Abstract
Objective:
To identify predictors associated with survival in civilian penetrating traumatic brain injury (pTBI) utilizing a contemporary, large, diverse 2-center cohort, and to develop a parsimonious survival prediction score for pTBI.
Methods:
Our cohort comprised 413 pTBI patients retrospectively identified from the local trauma registries at 2 US level 1 trauma centers, of which one was predominantly urban and the other predominantly rural. Predictors of in-hospital and 6-month survival identified in univariate and multivariable logistic regression were used to develop the simple Surviving Penetrating Injury to the Brain (SPIN) score.
Results:
The mean age was 33 ± 16 years and patients were predominantly male (87%). Survival at hospital discharge as well as 6 months post pTBI was 42.4%. Higher motor Glasgow Coma Scale subscore, pupillary reactivity, lack of self-inflicted injury, transfer from other hospital, female sex, lower Injury Severity Score, and lower international normalized ratio were independently associated with survival (all p < 0.001; model area under the curve 0.962). Important radiologic factors associated with survival were also identified but their addition to the full multivariable would have resulted in model overfitting without much gain in the area under the curve.
Conclusions:
The SPIN score, a logistic regression–based clinical risk stratification scale estimating survival after pTBI, was developed in this large, diverse 2-center cohort. While this preliminary clinical survival prediction tool does not include radiologic factors, it may support clinical decision-making after civilian pTBI if external validation confirms the probability estimates.
The predominant experience of penetrating traumatic brain injury (pTBI) derives from battlefield settings, but the contemporary civilian experience in patients treated after 2005 is limited to small and single-center studies.1–5 As a result, the epidemiology of civilian pTBI in modern trauma and neurocritical care settings remains incompletely understood, as does the neuroanatomical and biological basis for secondary brain injury. pTBI is often devastating, yet mortality rates among trauma centers vary widely, between 44% and 100%.2 Gunshot wounds (GSW), the primary mechanism for pTBI, are a serious public health problem. They account for a significant proportion of deaths across all head trauma, and claim over 32,000 lives in the United States annually,6 among >62,000 victims of firearms-related injuries.7 According to the most updated 2013 Centers for Disease Control and Prevention statistics, death rates by firearms comprised 5.1/100,000 for homicides, and 6.7/100,000 for suicides.6 A comprehensive, multivariable assessment of disease-, patient- and hospital-specific characteristics are lacking at a multicenter and population level. Recent and recurrent civilian mass shootings, and the severe wounding of US Congresswoman Gabrielle Giffords in 2011, have raised the public's desire for understanding the prognosis after pTBI.8,9 Defining pTBI outcome determinants is the necessary first step to identify potential strategies to improve patient outcomes.
In blunt TBI, clinical and anatomical predictors of outcome have been validated.10,11 However, the pathophysiology of pTBI is distinct from blunt TBI, and independent identification and validation of predictors for survival are crucial for families and physicians deciding about the allocation of scarce resources and interventions.
The objectives of the current study were to identify predictors associated with survival in a modern, large, diverse 2-center cohort, and to develop a parsimonious survival prediction score for civilian pTBI.
METHODS
Study design and population.
We conducted a retrospective study of consecutive patients with pTBI at the level 1 trauma centers University of Maryland Shock-Trauma Center ([UMSTC]; n = 508) between January 1, 2000, and December 31, 2009, and the University of Massachusetts Medical School/UMASS Memorial Medical Center ([UMASS]; n = 63) between June 20, 2003, and April 17, 2013, identified from the local trauma registries. Data were obtained from the trauma registry and medical records. Inclusion criteria were age ≥18 years with confirmed pTBI involving perforation of the dura on head CT (hCT) and clinical evidence of brain injury on neurologic examination. The medical record was not available for review in 16 patients at UMSTC and 24 patients at UMASS. Patients who were dead on arrival were excluded (UMSTC, n = 115; UMASS, n = 3), totaling 413 patients (n = 377 from UMSTC; n = 36 from UMASS).
Standard protocol approvals, registrations, and patient consents.
The institutional review boards at both centers approved this study with a waiver of consent due to the retrospective design.
Clinical management.
Routine clinical care was provided analogously at both centers with attention to close adherence to the Brain Trauma Foundation for severe traumatic brain injury (TBI) guidelines,12 as well as the American Association of Neurological Surgeons/Congress of Neurological Surgeons recommendations for the management and treatment of pTBI, including emergency neurosurgery.13–15 All patients were received as level 1 trauma activations with immediate resuscitation occurring in the trauma bays of the emergency departments (EDs). Support of ventilation/oxygenation by intubation and hemodynamic support to achieve a cerebral perfusion pressure ≥60 mm Hg with isotonic fluids and vasopressors as well as 30° head of the bed elevation was achieved. Coagulopathies were rapidly corrected using blood products and tranexamic acid or activated Factor VII (in patients on warfarin). Prothrombin complex concentrates were not yet available for clinical use during the study period. All patients were immediately admitted to the neurotrauma intensive care unit (ICU) under the care of board-certified neurointensivists or surgical intensivists with expertise in neurocritical care. Detailed descriptions of the ICU care for severe TBI patients at both centers have been published.16,17
Measurements.
