Abstract
Objective:
To characterize temporal trends in subarachnoid hemorrhage (SAH) incidence and outcomes over 5 time periods in a large population-based stroke study in the United States.
Methods:
All SAHs among residents of the Greater Cincinnati/Northern Kentucky region at least 20 years of age were identified and verified via study physician review in 5 distinct year-long study periods between 1988 and 2010. We abstracted demographics, care patterns, and outcomes, and we compared incidence and case-fatality rates across the study periods.
Results:
The incidence of SAH in the 5 study periods (age-, race-, and sex-adjusted to the 2000 US population) was 8.8 (95% confidence interval 6.8–10.7), 9.2 (7.2–11.2), 10.0 (8.0–12.0), 9.0 (7.1–10.9), and 7.7 (6.0–9.4) per 100,000, respectively; the trend in incidence rates from 1988 to 2010 was not statistically significant (p = 0.22). Advanced neurovascular imaging, endovascular coiling, and neurologic intensive care unit availability increased significantly over time. All-cause 5-day (32%–18%, p = 0.01; for trend), 30-day (46%–25%, p = 0.001), and 90-day (49%–29%, p = 0.001) case-fatality rates declined from 1988 to 2010. When we included only proven or highly likely aneurysmal SAH, the declines in case-fatality were no longer statistically significant.
Conclusions:
Although the incidence of SAH remained stable in this population-based region, 5-day, 30-day, and 90-day case-fatality rates declined significantly. Advances in surgical and medical management, along with systems-based changes such as the emergence of neurocritical care units, are potential explanations for the reduced case-fatality.
There have been several advances in recent years in the diagnosis and care of subarachnoid hemorrhage (SAH) patients, including medical advances, new systems of standardized care in neurocritical care units, and new surgical and endovascular techniques for aneurysmal SAH.1,2 Recent studies have reported SAH outcomes over time within the same population,3,4 but to our knowledge there have been no such recent studies in the United States. We therefore sought to examine temporal trends in nontraumatic SAH incidence, care patterns, and outcomes in a large US region using population-based data.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the institutional review boards of all participating hospitals in all 5 time periods.
We identified incident nontraumatic SAHs among residents of the Greater Cincinnati/Northern Kentucky (GCNK) region who were 20 years old or older in 5 distinct 1-year study periods: calendar year 1988, July 1993 to June 1994, and calendar years 1999, 2005, and 2010. The GCNK region is composed of 2 southwestern Ohio counties and 3 northern Kentucky counties, all bordering the Ohio River. The biracial population is approximately 1.3 million and is largely representative of the United States in terms of age, proportion of African Americans, and socioeconomic status.5 Only residents of the 5 GCNK counties were included in our study. Previous work has demonstrated that patients who live in this 5-county region seek stroke care at the hospitals in the region.5
The 1988 cohort.
The detailed methodology of the 1988 study has been reported previously.6 Briefly, the records of all patients who possibly had SAH or intracerebral hemorrhage (ICH) were reviewed from local hospitals and the county coroner offices. ICD-9 codes for SAH (430), ICH (431 and 432.9), cerebral aneurysm (437.3), arteriovenous malformation (747.81), and cerebrovascular accident (436) were used to retrieve records. A trained nurse under the close supervision of a vascular neurologist reviewed the records. The neurologist reviewed the clinical data and all available CT and MRI. Patients with both SAH and ICH were not included if a parenchymal source of bleeding was more likely.
The 1993–1994, 1999, 2005, and 2010 cohorts.
The methods of the GCNK studies for 1993–1994, 1999, and 2005 have also been described previously.5,7,8 Ascertainment methods remained consistent over time and those for 2010 were as described for the other years. Briefly, study nurses screened the hospital charts of all patients with primary or secondary stroke-related ICD-9 codes of 430–436. Additional screening included monitoring of emergency departments, public health clinics, outpatient clinics, and family practice centers. The records of the 5 county coroner's offices were also monitored for stroke deaths. A study physician then reviewed every potential case to determine case status.
Aneurysmal SAH (aSAH) cohort.
