Abstract
Objective:
To evaluate microRNA let7i in ischemic stroke and its regulation of leukocytes.
Methods:
A total of 212 patients were studied: 106 with acute ischemic stroke and 106 controls matched for risk factors. RNA from circulating leukocytes was isolated from blood collected in PAXgene tubes. Let7i microRNA expression was assessed using TaqMan quantitative reverse transcription PCR. To assess let7i regulation of gene expression in stroke, messenger RNA (mRNA) from leukocytes was measured by whole-genome Human Transcriptome Array Affymetrix microarray. Given microRNAs act to destabilize and degrade their target mRNA, mRNAs that inversely correlated with let7i were identified. To demonstrate let7i posttranscriptional regulation of target genes, a 3′ untranslated region luciferase assay was performed. Target protein expression was assessed using ELISA.
Results:
Let7i was decreased in patients with acute ischemic stroke (fold change −1.70, p < 0.00001). A modest inverse correlation between let7i and NIH Stroke Scale score at admission (r = −0.32, p = 0.02), infarct volume (r = −0.21, p = 0.04), and plasma MMP9 (r = −0.46, p = 0.01) was identified. The decrease in let7i was associated with increased expression of several of its mRNA targets, including CD86, CXCL8, and HMGB1. In vitro studies confirm let7i posttranscriptional regulation of target genes CD86, CXCL8, and HMGB1. Functional analysis predicted let7i regulates pathways involved in leukocyte activation, recruitment, and proliferation including canonical pathways of CD86 signaling in T helper cells, HMGB1 signaling, and CXCL8 signaling.
Conclusions:
Let7i is decreased in circulating leukocytes of patients with acute ischemic stroke. Mechanisms by which let7i regulates inflammatory response post stroke include targeting CD86, CXCL8, and HMGB1.
Ischemic stroke remains a leading cause of disability. Although early reperfusion therapy can improve outcomes, it is available to a minority of stroke patients. Specific therapies are needed to reduce brain injury and improve outcomes after stroke. The immune system responds rapidly following cerebral ischemia. A range of damage-associated molecular patterns, cytokines, and chemokines are released to activate circulating leukocytes, vasculature, and brain cells.1,2 This inflammatory response has an important role in acute ischemic stroke and can contribute to the final extent of brain damage. Improved understanding of the immune response in stroke and its regulation may afford novel opportunities to optimize recovery in ischemic stroke.
microRNAs are important epigenetic regulators of the immune system. They fine tune gene expression with known roles in regulating nuclear factor κB signaling, toll-like receptor signaling, and cytokine expression. We previously identified let7i to be decreased in leukocytes of patients with acute ischemic stroke.3 In this study, we evaluate let7i regulation of the peripheral immune response in patients with acute ischemic stroke.
Let7i is part of the let7 microRNA family. It is a broadly conserved microRNA that has important roles in cell regulation, differentiation, and signaling. In stroke, members of the let7 family have been shown to modulate endothelial cells as well as brain insulin-like growth factor-1.4,5 However, let7i regulation of leukocytes following stroke remains unknown. Further understanding of let7i microRNA and its target gene regulation in stroke is required to understand its role in brain injury and its regulation of the immune system post stroke.
METHODS
Standard protocol approvals, registrations, and patient consents.
Participants were recruited from the University of California Davis from December 2012 to January 2015. The institutional review board at the University of California Davis approved the study protocol, and all participants provided written informed consent.
Study population.
In total, 106 patients with acute ischemic stroke and 106 controls with vascular risk factors were studied. The diagnosis of ischemic stroke was made based on clinical presentation consistent with stroke and evidence of diffusion restriction on brain MRI (restricted diffusion on diffusion-weighted MRI). Two board-certified neurologists reviewed each patient independently, and agreement was required for diagnosis. Patients excluded from the study had infection (active or in the preceding 14 days of stroke), treatment with immunosuppression, leukemia, or lymphoma. Controls were patients similar in age, sex, and race/ethnicity with vascular risk factors but with no history of cardiovascular disease (stroke, peripheral vascular disease, or coronary ischemia).
microRNA isolation.
microRNA was isolated from whole blood acquired in PAXgene tubes (BD/PreAnalytiX, Franklin Lakes, NJ). PAXgene tubes contain an RNA stabilizing reagent that acts at time of blood draw to prevent RNA degradation. RNA in whole blood is derived mainly from peripheral blood cells (monocytes, neutrophils, T cells, B cells, and megakaryocytes). Blood samples were acquired in the first 72 hours from the onset of stroke and placed in storage at −80°C. RNA was isolated using a PAXgene microRNA kit (PreAnalytiX). Concentration of RNA was ascertained by NanoDrop (ThermoFisher, Waltham, MA) and quality by an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). All samples had a 28S/18S ribosomal RNA ratio ≥1.8, RNA integrity number ≥8, A260/A230 ratio ≥1.8, and an A260/A280 ratio ≥1.8.
Let7i measurement.