Besides basic patient and hospital demographics, we recorded time-to-hospital arrival, transfer from other hospital, mechanism of injury (GSW, knife, or other object), and self-infliction. Clinical parameters included admission Glasgow Coma Scale (GCS) score, Abbreviated Injury Scale–Head, and Injury Severity Score (ISS), calculated by the local trauma attending, pupillary reactivity, and arrival systolic blood pressure (SBP). Admission laboratory values were recorded. Illicit substance use was documented using an initial urine or serum toxicology screen. Each patient received a noncontrast hCT on arrival. All hCT images were reviewed by 2 trained evaluators, including one board-certified neurocritical care attending (K.N.S. or S.M.) at each site, for bullet trajectory (tangential, penetrating, perforating), injury anatomy (single lobe, multilobe/unilateral, bilateral, or posterior fossa), side of injury (neither, right, left, or both), presence of intracranial hemorrhage, intraventricular hemorrhage, subarachnoid hemorrhage, cisterns (open, compressed, absent), midline shift at the septum pellucidum, and bullet track through the ventricles (figure 1). Furthermore, placement of intracranial pressure monitor, craniectomy, use of prophylactic antibiotics and antiepileptic drugs, and placement of tracheostomy or gastrostomy tube were recorded. Discharge data included hospital length of stay, overall withdrawal of care, withdrawal of care on day 1, do not resuscitate status on admission, mechanism of death, and discharge disposition.
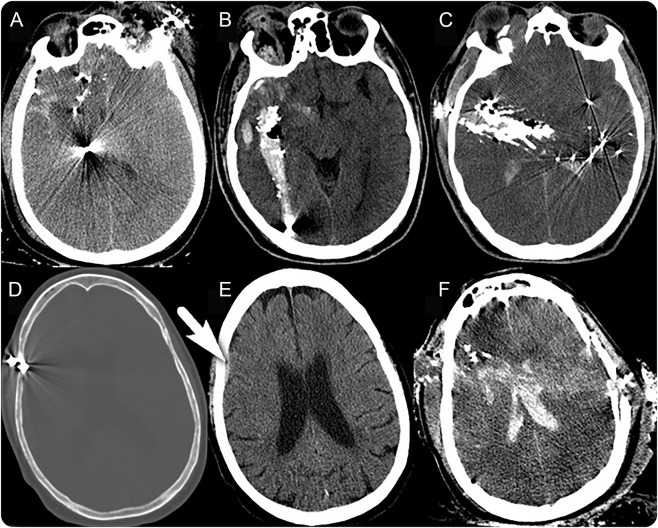
Figure 1. Head CT (hCT) examples of the radiologic grading for trajectory and anatomy.
(A–C) hCTs for 3 different patients, all with penetrating trajectories without perforation but involving different lobes (anatomy): (A) single lobe involvement; (B) unilateral involvement of multiple lobes (temporal, parietal, and occipital); (C) bilateral involvement of multiple lobes (bitemporal, parietal). (D, E) hCT of one patient with tangential trajectory: (D) bone window captures the bullet tangentially penetrating the dura; (E) brain window of same patient reveals an associated small subdural hematoma (arrow). (F) Perforating trajectory with through-and-through injury involving bilateral hemispheres and the ventricles.
Statistical analysis.
The primary outcome measure was inpatient survival; the secondary outcome measure was 6-month survival. Initial frequencies were compared using χ2 tests, t tests, or Wilcoxon rank sum tests, as appropriate. Associations of individual parameters with discharge and 6-month survival were calculated using univariate logistic regression. SBP was grouped using clinically meaningful cutoffs: <90 mm Hg, ≥90–139 mm Hg, ≥140–180 mm Hg, and ≥180 mm Hg. Motor GCS (mGCS) was first treated as an ordinal variable; however, mGCS 2–5 had similarly small observation rates, and each group separately did not have any statistically different associations with survival. Therefore, mGCS was collapsed into 3 groups: 1, 2–5, and 6. Variables with p value < 0.25 were candidates for the multivariable analysis. Multivariable logistic regression models were constructed incrementally by manually adding and removing variables due to the large number of univariate associations with survival. We created a base model using the validated International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) variables10 for blunt TBI: mGCS, pupillary reactivity, (hypotension [SBP <90 mm Hg], and hypoxia in the field or ED [O2 saturation <90%]). Of these, only mGCS and pupillary reactivity were significant predictors, and formed the final base model. Next, grouped variables from the radiologic, demographic, injury, and laboratory categories were manually added and eliminated stepwise. Variables independently associated with survival (p < 0.05 using Wald statistic) and resulting in the best model fit, assessed by −2 log-likelihood and C statistic, remained. Variables were examined for colinearity and interaction. We combined group variables with the base model as able without overfitting to construct a parsimonious multivariable model. From this, we developed a risk stratification scale. The nearest integer from the parameter estimates from the multivariable logistic model was used to assign the score points for the Surviving Penetrating Injury to the Brain (SPIN) score. Associations with survival were expressed in odds ratios with 95% confidence intervals. p Values < 0.05 were considered statistically significant. SAS 9.3 (SAS Institute Inc, Cary, NC) was used for all analyses.
RESULTS
Among the 413 patients, the mean age was 33 ± 16 years; 87% were male and 58% were black (table 1). A total of 175 patients (42.4%) survived to hospital discharge and 6 months after pTBI, with no additional deaths between hospital discharge and 6 months at either center. A total of 156 (41.4%) survived at UMSTC, and 19 (52.8%) at UMASS. There were several baseline differences between the UMSTC and UMASS cohorts (table 1): UMSTC patients were generally younger, predominantly black, more often had isolated pTBI with lower ISS, more commonly had prior traumas, less commonly had self-inflicted pTBI, were less commonly transferred from other centers, and had lower survival rates.
Table 1.