Because ruptured aneurysms are the leading cause of nontraumatic SAH, we also performed a subanalysis in which we identified proven or highly likely aSAH. We reviewed all cases with unknown etiology and categorized them as likely aneurysmal if the SAH was reported as “massive” or if the patient suddenly died shortly after SAH.
Incidence rates.
We calculated incidence rates for each time period and age-, race-, and sex-adjusted to the 2000 US population. The denominators for incidence rate calculations were based on population estimates from the US Census Bureau (census.gov) for the 5 study counties, with interpolation between census years accounting for migration, death, and birth. We estimated confidence intervals (CIs) assuming a Poisson distribution.
Demographics, care patterns, and outcomes.
We evaluated basic patient demographics, vascular risk factors, care characteristics, and functional status at baseline. Because SAH severity scales were not uniformly abstracted in the form of Hunt and Hess or World Federation of Neurological Surgeons scales, the admission Glasgow Coma Scale (GCS) served as a proxy for baseline SAH severity. Major clinical outcomes included modified Rankin Scale (mRS) scores at hospital discharge and all-cause case-fatality at 5, 30, and 90 days after initial SAH. Because treatment location was not uniformly abstracted at the patient level, we report the proportion of patients treated at hospitals with neurologic intensive care unit (ICU) availability in each time period.
Statistical analysis.
Data were managed and analyzed using SAS version 9.3 (SAS Institute, Cary, NC). Univariate comparisons were performed using analysis of variance, Kruskal-Wallis, or χ2 tests as appropriate. Regression modeling was utilized for examining trends over time, linear or logistic dependent upon the outcome of interest. SAS PROC GENMOD with the appropriate distribution and link function was used. The offset option was invoked for testing the trend in incidence rates over time. A Tukey-Kramer correction was used as appropriate to adjust for multiple comparisons. Some analyses were performed excluding 1998 due to unavailable data.
RESULTS
We identified 433 SAHs among residents of the GCNK region: 79 SAHs in 1988, 84 from July 1993 to June 1994, 95 in 1999, 91 in 2005, and 84 in 2010. Across the 5 time periods, 305 patients (70.4%) were women, 106 (24.5%) were black, 407 (94.0%) were diagnosed in an emergency room or hospital setting, and 26 (6.0%) were diagnosed on autopsy alone. In 1988, 10 SAHs were found at autopsy (12.7% of total), compared with 6 (7.1%) in 1993–1994, 4 (4.2%) in 1999, 3 (3.3%) in 2005, and 3 (3.6%) in 2010 (p value for trend is 0.01). Of the 433 SAHs, 319 (73.7%) were proven or highly likely aSAH. Although the distribution of aSAH cases varied over time—45 (57.0%) were identified as aSAH in 1988, 67 (79.8%) in 1993–1994, 79 (83.2%) in 1999, 65 (71.4%) in 2005, and 63 (75.0%) in 2010 (p = 0.001)—there was no distinct temporal trend (p = 0.11).
Incidence rates.
The incidence of SAH in the 5 study periods (age-, race-, and sex-adjusted to the 2000 US population) was 8.8 (95% CI 6.8–10.7), 9.2 (7.2–11.2), 10.0 (8.0–12.0), 9.0 (7.1–10.9), and 7.7 (6.0–9.4) per 100,000, respectively; the trend in incidence rates from 1988 to 2010 was not statistically significant (p = 0.22).
Demographics and care patterns.