A TaqMan microRNA assay was used to evaluate hsa-let-7i-5p (referred to as let7i) expression (TaqMan microRNA assay ID 002221) (mature microRNA sequence: UGAGGUAGUAGUUUGUGCUGUU) (Applied Biosystems, Foster City, CA). Total RNA (25 ng for each participant) was reverse transcribed into complementary DNA (cDNA) using a TaqMan microRNA RT Kit and RT Primers. cDNA was amplified by RT-PreAmp Primer and TaqMan PreAmp Master Mix (ThermoFisher). The PreAmp product was combined with TaqMan microRNA assay ID 002221 and TaqMan Universal PCR Master Mix and processed using a 7900HT Fast Real-Time PCR System (ThermoFisher). RQ manager was used to ascertain cycle thresholds with U75 serving as the endogenous control.
The expression of let7i in ischemic stroke was assessed in relation to controls. To identify factors that differed between patients with ischemic stroke and controls, univariate analysis was performed using 2-tailed t test, χ2, and Mann–Whitney test as appropriate (Stata 11.0; StataCorp, College Station, TX). Multivariate analysis using analysis of variance was then performed to evaluate the expression of let7i in ischemic stroke and controls including factors identified on univariate analysis with a p < 0.2 (Partek Genomics Suite 6.6; Partek Inc., St. Louis, MO).
Messenger RNA targets of let7i.
To identify the messenger RNA (mRNA) targets that may be regulated by let7i, we performed an analysis comparing the expression level of let7i to mRNA in circulating leukocytes. mRNA was measured using whole-genome Affymetrix Human Transcriptome Array (HTA) 2.0 microarray (Affymetrix, Santa Clara, CA). mRNA was isolated from whole blood collected and stabilized in PAXgene tubes (PreAnalytiX/BD). Total RNA was isolated using the PAXgene Blood mRNA isolation kits with quantity assessed by Nanodrop and quality by an Agilent 2100 Bioanalyzer. For each sample, 75 ng of total RNA was prepared using the GeneChip WT PLUS Reagent Kit following manufacturer's protocols. Labeled cDNA from each sample was hybridized to an HTA 2.0 microarray and scanned using a GeneChip Scanner 3000 7G (Affymetrix).
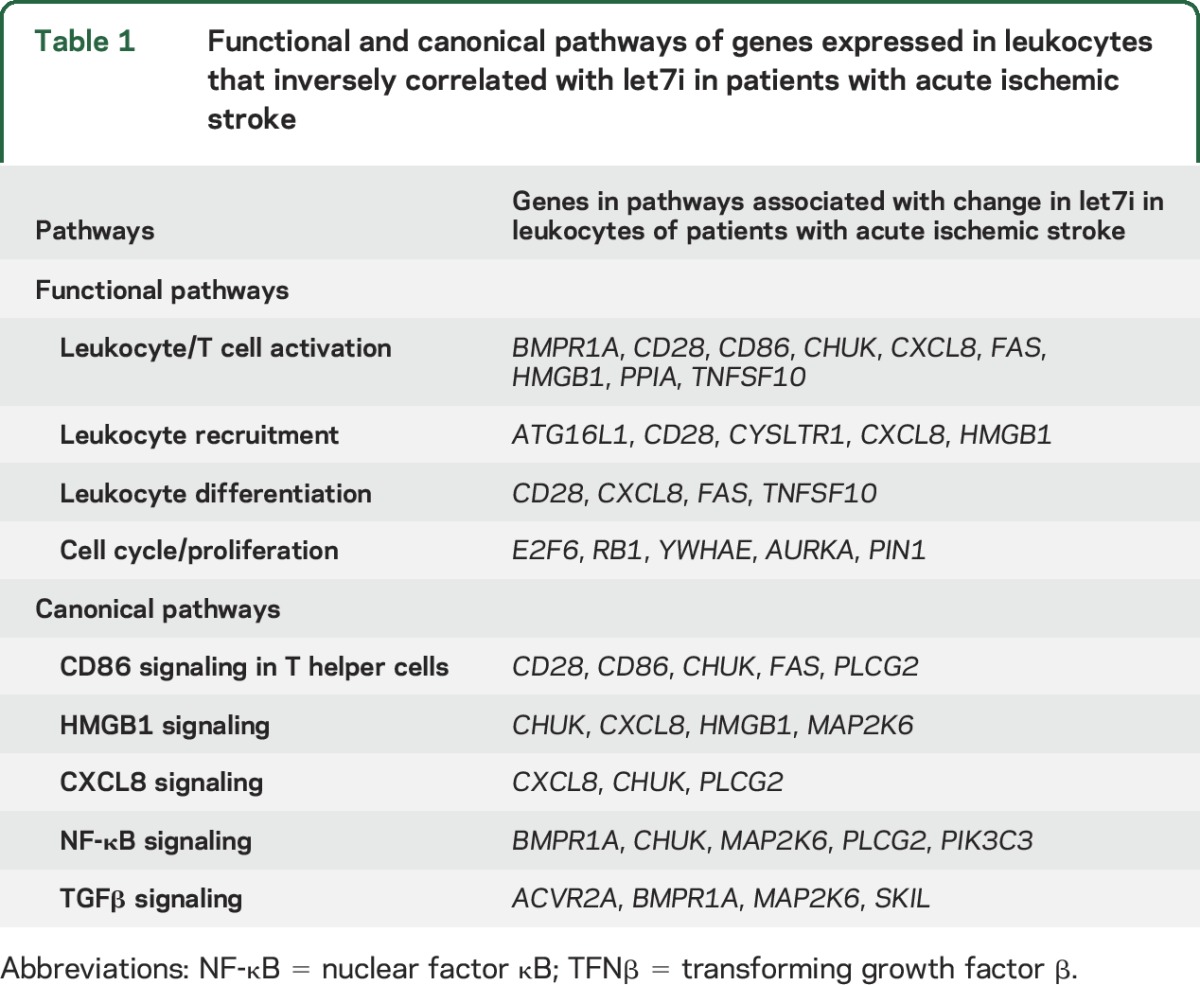
HTA 2.0 microarray data were normalized using Robust Multiarray Average (RMA), log2-transformed, and summarized to the gene transcript level (Partek Genomics Suite 6.6). Gene transcripts were filtered to targets of let7i based on databases of microRNA gene targets (TargetScan 7.0 homo sapiens, Ingenuity Pathway Analysis, Tarbase v7.0, miRecords). Gene transcripts were deemed targets if previous regulation by let7i had been reported or the weighted context++ score was less than −0.2 indicating increased likelihood of being a target.6 The expression of let7i was