Baseline characteristics
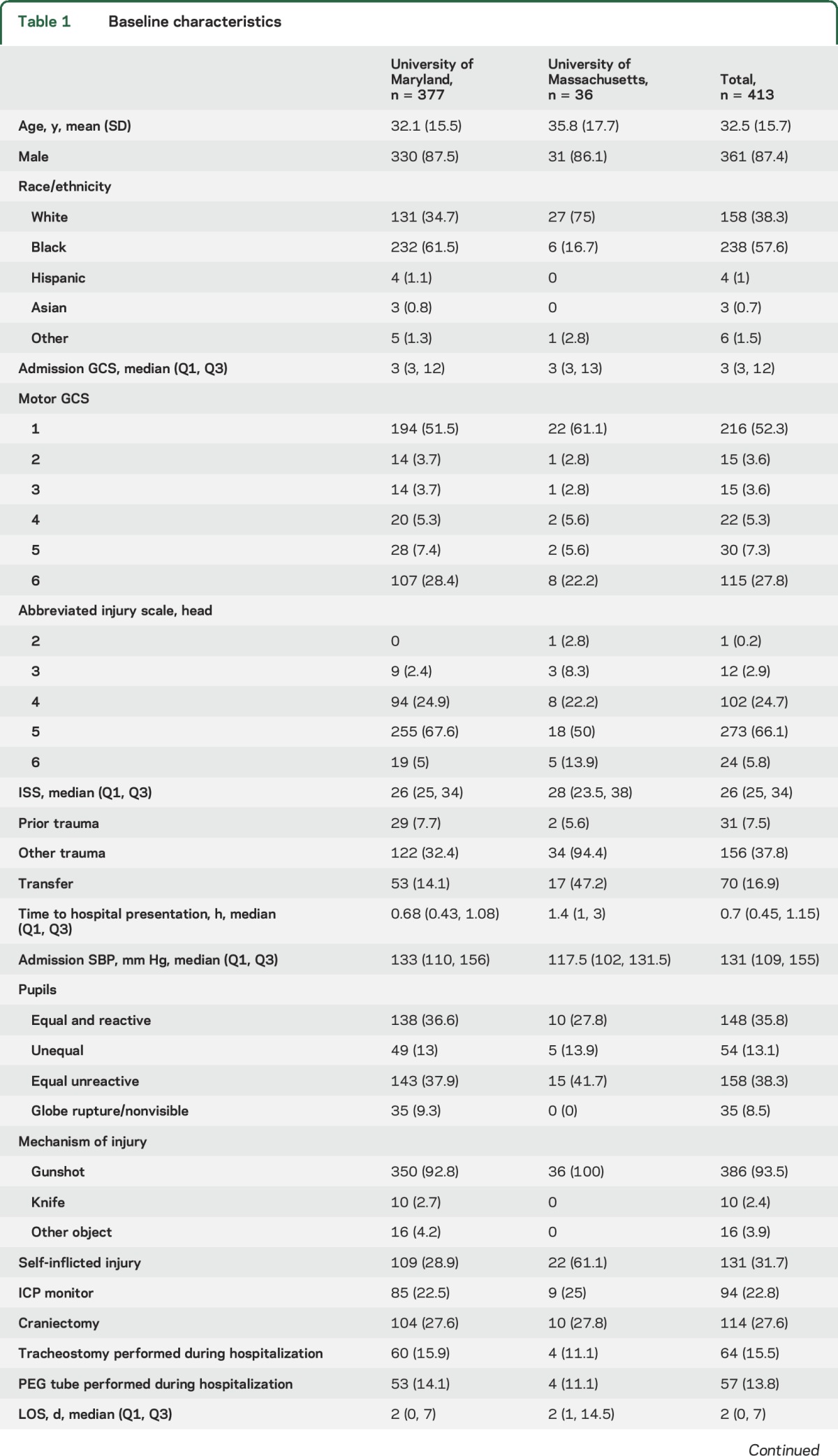
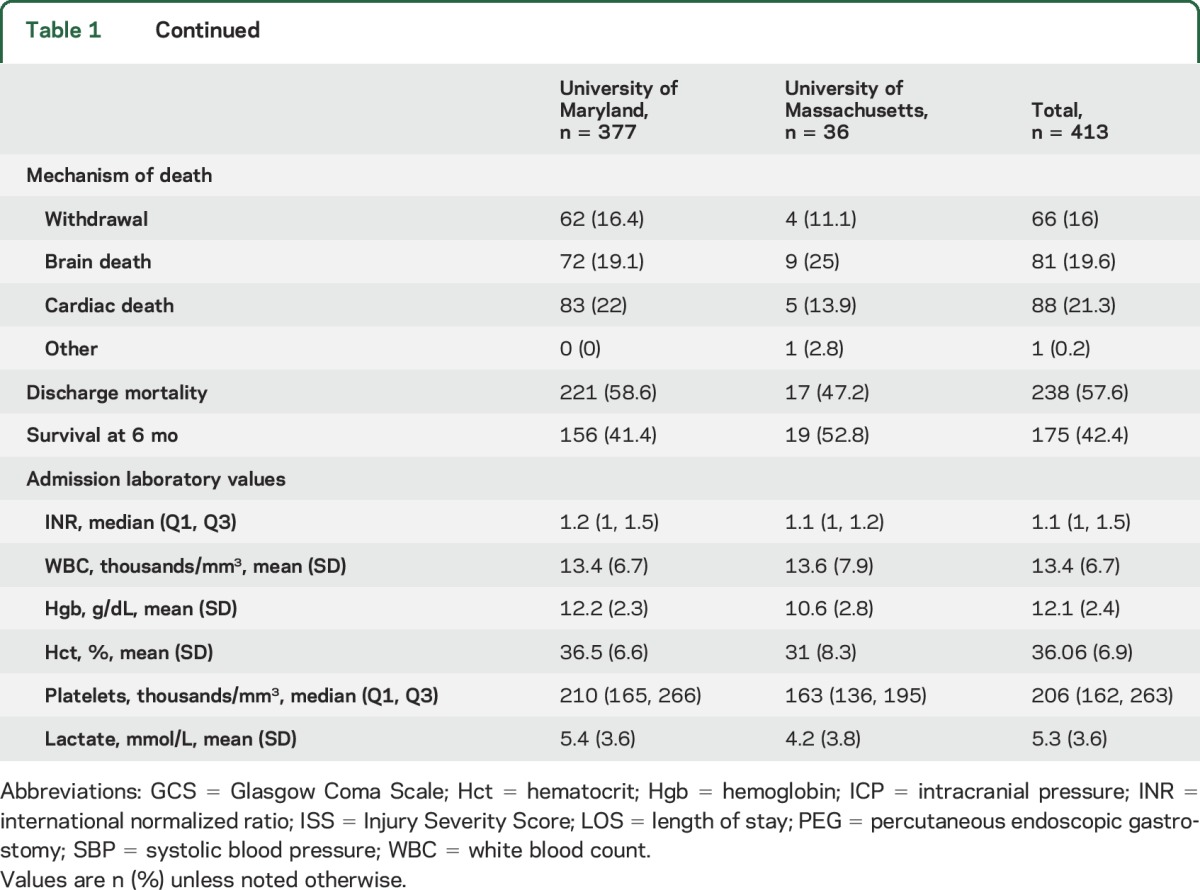
Laboratory values were similar between the centers, except UMASS patients were more anemic, had lower median platelet counts, and had more elevated alcohol levels (table 1). Additional baseline variables are shown in table e-1 at Neurology.org.
hCTs were available for review in 336 (89.1%) UMSTC patients and in 35 (97.2%) UMASS patients (table e-2). Most had penetrating (53.3%) compared to perforating (22.5%) and tangential (14.3%) trajectories. Admission hCTs were similar between the centers, except UMASS patients had higher proportions of penetrating or perforating injuries and intraventricular, intracranial, and subarachnoid hemorrhage.
Univariate associations with survival are shown in table e-3, and identified several candidate variables for the multivariable analysis.
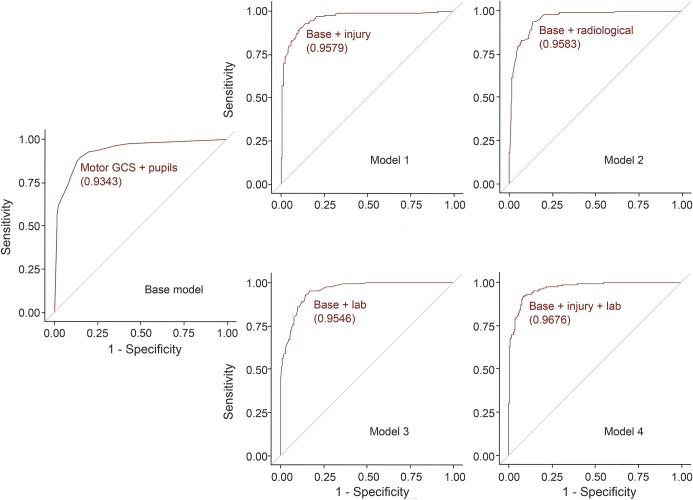
The multivariable base model (mGCS and pupillary reactivity) revealed a C statistic of 0.935 (figure 2). Three separate additional multivariable models were constructed: model 1 (base + injury variables), model 2 (base + radiologic variables), and model 3 (base + laboratory variables), which all attained slightly improved C statistics compared to the already very high base model's C statistic. Combining the base, injury, and laboratory variables (model 4) achieved the highest C statistic of 0.962 (figure 2) with lower ISS, lack of self-inflicted injury, transfer, female sex, and lower international normalized ratio (INR) as independent predictors of survival while adjusting for mGCS and pupillary reactivity (table 2). Adding radiologic variables into this combined model would have resulted in model overfitting, and therefore was not possible. After adjusting for mGCS and pupillary reactivity, trajectory, lack of intraventricular hemorrhage (IVH), lack of cisternal compression, and single (vs multiple) penetrating brain injury at one time remained as independent predictors of survival (table 2).
Figure 2. Receiver operating characteristic (ROC) curves for the multivariable models.
Base model: motor Glasgow Coma Scale (mGCS) and pupillary reactivity on admission. Model 1 (base + injury variables): base + Injury Severity Score (ISS) + self-inflicted + transfer + sex. Model 2 (base + radiologic variables): base + trajectory + intraventricular hemorrhage (IVH) + cisterns + multiple brain wounds. Model 3 (base + laboratory variables): base + international normalized ratio (INR). Model 4 (base + injury + laboratory variables): base + ISS + self-inflicted + transfer + sex + INR. The corresponding area under the curve values (C statistic) are shown in parentheses under each ROC.
Table 2.