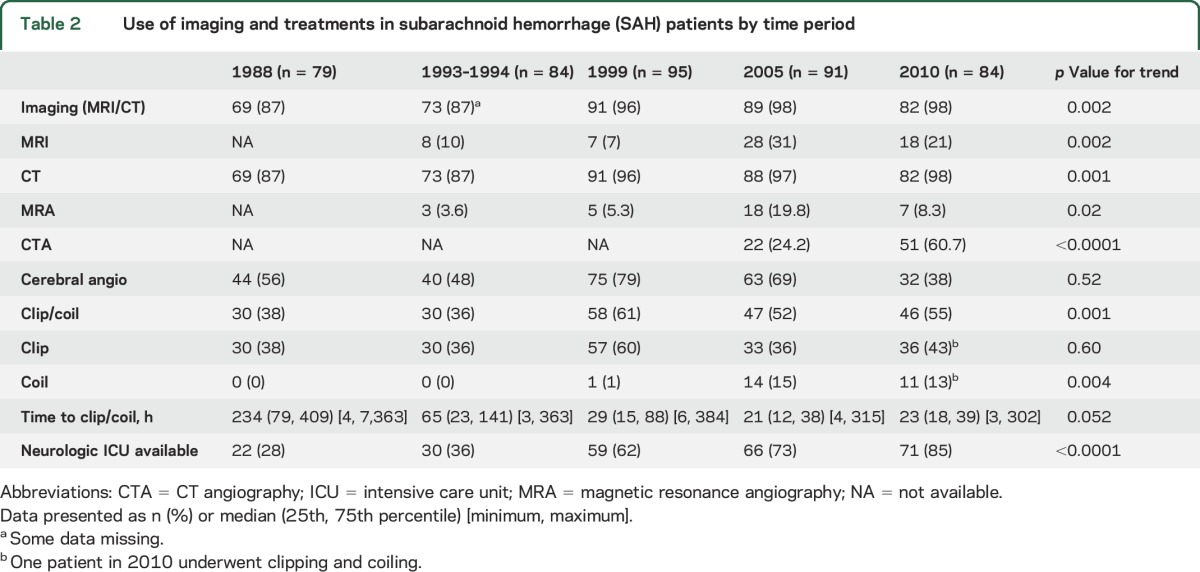
Demographics and risk factors across time periods are shown in table 1. Age, sex, race, and presenting GCS were not different among the time periods. There were no significant trends in baseline characteristics except for an increase in current smoking and a decrease in the proportion of baseline mRS scores of 0–1. Diagnostic imaging and treatment trends over time are shown in table 2. The use of both CT and MRI increased significantly from 1993–1994 to 2010, as did CT angiography and magnetic resonance angiography. The proportion of patients who underwent coiling increased to 13% in 2010 from 0% in 1993–1994. There was a trend toward reduction of time to intervention as well. Care at a hospital with a neurologic ICU available also increased substantially over the study periods.
Table 1.
Baseline characteristics of subarachnoid hemorrhage (SAH) patients by time period
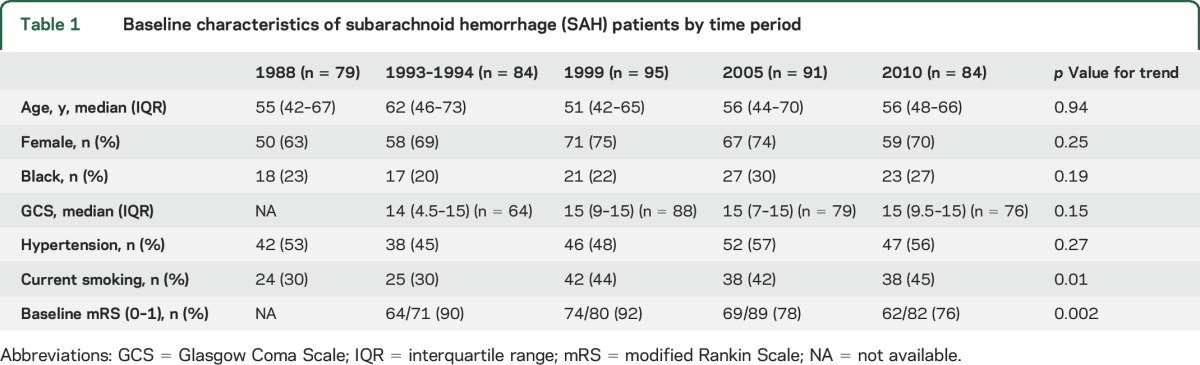
Table 2.
Use of imaging and treatments in subarachnoid hemorrhage (SAH) patients by time period
Outcomes.