compared to the expression of predicted gene targets using Pearson correlation. Gene targets that inversely correlated with let7i expression with an r ≤ −0.2 and p < 0.05 were considered regulated targets in stroke. This is based on the principle that a microRNA causes mRNA degradation and translational repression. Functional analysis of the identified targets was performed in Ingenuity Pathway Analysis. Overrepresented pathways predicted to be regulated by let7i in ischemic stroke were identified (table 1).
Table 1.
Functional and canonical pathways of genes expressed in leukocytes that inversely correlated with let7i in patients with acute ischemic stroke
Enzyme-linked immunosorbent assay.
Plasma protein expression was determined using ELISA for human MMP9 according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). Plasma from patients was acquired in an EDTA tube. Samples were centrifuged at 1,000g for 10 minutes with plasma removed and stored frozen at −80°C within 1 hour of collection.
The 3′ untranslated region luciferase assay.
Although a genome-wide transcript analysis can identify let7i target transcripts, further study is needed to demonstrate that let7i alters a transcript's stability or translational efficiency. For selected genes identified that were previously associated with ischemic stroke, a let7i targeting experiment was performed using a luciferase reporter assay. A microRNA reporter vector construct was used (Active Motif; SwitchGear Genomics, Carlsbad, CA). This vector contains a constitutive promotor expressing Renilla luciferase with the 3′ untranslated region (3′UTR) for the transcript of interest cloned downstream. microRNA binding the 3′UTR would be expected to reduce luciferase expression. The 3′UTR sequences for CD86, CXCL8, HMGB1, MMP9, ACTN (housekeeping gene), or empty control cloned downstream of a Renilla luciferase sequence were used (Active Motif, SwitchGear Genomics). HT1080 cells at 80% confluency were transfected with 100 ng of the 3′UTR constructs with DharmaFECT Duo (GE Dharmacon, Lafayette, CO) in serum-free media. Reporter vectors were cotransfected with let7i mimic (7.5 nM or 15 nM), or nontargeting scrambled microRNA control (15 nM), or no microRNA (Life Technologies, mirVana microRNA, Applied Biosystems). After 24 hours, luciferase activity was measured by LightSwitch luciferase assay (Active Motif) using a Biotek Synergy HT Multi-Mode Microplate Reader (Biotek, Winooski, VT). The decrease in translation induced by the let7i effect on the 3′UTR of interest was assessed by comparing the luciferase signal in let7i-treated samples relative to untreated control. Experiments were performed in triplicate and repeated twice.