Final multivariable models
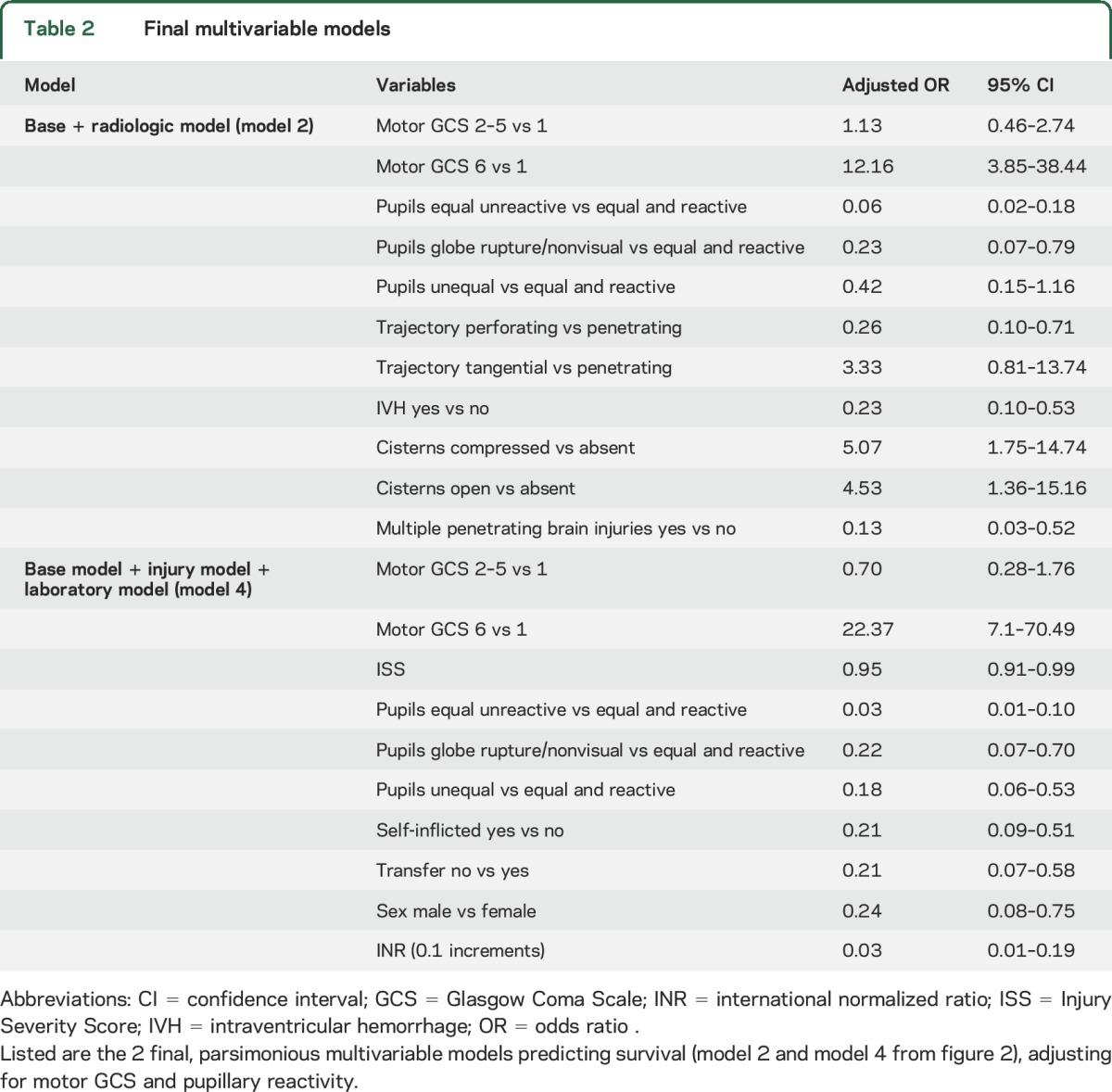
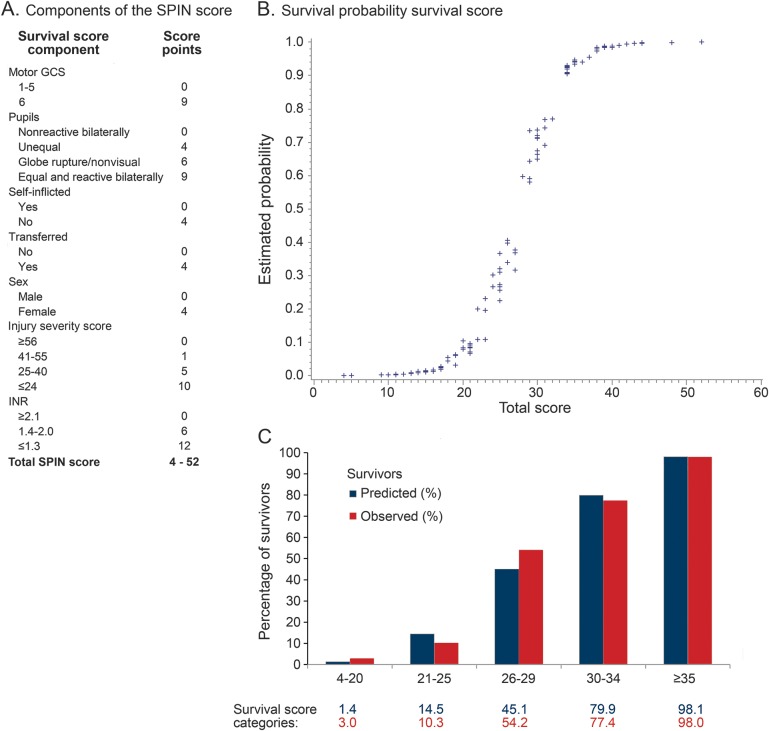
The SPIN score, a survival risk stratification scale, was developed as a sum of individual points (figure 3) from model 4 (base + injury + laboratory model). The score ranged from 4 to 52, with higher scores indicating stronger likelihood of survival. To facilitate the score's usefulness, mGCS, ISS, and INR were divided into the clinically most meaningful categories. In the model, 98% of the patients with a SPIN score of ≥35 survived, while only 3% of the patients with a score of ≤20 survived (figure 3). In fact, there were no patients with a SPIN score of ≤16 who survived.