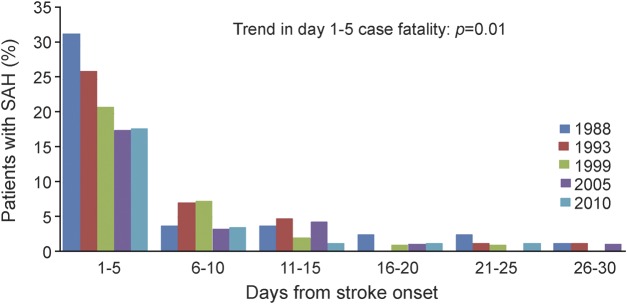
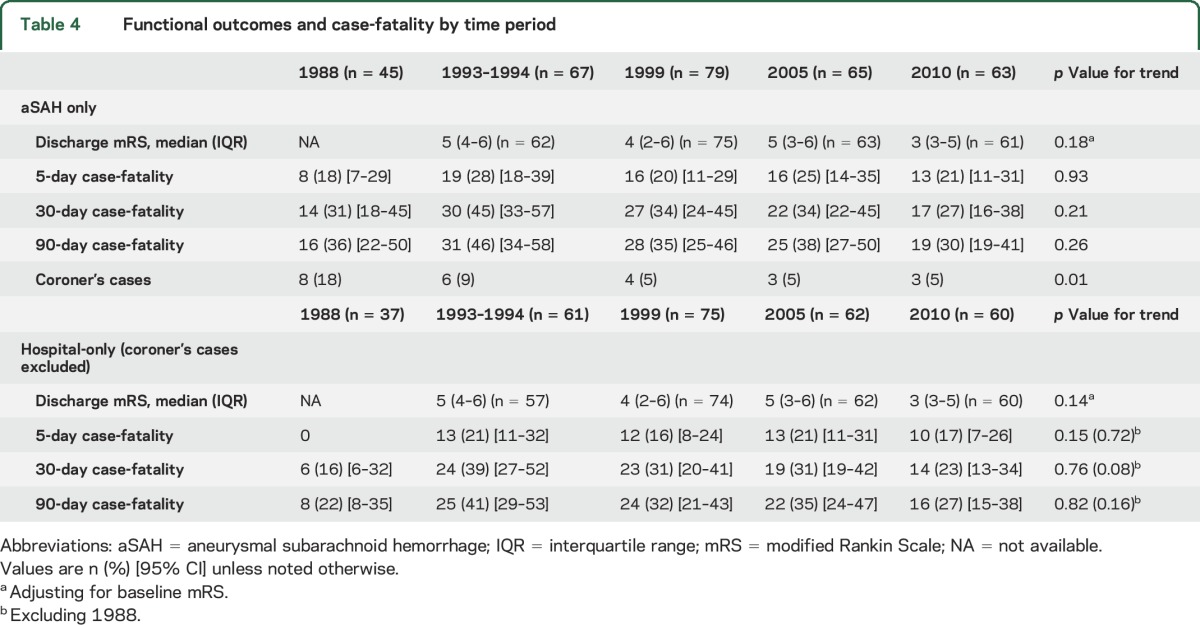
Short-term SAH outcomes over time are shown in table 3 and in the figure. Median discharge mRS scores at hospital discharge declined significantly from 5 to 3. All-cause 5-day, 30-day, and 90-day case-fatality rates declined significantly from 1988 to 2010, even when all autopsy-alone cases were excluded. The largest decreases in case-fatality rates occurred between 1988 and 1999. The majority of deaths occurred within 5 days post onset in all time periods. We performed an additional analysis with those patients with proven or highly likely aSAH with the results shown in table 4. Though the trends were generally similar to those in table 3, the results were no longer statistically significant. Because so many SAHs from 1988 had an unknown etiology, we performed an additional analysis excluding 1988 and again found that improved case-fatality rates did not reach statistical significance.
Table 3.
