilbody S, et al. Cost-effectiveness of combining systematic identification and treatment of co-morbid major depression for people with chronic diseases: The example of cancer. Psychol Med. 2014;44:1451–60. doi: 10.1017/S0033291713002079. [DOI] [PubMed] [Google Scholar]
- 35.Duarte A, Walker J, Walker S, Richardson G, Holm Hansen C, Martin P, et al. Cost-effectiveness of integrated collaborative care for comorbid major depression in patients with cancer. J Psychosom Res. 2015;79:465–70. doi: 10.1016/j.jpsychores.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strong V, Waters R, Hibberd C, Murray G, Wall L, Walker J, et al. Management of depression for people with cancer (SMaRT oncology 1): A randomised trial. Lancet. 2008;372:40–8. doi: 10.1016/S0140-6736(08)60991-5. [DOI] [PubMed] [Google Scholar]
- 37.Mandelblatt JS, Cullen J, Lawrence WF, Stanton AL, Yi B, Kwan L, et al. Economic evaluation alongside a clinical trial of psycho-educational interventions to improve adjustment to survivorship among patients with breast cancer. J Clin Oncol. 2008;26:1684–90. doi: 10.1200/JCO.2007.14.0822. [DOI] [PubMed] [Google Scholar]
- 38.Lemieux J, Topp A, Chappell H, Ennis M, Goodwin PJ. Economic analysis of psychosocial group therapy in women with metastatic breast cancer. Breast Cancer Res Treat. 2006;100:183–90. doi: 10.1007/s10549-006-9249-1. [DOI] [PubMed] [Google Scholar]
- 39.Sabariego C, Brach M, Herschbach P, Berg P, Stucki G. Cost-effectiveness of cognitive-behavioral group therapy for dysfunctional fear of progression in cancer patients. Eur J Health Econ. 2011;12:489–97. doi: 10.1007/s10198-010-0266-y. [DOI] [PubMed] [Google Scholar]
- 40.van Gils PF, Schoemaker CG, Polder JJ. How much should a gained life-year cost?. Study on the assessment of a QALY. Ned Tijdschr Geneeskd. 2013;157:A6507. [PubMed] [Google Scholar]
- 41.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: What it is and what that means. Pharmacoeconomics. 2008;26:733–44. doi: 10.2165/00019053-200826090-00004. [DOI] [PubMed] [Google Scholar]
- 42.National Institute for Health and Care Excellence (NICE). Process and Methods Guides. The Social Care Guidance Manual. 2013 [PubMed] [Google Scholar]
- 43.The Netherlands: National Health Care Institute; 2015. National Health Care Institute. Guideline for conducting economic evaluations in healthcare. [Google Scholar]
- 44.Tan-Torres Edejer T, Baltussen T, Adam T, Hutubessy R, Acharya A, Evans DB, et al. Geneva: World Health Organization; 2003. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. [Google Scholar]
- 45.Compen FR, Bisseling EM, Van der Lee ML, Adang EM, Donders AR, Speckens AE. Study protocol of a multicenter randomized controlled trial comparing the effectiveness of group and individual internet-based mindfulness-based cognitive therapy with treatment as usual in reducing psychological distress in cancer patients: The BeMind study. BMC Psychol. 2015;3:27. doi: 10.1186/s40359-015-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schellekens MP, van den Hurk DG, Prins JB, Molema J, Donders AR, Woertman WH, et al. Study protocol of a randomized controlled trial comparing mindfulness-based stress reduction with treatment as usual in reducing psychological distress in patients with lung cancer and their partners: The MILON study. BMC Cancer. 2014;14:3. doi: 10.1186/1471-2407-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nordin K, Rissanen R, Ahlgren J, Burell G, Fjällskog ML, Börjesson S, et al. Design of the study: How can health care help female breast cancer patients reduce their stress symptoms?. A randomized intervention study with stepped-care. BMC Cancer. 2012;12:167. doi: 10.1186/1471-2407-12-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boele FW, Verdonck-de Leeuw IM, Cuijpers P, Reijneveld JC, Heimans JJ, Klein M. Internet-based guided self-help for glioma patients with depressive symptoms: Design of a randomized controlled trial. BMC Neurol. 2014;14:81. doi: 10.1186/1471-2377-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Spek N, Vos J, van Uden-Kraan CF, Breitbart W, Cuijpers P, Knipscheer-Kuipers K, et al. Effectiveness and cost-effectiveness of meaning-centered group psychotherapy in cancer survivors: Protocol of a randomized controlled trial. BMC Psychiatry. 2014;14:22. doi: 10.1186/1471-244X-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattsson S, Alfonsson S, Carlsson M, Nygren P, Olsson E, Johansson B. U-CARE: Internet-based stepped care with interactive support and cognitive behavioral therapy for reduction of anxiety and depressive symptoms in cancer — A clinical trial protocol. BMC Cancer. 2013;13:414. doi: 10.1186/1471-2407-13-414. [DOI] [PMC free article] [PubMed] [Google Scholar]