RESULTS
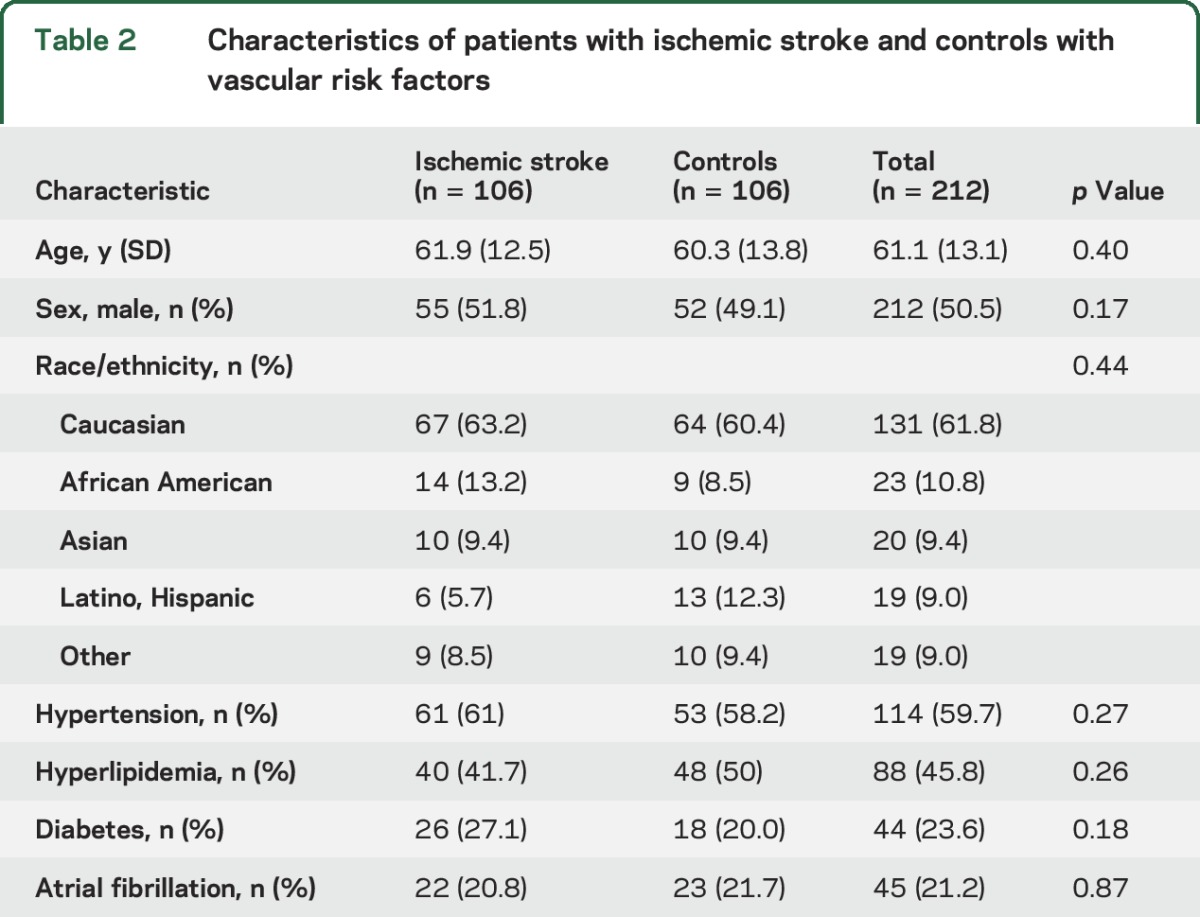
A total of 212 patients were studied: 106 patients with acute ischemic stroke and 106 controls. Patient characteristics are summarized in table 2. The average age was 61.1 years, 49.5% were men, 61.8% Caucasian, 9.4% Asian, 10.8% African American, 9.0% Hispanic, and 9.0% of other race. Controls were similar in age, sex, and vascular risk factor status compared to patients with ischemic stroke (table 2). Hypertension was present in 59.7% of patients, hyperlipidemia in 45.8%, diabetes in 23.6%, and atrial fibrillation in 21.2%. The median NIH Stroke Scale (NIHSS) score was 5.3 (interquartile range 2–9) with 8 participants having an NIHSS score between 10 and 15 and 6 with an NIHSS score >15. There were 25 (23.6%) cardioembolic strokes, 25 (23.6%) large vessel strokes, 25 (23.6%) small vessel lacunar strokes, and 31 (29.2%) cryptogenic strokes.
Table 2.
Characteristics of patients with ischemic stroke and controls with vascular risk factors
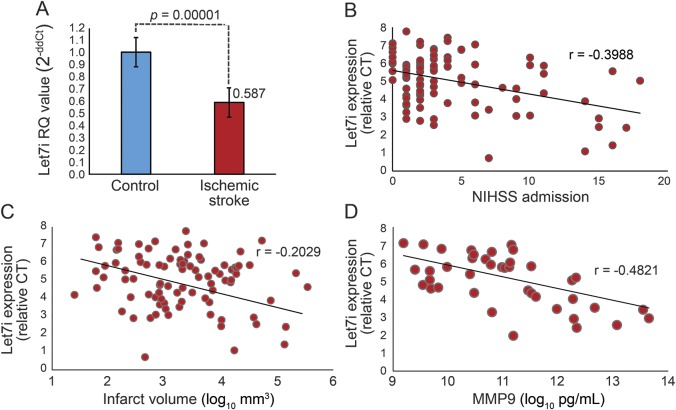
microRNA let7i was significantly lower in ischemic stroke relative to controls (p = 0.00001, fold change −1.70) (figure 1A). Let7i remained significantly lower in stroke after adjusting for age, sex, hypertension, diabetes, and hyperlipidemia. Over the 72-hour period after stroke, let7i was significantly decreased in participants acquired within the first 24 hours of stroke onset and continued to be decreased from 24–48 hours and 48–72 hours (figure e-1 at Neurology.org). The decreased expression of let7i correlated modestly with an increase in admission NIHSS score (r = −0.39, p = 0.02), infarct volume (r = −0.20, p = 0.04), and plasma MMP9 (r = −0.48, p = 0.01) (figure 1). Other clinical characteristics were not found to correlate with let7i including age, sex, systolic blood pressure, and admission serum glucose.