Functional outcomes and case-fatality by time period
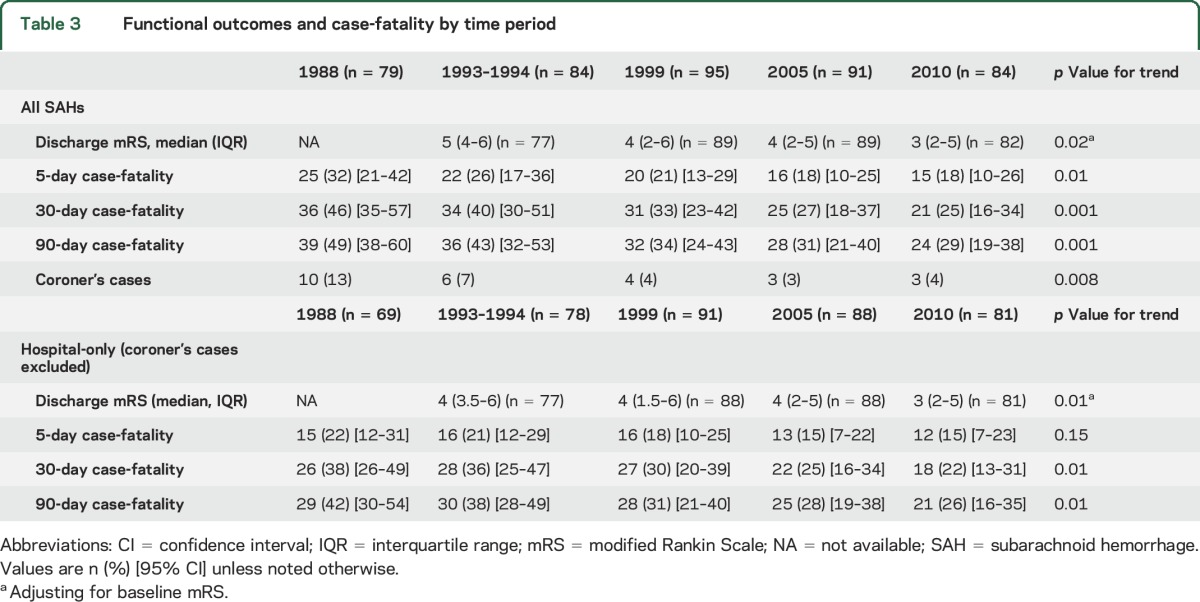
Figure. Short-term subarachnoid hemorrhage (SAH) outcomes over time.
Table 4.
Functional outcomes and case-fatality by time period
DISCUSSION
In this large population-based study spanning multiple study periods, we found that although SAH incidence rates remained unchanged, all-cause case-fatality rates declined significantly. We also found a significant shift in SAH care across these time periods, which included changes in autopsy rates, imaging, interventions, and systemic changes such as neurologic ICU utilization.
Recent systematic reviews have evaluated the incidence of SAH in several countries.9,10 In general, these studies have found that the incidence of SAH has remained relatively stable over the last several decades. The incidence is generally higher in Japan and Scandinavia (∼20 per 100,000) and lower in South and Central America (∼5 per 100,000). The only study that has evaluated temporal trends in incidence in the United States of which we are aware is the Rochester, Minnesota, study, in which investigators identified 185 SAHs in Olmsted County from 1955 to 1989. The incidence rate ranged from 7.5 to 12.9 per 100,000, and the changes across 5-year time periods were not statistically significant.11 The current study includes more than twice the number of SAHs, as well as a more recent cohort with a minority population as well.
Systematic reviews have also found reductions in SAH case-fatality in multiple population-based studies in several countries,4,12 though few studies have reported case-fatality rates across multiple time periods, and none in the United States.4 (The Rochester study reported 30-day and 1-year survival stratified by sex and 5-year period, but the overall case-fatality per time period was not reported.) Most of these studies also began surveillance in the 1980s and generally found declines in early case-fatality rates that mirror the declines we report, though most did not find these declines to be statistically significant.
Several changes in SAH care over the last 25 years are mirrored in our data. The numbers of SAHs diagnosed by autopsy declined as autopsy rates have declined nationwide.13 Noninvasive angiography is replacing conventional angiography for SAH diagnosis.14 Surgical intervention was commonly delayed 10–12 days after SAH until the 1980s, but is now often performed as early as feasible to reduce rebleeding risk.2 Less-invasive endovascular coiling is more common.15,16 Specialized stroke units and neurologic ICUs, with an emphasis on multidisciplinary collaboration, protocolization, and continuous quality improvement, are more prevalent17 and associated with improved patient outcomes.18 At the systems of care level, the development of primary stroke centers19 and comprehensive stroke centers20 has also driven quality improvement in stroke care.