Figure 3. Surviving Penetrating Injury to the Brain (SPIN) score.
(A) Components of the SPIN score and assigned score points. (B) Estimated survival probability (y-axis) plotted against the total survival (SPIN score). (C) Comparison of the observed (red bars) to predicted (blue bars) proportion of survivors by SPIN score categories. Y-axis: percent of patients who survive at hospital discharge and 6 months after penetrating traumatic brain injury. X-axis: SPIN score categories. Data table: percent of predicted and observed patients in the entire cohort per SPIN score category. GCS = Glasgow Coma Scale; INR = international normalized ratio.
DISCUSSION
In this contemporary, large, ethnically diverse, civilian pTBI population from 2 centers, we found that admission mGCS and pupillary reactivity (base model) were by far the strongest predictors of survival. We identified several additional independent predictors of survival. However, mGCS and pupillary reactivity were such dominant predictors that the other variables improved the survival prediction accuracy only minimally.
Other studies examining specifically GSW have previously identified an association with lower presenting GCS and pupillary reactivity with higher mortality and worse functional outcomes.2,3,5 We applied this to our large mixed pTBI cohort, which included mostly but not exclusively GSWs. Our sequential model building approach confirmed both mGCS and pupillary reactivity as the strongest predictors of survival with a very high area under the curve. Unlike all other studies on GSW, which used total GCS, we intentionally used mGCS, as landmark studies in blunt TBI have shown that in cohorts with mostly intubated patients (as is the case in pTBI), the verbal subscore cannot be obtained due to the presence of the endotracheal tube, and the eye opening subscore is not consistently and reliably recorded, partially due to several external factors, including drug or alcohol intoxication.18,19 We did not equate the verbal GCS subscore to 1 in intubated patients, as it underestimates GCS. Therefore, as in the IMPACT score,10 we used mGCS in our model.
We found that increasing ISS was independently associated with lower odds for survival. ISS indicates the presence of additional body injuries and polytrauma, which portends a higher mortality risk due to systemic injury. Further, self-inflicted pTBI was independently associated with lower odds for survival. In fact, our multivariable adjustment revealed an almost 80% higher odds for dying when the pTBI was self-inflicted. This is likely explained by the close proximity between the entry point of the penetrating object and the brain, with a higher ballistic and more destructive energy. Lower INR indicated higher odds for survival, due to improved hemostasis. We are unable to conclude from our data whether our patients' admission INRs were elevated due to warfarin intake or the presence of disseminated intravascular coagulation from the pTBI.
Female patients had 76% higher odds for survival compared to men, even after adjusting for severity of injury. Female sex has been shown to be protective in blunt TBI,20 although the reason for this remains unclear. While several preclinical and uncontrolled clinical studies have suggested that progesterone provides neuroprotection,21–23 this was not confirmed in large clinical trials in early severe blunt TBI.24,25 However, one additional explanation may be that female patients may have less severe pTBI as they commonly practice less risky behaviors that predispose to pTBI. Alternatively, our injury severity adjustment using ISS may not be robust enough to control for pTBI severity.
We found it surprising that transfer from another hospital was associated with survival; one would assume that direct admission to a level 1 trauma center would result in improved outcomes due to faster specialty trauma care and resuscitation. Our finding may be explained by the fact that patients were only transferred if they survived the initial resuscitation. Further, we excluded all patients who were dead on arrival. Therefore, we call for the attempt to validate transfer from other hospital as a predictor of survival in other cohorts.
Unexpectedly, age was not associated with survival in our adjusted base model. Patients with pTBI are younger in general (mean age ≤35 years), and therefore age is not an explanatory predictor of outcome in pTBI as seen in other diseases afflicting elderly, including blunt TBI, stroke, or cancer.
Other smaller studies examining factors on hCTs have previously revealed that compression of the basal cisterns, IVH, and bullet trajectory with involvement of the zona fatalis, i.e., perforation through bilateral lobes (except bilateral frontal lobes), brainstem, and the ventricles indicate much worse outcomes.1,2,5,26 These studies, however, included only 119 hCTs or fewer, and did not have the statistical power to examine the associations of specific neuroanatomical CT findings to outcomes in a more refined way. Our study comprising 371 hCTs revealed that survival was independently and incrementally associated with a penetrating or tangential trajectory, compared to a perforating trajectory. Similarly, the presence of open or compressed cisterns each carried a 5 times higher odds for survival compared to absent cisterns on hCT. This suggests that aggressive resuscitation should not be withheld based upon presence of a penetrating (but not perforating) trajectory, or compressed (but not absent) cisterns. Importantly, none of the radiologic factors we found to be associated with survival was included in the SPIN score to prevent model overfitting. Therefore, the SPIN score remains preliminary. Even in examples of very low SPIN scores, the radiologic findings should still be considered before making clinical decisions about a patient.