Figure 1. Expression of let7i microRNA in ischemic stroke.
(A) Relative expression of let7i in patients with acute ischemic stroke (n = 106) compared to vascular risk controls (n = 106). Let7i decreased in leukocytes of patients with acute ischemic stroke compared to controls (fold change = −1.70, p = 0.00001). A decrease in level of let7i correlated modestly with (B) an increase in NIHSS score at admission, (C) an increase in infarct volume (log10 mm3), and (D) an increase in plasma MMP9 (log10 pg/mL) in patients with acute ischemic stroke. CT = cycle threshold; NIHSS = NIH Stroke Scale; RQ = relative quantification.
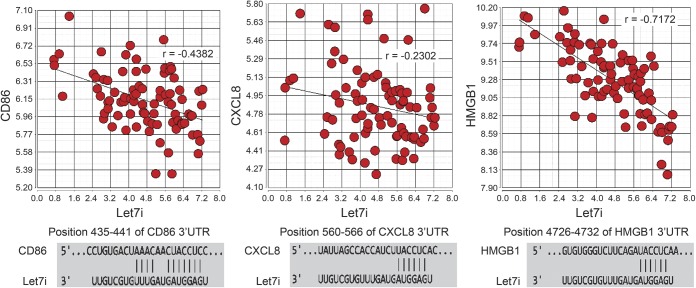
To identify gene targets regulated by let7i in patients with acute ischemic stroke, the expression of genes in circulating leukocytes was assessed. A decrease in let7i correlated with an increase in several of its predicted gene targets (table e-1). CD86 (r = −0.43, p = 3.1 × 10−5), CXCL8 (r = −0.23, p = 0.029), and HMGB1 (r = −0.71, p = 1.6 × 10−14) (figure 2) were among the targets identified with previously described roles in stroke. Although plasma MMP9 protein correlated with let7i, leukocyte MMP9 mRNA did not (r = 0.02).
Figure 2. Correlation of let7i with gene targets CD86, CXCL8, HMGB1 in circulating leukocytes of patients with acute ischemic stroke.
The y-axis is the relative expression of messenger RNA transcript based on normalized intensity values from the Human Transcriptome Array 2.0 Affymetrix microarray. The x-axis is the relative expression of let7i based on delta Ct values from TaqMan reverse transcription PCR. As let7i decreased, levels of CD86, CXCL8, and HMGB1 increased suggesting a regulatory effect of let7i for these targets in ischemic stroke. The binding site of let7i in the 3′UTR of CD86, CXCL8, and HMGB1 is shown below each scatterplot. 3′UTR = 3′ untranslated region.
To determine functional pathways regulated by let7i in circulating leukocytes of patients with ischemic stroke, gene targets that inversely correlated with let7i expression were evaluated for common overrepresented pathways. Functional pathways identified included leukocyte activation, recruitment, and proliferation (table 1). Canonical pathways identified included CD86 signaling in T helper cells, HMGB1 signaling, and CXCL8 signaling.
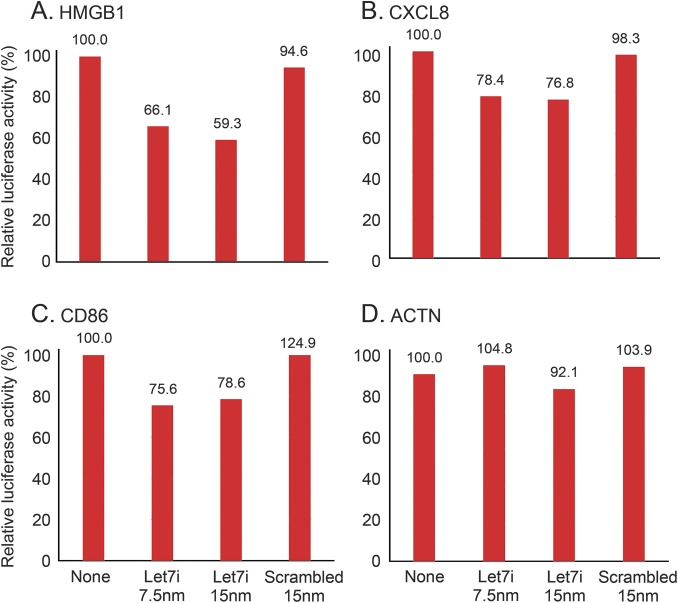
To test the posttranscriptional regulation of let7i on CD86, CXCL8, and HMGB1, a luciferase reporter assay was performed. The 3′UTR for CD86, CXCL8, or HMGB1 fused to a luciferase reporter gene was cotransfected with let7i microRNA or scrambled microRNA, or no microRNA and assayed 24 hours after transfection. No significant change was observed when an empty reporter vector was cotransfected with let7i or scrambled microRNA. No significant change occurred when let7i mimic or scrambled microRNA mimic was cotransfected with ACTN control 3′UTR or the 3′UTR of MMP9. Let7i did produce a 22% to 24% reduction in luciferase activity (p < 0.05) when cotransfected with the 3′UTR of CXCL8, 33% to 41% reduction in luciferase activity for HMGB1 (p < 0.05), and a 22% to 25% reduction for CD86 (p < 0.05) (figure 3).