Though aneurysmal rupture is the most frequent, other etiologies of nontraumatic SAH include perimesencephalic nonaneurysmal SAH, arteriovenous malformations, dissection, amyloid angiopathy, and reversible cerebral vasoconstriction syndrome. Using a conservative threshold for aSAH, we found that at least 75% of SAHs were highly likely aneurysmal in etiology in our population, which is in line with previous reports.21 The trends in case-fatality decline in the aSAH subanalysis appeared generally similar to that of the overall cohort, though the statistical significance disappeared likely due to the smaller sample size. Another potential explanation is that improved imaging identified smaller nonaneurysmal SAHs, which typically are not life-threatening. As noted above, though, we did not identify a trend toward more nonaneurysmal SAHs in the later time periods.
The GCNK Stroke Study is the largest population-based stroke study in the United States, with the same population evaluated over multiple time periods and with many of the same study personnel, using a stable methodology. However, there are several limitations to this study. First, in this observational study the data are retrospective and descriptive in nature; this study cannot answer why case-fatality rates are declining. There are several data points missing from 1988, including severity scores and baseline functional status. There was no consistent formal grading of SAH severity documented in the medical records across time periods, though presenting GCS was used as a surrogate. Emerging evidence suggests that psychological and cognitive burden of disease in SAH is significant,22 but we cannot comment on those outcome domains in the current study. We also did not collect data on either aggressiveness of care or withdrawal of care across all time periods, so we cannot comment on whether case-fatality rates may be declining because fewer families or physicians are withdrawing care. Case-fatality rates may appear lower because cases formerly identified on autopsy are not now identified. We also cannot comment on overall autopsy rates over time in our population. The increasing use of sophisticated diagnostic imaging raises the possibility of detection bias for smaller SAH. Finally, we were unable to determine SAH etiology with certainty in a substantial portion across time periods, especially in 1988.
In our population, SAH incidence remained unchanged across several time points but case-fatality declined. Future studies should explore potential differential effects of sex and race on SAH incidence and outcomes. Future studies should also explore how specific factors and processes of care lead to improved outcomes and the effect of these interventions on patient-centered outcomes such as quality of life and neuropsychological outcomes.
ACKNOWLEDGMENT
The authors thank the following for their participation in the Greater Cincinnati/Northern Kentucky Stroke Study: University Hospital; Good Samaritan Hospital; Bethesda North Hospital; Christ Hospital; St. Elizabeth Edgewood, Covington, Florence, and Ft. Thomas Hospitals; Mercy Anderson, Clermont, Fairfield, Mt. Airy, and Western Hills Hospitals; the Jewish Hospital; Deaconess Hospital; Cincinnati Children's Medical Center; and the Cincinnati Veterans Affairs Medical Center.
GLOSSARY
- aSAH
aneurysmal subarachnoid hemorrhage
- CI
confidence interval
- GCNK
Greater Cincinnati/Northern Kentucky
- GCS
Glasgow Coma Scale
- ICD-9
International Classification of Diseases–9
- ICH
intracerebral hemorrhage
- ICU
intensive care unit
- mRS
modified Rankin Scale
- SAH
subarachnoid hemorrhage
AUTHOR CONTRIBUTIONS
Jason Mackey: study concept and design, drafting/revising of the manuscript, acquisition of data. Jane C. Khoury: analysis and interpretation of the data, drafting/revising of the manuscript. Kathleen Alwell: acquisition of the data, drafting/revising of the manuscript. Charles J. Moomaw: drafting/revising of the manuscript. Brett M. Kissela: acquisition of data, study supervision, drafting/revising of the manuscript. Matthew L. Flaherty: acquisition of data, drafting/revising of the manuscript. Opeolu Adeoye: acquisition of data, drafting/revising of the manuscript. Daniel Woo: acquisition of data, drafting/revising of the manuscript. Simona Ferioli: acquisition of data, drafting/revising of the manuscript. Felipe De Los Rios La Rosa: acquisition of data, drafting/revising of the manuscript. Sharyl Martini: acquisition of data, drafting/revising of the manuscript. Pooja Khatri: acquisition of data, drafting/revising of the manuscript. Joseph P. Broderick: study supervision, drafting/revising of the manuscript. Mario Zuccarello: drafting/revising of the manuscript. Dawn Kleindorfer: study supervision, drafting/revising of the manuscript.