Our study also revealed that single rather than concomitant multiple pTBI was independently associated with 87% higher odds for survival in the adjusted model. This association has not been reported before, although clinically it is not surprising. It is rare to have multiple pTBI at once, and only 8% of patients in our cohort presented with multiple pTBI. However, our study's power was sufficient to document its association with outcome for the first time.
Interestingly, at both centers, the proportion of patients surviving pTBI was the same at hospital discharge as at 6 months postinjury. We paid particular attention to whether this may be due to incomplete follow-up information. At both centers, however, we confirmed through inpatient and outpatient chart review, as well as the public death certificate registry, that survivors were still alive at 6 months, while the deceased had died during the hospital admission within the early period after injury.
Our study has important limitations. We did not internally or externally validate the SPIN score. We considered but decided against internal validation to maximize the power of the full cohort for score development. External validation is ideal, and in planning. Until then, the SPIN score remains preliminary. We excluded patients who were dead on arrival, because we were looking for factors associated with survival for the purpose of bedside prognostication. We may have overlooked other important variables that are associated with imminent death prior to hospital arrival. Our cohort is mostly composed of UMSTC patients, thereby potentially skewing our models towards the urban pTBI population and underestimating factors unique to a rural population. Both subcohorts stem from academic US East Coast centers, and therefore findings may not be generalizable to other geographic areas. Importantly, our cohort comprises a large proportion of minorities. In the United States, pTBI is more prevalent in minority populations7; hence our cohort is representative of the population at highest risk for pTBI. Our study does not prove causality or modifiability. It remains unclear whether early and aggressive interventions to improve mGCS and pupillary reactivity by use of aggressive resuscitation, osmotherapy, or neurosurgery ultimately alters outcome. Due to the study's retrospective nature, some variables were incompletely collected because of unavailability. For the same reasons, we were inherently unable to confirm reliably whether the care at both centers was truly identical, although clinical care was provided at each institution according to level 1 trauma center standards following published guidelines. Therefore, it is conceivable that institutional differences in the routine clinical care of patients existed, resulting in unadjusted biases. We standardized the definitions for our variables pre hoc so that the data acquisition was as uniform as possible. While the attempt at both centers is not to discuss withdrawal of care with the family or setting treatment limitations before 48 hours after admission, we cannot adjust for center- or physician-specific biases that may have led to early limitations of care. Therefore, we were not able to control for early self-fulfilling prophecies or therapeutic nihilism, a common dilemma in all studies involving critically ill brain-injured patients.27–29 Finally, we did not examine whether the GCS and pupillary findings were irreversible or improved serially. Future studies should address whether patients with early clinical findings suggestive of poor outcome improve in the first 12–24 hours, and whether this improvement carries further prognostic value.
In this large, contemporary 2-center US cohort, we identified important clinical and radiologic predictors associated with survival after pTBI. Higher mGCS and pupillary reactivity on admission were by far the strongest independent predictors of survival, with all other independent predictors adding only very little to the improvement of the models' C statistic. The SPIN score has not been validated and does not contain radiologic factors, and therefore is currently a preliminary tool that may provide guidance for physicians and families in their direction-of-care decision-making in patients with pTBI.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Heena Santry for guidance on this study.
GLOSSARY
- ED
emergency department
- GCS
Glasgow Coma Scale
- GSW
gunshot wounds
- hCT
head CT
- ICU
intensive care unit
- IMPACT
International Mission for Prognosis and Analysis of Clinical Trials in TBI
- INR
international normalized ratio
- ISS
Injury Severity Score
- IVH
intraventricular hemorrhage
- mGCS
motor Glasgow Coma Scale
- pTBI
penetrating traumatic brain injury
- SBP
systolic blood pressure
- SPIN
Surviving Penetrating Injury to the Brain
- TBI
traumatic brain injury
- UMASS
University of Massachusetts Medical School/UMASS Memorial Medical Center
- UMSTC
University of Maryland Shock-Trauma Center
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
S.M., K.N.S., T.M.S., D.M.S., and T.E. were responsible for study concept and design. D.A., A.A., and S.I. were responsible for acquisition and analysis of data. S.M., D.A., and K.N.S. were responsible for drafting the manuscript and figures.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
S. Muehlschlegel is funded by NIH/NINDS grant K23HD080971. During the study period, but no longer currently, she received research funding from the American Heart/Stroke Association, Worcester Research Foundation, and the University of Massachusetts Medical School. D. Ayturk, A. Ahlawat, and S. Izzy report no disclosures relevant to the manuscript. T. Scalea receives research support from the National Institutes of Health, National Highway Traffic Safety Administration, United States Army, National Trauma Institute/Department of Defense, and the University of Pittsburgh. During the study period, but no longer currently, he received additional research support from the Maryland Motor Vehicle Administration, TEI Biosciences, US Air Force, American Association for the Surgery of Trauma, University of California, San Diego, and the University of Texas Health Science Center at Houston. D. Stein is supported by a US Air Force grant and serves as an advisor to Decisio Health, Inc., from which entity she receives paid travel. T. Emhoff reports no disclosures relevant to the manuscript. K. Sheth receives research support from Remedy Pharmaceuticals as co-PI for GAMES Pilot, GAMES-RP, and the planned phase III CHARM study, was supported by an American Brain Foundation Research grant at the time of data collection, and is currently a coinvestigator on NIH/NINDS grant R21NS095147 and the American Heart Association Grant-In-Aid 15GRNT25290018. He serves as the Clinical Events Chair in the DAWN Stroke Study by Stryker Neurovascular, as member of the Advisory Committee for intracerebral hemorrhage for Novartis Pharmaceuticals, and performs consulting for C30 Telemedicine. He is an editorial board member for Neurology®, Stroke, Neurocritical Care, and Current Treatment Options in Neurology. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Aldrich EF, Eisenberg HM, Saydjari C, et al. Predictors of mortality in severely head-injured patients with civilian gunshot wounds: a report from the NIH Traumatic Coma Data Bank. Surg Neurol 1992;38:418–423. [DOI] [PubMed] [Google Scholar]
- 2.Aarabi B, Tofighi B, Kufera JA, et al. Predictors of outcome in civilian gunshot wounds to the head. J Neurosurg 2014;120:1138–1146. [DOI] [PubMed] [Google Scholar]
- 3.Martins RS, Siqueira MG, Santos MT, Zanon-Collange N, Moraes OJ. Prognostic factors and treatment of penetrating gunshot wounds to the head. Surg Neurol 2003;60:98–104; discussion 104. [DOI] [PubMed] [Google Scholar]
- 4.Joseph B, Aziz H, Pandit V, et al. Improving survival rates after civilian gunshot wounds to the brain. J Am Coll Surg 2014;218:58–65. [DOI] [PubMed] [Google Scholar]
- 5.Gressot LV, Chamoun RB, Patel AJ, et al. Predictors of outcome in civilians with gunshot wounds to the head upon presentation. J Neurosurg 2014;121:645–652. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Centers for Disease Control and Prevention: fast stats injury [online]. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Accessed May 31, 2016.