Figure 3. Let7i regulation of the 3′UTR for HMGB1, CXCL8, CD86, and ACTN.
A luciferase reporter vector with either (A) CXCL8-3′UTR or (B) CD86-3′UTR, or (C) HMGB1-3′UTR, or (D) ACTN-3′UTR was cotransfected into cells with let7i mimic at 7.5 nm or 15 nm, or a nontargeting scrambled microRNA mimic, or no microRNA (none). Following a 24-hour incubation, luciferase activity was measured. Ratio is relative to untreated control. Let7i produced a 22% to 24% reduction in CXCL8 (p < 0.05), a 33% to 41% reduction in HMGB1 (p < 0.05), and a 22% to 25% reduction in CD86 (p < 0.05). 3′UTR = 3′ untranslated region.
DISCUSSION
Let7i microRNA decreased in circulating leukocytes of patients with acute ischemic stroke. A primary function of microRNA is to bind mRNA to trigger degradation and translational repression. To evaluate this regulatory role of let7i microRNA in ischemic stroke, let7i gene targets expressed in leukocytes were analyzed. Let7i gene targets identified were involved in leukocyte proliferation and activation including HMGB1, CD86, and CXCL8. Let7i also decreased as infarct severity and plasma MMP9 increased. These data suggest that let7i regulates the peripheral immune system in patients with acute ischemic stroke, and this regulation may relate to severity of ischemic brain injury.
The regulation of the immune response by let7i in ischemic stroke is supported by prior studies demonstrating that let7i regulates immune cell response to infection.7–9 In stroke, let7i regulated leukocytes through target genes involved in proliferation, activation, and recruitment. This raises the question as to whether let7i could be used as a stroke therapy to modulate the leukocyte response. It could potentially modulate inflammatory response to ischemic brain and blood–brain barrier. microRNA has shown promise as a treatment for a number of diseases including cancer, atherosclerosis, heart failure, hepatitis C, and spinal and bulbar muscular atrophy.10–15 microRNAs have several properties that make them attractive as a therapeutic agent. Their nucleotide sequence permits specific gene regulation, while their small size facilitates delivery. microRNAs generally target several genes in the same pathway or network permitting modulation of a disease state at multiple levels. While potentially useful in complex disease, this property may also produce off-target effects whereby a microRNA regulates expression of genes not involved in the disease process. With further study, improved targeting and delivery of microRNA may reduce these off-target effects.
CD86 promotes inflammation post stroke and may contribute to brain injury. Our data indicate that let7i is a regulator of CD86-related inflammation in stroke. CD86 increased as let7i decreased (r = −0.43, p = 0.002) as did CD28, the receptor for CD86 (r = −0.36, p = 0007). CD86 is expressed by activated monocytes and B cells to promote inflammation and T cell activation. In ischemic stroke, an increase in CD86 monocytes (M1 proinflammatory phenotype) is associated with worse functional outcome,16,17 and CD86 monocytes can promote neurotoxicity that worsens neurologic injury.18 CD86 stimulation of CD28 results in the production of inflammatory cytokines (interleukin [IL]-6, tumor necrosis factor α [TNF-α], CCL2, TNFSF11, transforming growth factor β) as well as promotes leukocyte activation, chemotaxis, and recruitment.19 Blocking CD28 post stroke reduces infarct size, improves behavioral outcomes, and reduces the inflammatory response.20,21 Our study suggests that a decrease in let7i may promote this detrimental immune response post stroke by increasing expression of CD86.
Let7i may also regulate CXCL8 (IL-8, neutrophil chemotactic factor) in stroke. CXCL8 is a chemokine produced by activated macrophages and endothelial cells. It binds CXCR1 and CXCR2 to promote neutrophil chemotaxis, activation, degranulation, and release of MMP9.22 CXCL8 can promote tissue injury23 and is associated with infarct severity in humans24 and rodents.25,26 Thus, regulation of CXCL8 may be essential to limit injury to ischemic brain. We found the decrease of let7i in stroke to be associated with an increase in CXCL8 as well as an increase in stroke severity. This suggests let7i may permit an increase in CXCL8, promoting neutrophil activation in relation to stroke severity.