STUDY FUNDING
NIH/NINDS R01 NS030678.
DISCLOSURE
J. Mackey: NIH/NINDS R01 NS030678 (modest). J. Khoury: NIH/NINDS R01 NS030678 (significant). K. Alwell: NIH/NINDS R01 NS030678 (significant). C. Moomaw: NIH/NINDS R01 NS030678 (significant). B. Kissela: NIH/NINDS R01 NS030678 (significant). M. Flaherty: NIH/NINDS R01 NS030678 (modest). O. Adeoye reports no disclosures relevant to the manuscript. D. Woo: NIH/NINDS R01 NS030678 (modest). S. Ferioli: NIH/NINDS R01 NS030678 (modest). F. De Los Rios La Rosa, S. Martini, P. Khatri, J. Broderick, and M. Zuccarello report no disclosures relevant to the manuscript. D. Kleindorfer: NIH/NINDS R01 NS030678 (significant). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke 2012;43:1711–1737. [DOI] [PubMed] [Google Scholar]
- 2.Bederson JB, Connolly ES, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 2009;40:994–1025. [DOI] [PubMed] [Google Scholar]
- 3.Sandvei MS, Mathiesen EB, Vatten LJ, et al. Incidence and mortality of aneurysmal subarachnoid hemorrhage in two Norwegian cohorts, 1984–2007. Neurology 2011;77:1833–1839. [DOI] [PubMed] [Google Scholar]
- 4.Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology 2010;74:1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke 1998;29:415–421. [DOI] [PubMed] [Google Scholar]
- 6.Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med 1992;326:733–736. [DOI] [PubMed] [Google Scholar]
- 7.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke 2004;35:426–431. [DOI] [PubMed] [Google Scholar]
- 8.Kleindorfer DO, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke 2010;41:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007;78:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–369. [DOI] [PubMed] [Google Scholar]
- 11.Brown RD Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke 1996;27:373–380. [PubMed] [Google Scholar]
- 12.Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 2009;8:635–642. [DOI] [PubMed] [Google Scholar]
- 13.Shojania KG, Burton EC. The vanishing nonforensic autopsy. N Engl J Med 2008;358:873–875. [DOI] [PubMed] [Google Scholar]
- 14.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001;124:249–278. [DOI] [PubMed] [Google Scholar]
- 15.Brinjikji W, Rabinstein AA, Nasr DM, Lanzino G, Kallmes DF, Cloft HJ. Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001–2008. AJNR Am J Neuroradiol 2011;32:1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andaluz N, Zuccarello M. Recent trends in the treatment of cerebral aneurysms: analysis of a nationwide inpatient database. J Neurosurg 2008;108:1163–1169. [DOI] [PubMed] [Google Scholar]
- 17.Suarez JI. Outcome in neurocritical care: advances in monitoring and treatment and effect of a specialized neurocritical care team. Crit Care Med 2006;34:S232–S238. [DOI] [PubMed] [Google Scholar]
- 18.Kramer AH, Zygun DA. Do neurocritical care units save lives? Measuring the impact of specialized ICUs. Neurocrit Care 2011;14:329–333. [DOI] [PubMed] [Google Scholar]
- 19.Alberts MJ, Hademenos G, Latchaw RE, et al. Recommendations for the establishment of primary stroke centers. Brain attack coalition. JAMA 2000;283:3102–3109. [DOI] [PubMed] [Google Scholar]
- 20.Alberts MJ, Latchaw RE, Selman WR, et al. Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition. Stroke 2005;36:1597–1616. [DOI] [PubMed] [Google Scholar]
- 21.Rinkel GJ, Wijdicks EF, Hasan D, et al. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet 1991;338:964–968. [DOI] [PubMed] [Google Scholar]
- 22.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 2010;41:e519–536. [DOI] [PubMed] [Google Scholar]