- 7.CDC. Centers for Disease Control and Prevention: fast stats injury [online]. Available at: http://webappa.cdc.gov/sasweb/ncipc/nfilead2001.html. Accessed May 31, 2016.
- 8.Lin DJ, Lam FC, Siracuse JJ, Thomas A, Kasper EM. “Time is brain”: the Gifford factor: or: why do some civilian gunshot wounds to the head do unexpectedly well? A case series with outcomes analysis and a management guide. Surg Neurol Int 2012;3:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giffords G, Kelly M. Gabby: A Story of Courage and Hope. New York: Simon & Schuster; 2011. [Google Scholar]
- 10.Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 2008;5:e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007;24:329–337. [DOI] [PubMed] [Google Scholar]
- 12.Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery Epub 2016 Sep 20. [DOI] [PubMed] [Google Scholar]
- 13.Part 1: guidelines for the management of penetrating brain injury: introduction and methodology. J Trauma 2001;51:S3–S6. [PubMed] [Google Scholar]
- 14.Surgical management of penetrating brain injury. J Trauma 2001;51:S16–S25. [PubMed] [Google Scholar]
- 15.Intracranial pressure monitoring in the management of penetrating brain injury. J Trauma 2001;51:S12–S15. [PubMed] [Google Scholar]
- 16.Muehlschlegel S, Carandang R, Ouillette C, Hall W, Anderson F, Goldberg R. Frequency and impact of intensive care unit complications on moderate-severe traumatic brain injury: early results of the Outcome Prognostication in Traumatic Brain Injury (OPTIMISM) study. Neurocrit Care 2013;18:318–331. [DOI] [PubMed] [Google Scholar]
- 17.Sheth KN, Stein DM, Aarabi B, et al. Intracranial pressure dose and outcome in traumatic brain injury. Neurocrit Care 2013;18:26–32. [DOI] [PubMed] [Google Scholar]
- 18.Marmarou A, Lu J, Butcher I, et al. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: an IMPACT analysis. J Neurotrauma 2007;24:270–280. [DOI] [PubMed] [Google Scholar]
- 19.Meredith W, Rutledge R, Fakhry SM, Emery S, Kromhout-Schiro S. The conundrum of the Glasgow Coma Scale in intubated patients: a linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J Trauma 1998;44:839–844. [DOI] [PubMed] [Google Scholar]
- 20.Wohltmann CD, Franklin GA, Boaz PW, et al. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg 2001;181:297–300. [DOI] [PubMed] [Google Scholar]
- 21.Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res 1993;607:333–336. [DOI] [PubMed] [Google Scholar]
- 22.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J neurotrauma 2000;17:367–388. [DOI] [PubMed] [Google Scholar]
- 23.Deutsch ER, Espinoza TR, Atif F, Woodall E, Kaylor J, Wright DW. Progesterone's role in neuroprotection, a review of the evidence. Brain Res 2013;1530:82–105. [DOI] [PubMed] [Google Scholar]
- 24.Skolnick BE, Maas AI, Narayan RK, et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med 2014;371:2467–2476. [DOI] [PubMed] [Google Scholar]
- 25.Wright DW, Yeatts SD, Silbergleit R, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med 2014;371:2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KA, Wang MY, McNatt SA, et al. Vector analysis correlating bullet trajectory to outcome after civilian through-and-through gunshot wound to the head: using imaging cues to predict fatal outcome. Neurosurgery 2005;57:737–747. [PubMed] [Google Scholar]
- 27.Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology 2001;56:766–772. [DOI] [PubMed] [Google Scholar]
- 28.Izzy S, Compton R, Carandang R, Hall W, Muehlschlegel S. Self-fulfilling prophecies through withdrawal of care: do they exist in traumatic brain injury, too? Neurocrit Care 2013;19:347–363. [DOI] [PubMed] [Google Scholar]
- 29.Hemphill JC III, White DB. Clinical nihilism in neuroemergencies. Emerg Med Clin North Am 2009;27:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.