HMGB1 expressed in circulating leukocytes post stroke was found to be regulated by let7i. HMGB1 is released following tissue injury to mediate innate immunity and repair. It promotes leukocyte proliferation and chemotaxis to sites of injury. It is produced by most cells including brain and leukocytes. Previous studies show that HMGB1 increases in patients with ischemic stroke and correlates with the number of circulating leukocytes,27 stroke severity (NIHSS score, infarct size), and inflammatory markers (TNF-α, IL-1, IL-6, MMP9).28–31 These findings are consistent with our study whereby a decrease in let7i was associated with a rise in HMGB1 as well as an increase in inflammatory response, MMP9, and infarct size.
Plasma levels of MMP9 increased with a decrease in let7i. The explanation for this relationship requires further study, as we did not find let7i to correlate with MMP9 mRNA or to regulate MMP9 via its 3′UTR. One explanation might be that a decrease in let7i permits a proliferation of neutrophils, a major source of plasma MMP9 post stroke.1 However, let7i remained correlated with MMP9 when neutrophil count was adjusted for. Alternatively, MMP9 may have increased because of let7i regulation of genes that affect the production and release of MMP9.32 The decrease in let7i did correlate with an increase in several genes that affect MMP9, including CHUK, PIN1, CRK, TNFSF10, IGFBP7. Potentially MMP9 plasma levels are indirectly affected by let7i's effect on these targets.
In patients with more severe strokes (increased infarct size, increased NIHSS score), let7i levels were lower. While the significance of this finding requires further study, it may indicate that let7i regulates the immune response in relation to severity of stroke. A relationship between brain and leukocyte gene expression and microRNA regulation has been observed in experimental stroke.33,34 In stroke patients, infarct size has been associated with inflammatory response, with levels of TNF-α,35 IL-6,36,37 ICAM1,35 MMP2, and MMP935,38 correlating with infarct volume. Let7i may regulate this inflammatory response post stroke through its effect on CD86, CXCL8, and HMGB1. In addition, let7i may regulate leukocyte proliferation through its effect on cell-cycle genes (E2F6, RB1, YWHAE, AURKA, and PIN1) (table 1).
This study improves our understanding of microRNA regulation of leukocyte response in human ischemic stroke. Strengths include analysis of a large cohort of patients with rapidly stabilized RNA, whole-genome evaluation of let7i gene targets, and validation of let7i targets by in vitro analysis. Further studies are needed to evaluate the regulatory role of let7i at the individual immune cell level. Functional analysis was based on gene targets that inversely correlated with let7i and predicted to be likely targets of let7i. Selected mRNA targets were analyzed by in vitro analysis but additional target analyses are needed to comprehensively understand let7i regulation of targets and pathways important in ischemic stroke. The representation of larger strokes was small relative to the mild–moderate group, thus further evaluation of let7i in severe strokes is needed.
Let7i microRNA is shown to decrease in patients with acute ischemic stroke and to modulate the peripheral immune system. Mechanisms by which let7i regulates inflammatory response in stroke include targeting CD86, CXCL8, and HMGB1.
Supplementary Material
ACKNOWLEDGMENT
The authors appreciate the support of the MIND Institute, the Genomics Shared Resource, and the Department of Neurology at the University of California Davis, as well as the patients who participated in this study.
GLOSSARY
- cDNA
complementary DNA
- HTA
Human Transcriptome Array
- IL
interleukin
- mRNA
messenger RNA
- NIHSS
NIH Stroke Scale
- 3′UTR
3′ untranslated region
- TNF-α
tumor necrosis factor α
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Conception and designed the experiments (G.J. and F.S.), acquisition of data (G.J., N.S., M.O., H.H., B.A., C.D.-A), analysis and interpretation of data (G.J., B.S., D.L., F.S.), drafted (G.J.) or revised (all authors) the manuscript, and gave final approval of the version to be published (all authors).
STUDY FUNDING
The presented research was done with support from the American Heart Association (G.C.J.) and the NIH (NS075035, NS079153, F.R.S.).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 2015;35:888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011;17:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jickling GC, Ander BP, Zhan X, Noblett D, Stamova B, Liu D. microRNA expression in peripheral blood cells following acute ischemic stroke and their predicted gene targets. PLoS One 2014;9:e99283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One 2012;7:e32662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rom S, Dykstra H, Zuluaga-Ramirez V, Reichenbach NL, Persidsky Y. miR-98 and let-7g* protect the blood-brain barrier under neuroinflammatory conditions. J Cereb Blood Flow Metab 2015;35:1957–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015:4:e05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar M, Ahmad T, Sharma A, et al. Let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. J Allergy Clin Immunol 2011;128:1077–1085.e10. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Chen Q, Song Y, et al. MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett 2011;585:1963–1968. [DOI] [PubMed] [Google Scholar]
- 9.Schulte LN, Eulalio A, Mollenkopf HJ, Reinhardt R, Vogel J. Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family. EMBO J 2011;30:1977–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ai C, Jiang R, Fu L, Chen Y. MicroRNA-495 mimics delivery inhibits lung tumor progression. Tumour Biol 2015;36:729–735. [DOI] [PubMed] [Google Scholar]
- 11.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. New Engl J Med 2013;368:1685–1694. [DOI] [PubMed] [Google Scholar]
- 12.Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci USA 2010;107:12228–12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki Y, Adachi H, Katsuno M, et al. Viral delivery of miR-196a ameliorates the SBMA phenotype via the silencing of CELF2. Nat Med 2012;18:1136–1141. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery RL, Hullinger TG, Semus HM, et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 2011;124:1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue W, Dahlman JE, Tammela T, et al. Small RNA combination therapy for lung cancer. Proc Natl Acad Sci USA 2014;111:E3553–E3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urra X, Cervera A, Villamor N, Planas AM, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience 2009;158:1174–1183. [DOI] [PubMed] [Google Scholar]
- 17.Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke 2009;40:1262–1268. [DOI] [PubMed] [Google Scholar]
- 18.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 2009;29:13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantani PT, Ljungcrantz I, Andersson L, et al. Circulating CD40+ and CD86+ B cell subsets demonstrate opposing associations with risk of stroke. Arterioscler Thromb Vasc Biol 2014;34:211–218. [DOI] [PubMed] [Google Scholar]
- 20.Na SY, Mracsko E, Liesz A, Hunig T, Veltkamp R. Amplification of regulatory T cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice. Stroke 2015;46:212–220. [DOI] [PubMed] [Google Scholar]
- 21.Schuhmann MK, Kraft P, Stoll G, et al. CD28 superagonist-mediated boost of regulatory T cells increases thrombo-inflammation and ischemic neurodegeneration during the acute phase of experimental stroke. J Cereb Blood Flow Metab 2015;35:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol 2005;78:279–288. [DOI] [PubMed] [Google Scholar]
- 23.Reeves EP, Williamson M, O'Neill SJ, Greally P, McElvaney NG. Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis. Am J Respir Crit Care Med 2011;183:1517–1523. [DOI] [PubMed] [Google Scholar]
- 24.Kostulas N, Pelidou SH, Kivisakk P, Kostulas V, Link H. Increased IL-1beta, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke 1999;30:2174–2179. [DOI] [PubMed] [Google Scholar]
- 25.Garau A, Bertini R, Colotta F, et al. Neuroprotection with the CXCL8 inhibitor repertaxin in transient brain ischemia. Cytokine 2005;30:125–131. [DOI] [PubMed] [Google Scholar]
- 26.Villa P, Triulzi S, Cavalieri B, et al. The interleukin-8 (IL-8/CXCL8) receptor inhibitor reparixin improves neurological deficits and reduces long-term inflammation in permanent and transient cerebral ischemia in rats. Mol Med 2007;13:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulze J, Zierath D, Tanzi P, et al. Severe stroke induces long-lasting alterations of high-mobility group box 1. Stroke 2013;44:246–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liesz A, Dalpke A, Mracsko E, et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci 2015;35:583–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang QW, Lu FL, Zhou Y, et al. HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J Cereb Blood Flow Metab 2011;31:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogelgesang A, May VE, Grunwald U, et al. Functional status of peripheral blood T-cells in ischemic stroke patients. PLoS One 2010;5:e8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sapojnikova N, Kartvelishvili T, Asatiani N, et al. Correlation between MMP-9 and extracellular cytokine HMGB1 in prediction of human ischemic stroke outcome. Biochim Biophys Acta 2014;1842:1379–1384. [DOI] [PubMed] [Google Scholar]
- 32.Ventayol M, Vinas JL, Sola A, et al. miRNA let-7e targeting MMP9 is involved in adipose-derived stem cell differentiation toward epithelia. Cell Death Dis 2014;5:e1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu DZ, Tian Y, Ander BP, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab 2010;30:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 2008;39:959–966. [DOI] [PubMed] [Google Scholar]
- 35.Sotgiu S, Zanda B, Marchetti B, et al. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur J Neurol 2006;13:505–513. [DOI] [PubMed] [Google Scholar]
- 36.Vila N, Castillo J, Davalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 2000;31:2325–2329. [DOI] [PubMed] [Google Scholar]
- 37.Montaner J, Rovira A, Molina CA, et al. Plasmatic level of neuroinflammatory markers predict the extent of diffusion-weighted image lesions in hyperacute stroke. J Cereb Blood Flow Metab 2003;23:1403–1407. [DOI] [PubMed] [Google Scholar]
- 38.Rosell A, Alvarez-Sabin J, Arenillas JF, et al. A matrix metalloproteinase protein array reveals a strong relation between MMP-9 and MMP-13 with diffusion-weighted image lesion increase in human stroke. Stroke 2005;36:1415–1420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.