Abstract
Objective:
To explore temporal trends in tissue plasminogen activator (tPA) administration for acute ischemic stroke (AIS) in a biethnic community without an academic medical center and variation in trends by age, sex, ethnicity, and stroke severity.
Methods:
Cases of AIS were identified from 7 hospitals in the Brain Attack Surveillance in Corpus Christi (BASIC) project, a population-based surveillance study between January 1, 2000, and June 30, 2012. tPA, demographics, and stroke severity as assessed by the NIH Stroke Scale (NIHSS) were ascertained from medical records. Temporal trends were explored using generalized estimating equations, and adjustment made for age, sex, ethnicity, and NIHSS. Interaction terms were included to test for effect modification.
Results:
There were 5,277 AIS cases identified from 4,589 unique individuals. tPA use was steady at 2% and began increasing in 2006, reaching 11% in subsequent years. Stroke severity modified temporal trends (p = 0.003) such that cases in the highest severity quartile (NIHSS > 8) had larger increases in tPA use than those in lower severity quartiles. Although ethnicity did not modify the temporal trend, Mexican Americans (MAs) were less likely to receive tPA than non-Hispanic whites (NHWs) due to emerging ethnic differences in later years.
Conclusions:
Dramatic increases in tPA use were apparent in this community without an academic medical center. Primary stroke center certification likely contributed to this rise. Results suggest that increases in tPA use were greater in higher severity patients compared to lower severity patients, and a gap between MAs and NHWs in tPA administration may be emerging.
Stroke is a leading cause of death and long-term adult disability in the United States.1,2 Due to reductions in stroke mortality and increased life expectancy, the prevalence of stroke is climbing and predicted to increase by 20.5% by 2030, with the largest increase among Hispanic men (29%).1 Disparities exist in stroke care in the United States with differences in tissue plasminogen activator (tPA) use based on sex and race-ethnicity demonstrated in academic settings.3–7 Temporal trends show that tPA use has more than doubled at large, high-volume academic hospitals since the early 2000s, with data suggesting that treatment gaps based on age and race are narrowing.8–10 Yet there is a paucity of information concerning trends in community hospitals, which have been shown to exhibit different trends than academic centers.11
Mexican Americans (MA) have an increased stroke burden when compared to their non-Hispanic white (NHW) counterparts, including increased stroke incidence and poorer neurologic, functional, and cognitive outcomes.12–14 In the face of increasing prevalence of stroke in the US population, in particular among Hispanics, increasing the use of tPA in those who are clinically eligible is essential.1 Our study evaluated temporal trends in tPA use from 2000 to 2012 in a biethnic community without an academic medical center, and the effect of race-ethnicity, sex, age, and stroke severity on these trends.
METHODS
Population and setting.
Brain Attack Surveillance in Corpus Christi (BASIC) is a population-based surveillance project aimed at capturing all strokes in Nueces County, Texas, employing both active and passive surveillance components. The project has been in continuous operation since 2000. Most residents of Nueces County live in the Corpus Christi metropolitan area, and the surrounding counties are sparsely populated. The population of Nueces County is approximately 347,691 (2012 estimate) with 61.5% identifying as Hispanic, most of whom are MA.15 The population is served by 2 health systems with 7 acute-care community hospitals between them, none of which is an academic medical center. The closest potential referral centers are located in San Antonio and Houston, which are both more than 150 miles away, meaning that exceedingly few cases from Nueces County will be sent to one of these areas without passing through one of the area's 7 acute-care facilities. All hospitals have CT and MRI capabilities. During the study, 2 of the hospitals were certified as Joint Commission primary stroke centers (PSCs). One center was certified in 2009 and the other in 2010, and both have maintained certification to the present date. In order to be eligible for the BASIC project, a patient must be 45 years old or greater and reside in Nueces County for at least 6 months of the year. Cases in which the immediate cause of stroke was head trauma were excluded. For our analysis we included only those cases with race/ethnicity of MA or NHW. Other races/ethnicities were excluded due to their low sample size, which prohibited the examination of trends in these subgroups.
Standard protocol approvals, registrations, and patient consents.
The University of Michigan internal review board (IRB) and the IRBs of both Nueces County health systems approved the BASIC project.
Case ascertainment.
The BASIC project utilizes both active and passive surveillance techniques to identify cases of stroke. The study procedures were previously described in full, and are briefly described here.12,16,17 In the active component, trained abstractors review hospital admission logs on a daily basis using validated screening terms.18 In addition, the hospital wards and intensive care units are reviewed to identify in-house strokes and those missed upon admission. Emergency department (ED) logs are frequently reviewed as well using the aforementioned screening terms. Abstractors are trained to review medical records and generate electronic reports of possible cases. In the passive component, hospital discharge records are collected monthly and reviewed searching for ICD-9 codes for stroke diagnoses (430–438, excluding 433.x0, 434.x0 [x 5 1–9], 437.0, 437.2, 437.3, 437.4, 437.5, 437.7, 437.8, and 438). There have been minor changes to the case ascertainment procedures during the project, which have been described previously.12
Case validation.
Cases are validated by neurologists or a stroke fellowship–trained emergency physician. Since tPA is only appropriate for the treatment of ischemic stroke, we excluded cases of hemorrhagic strokes. Stroke cases are validated using previously published international guidelines that define stroke as the onset of focal neurologic deficits following a defined vascular pattern without resolution within 24 hours, unless tPA is administered, and is not explainable by nonvascular etiology.19 This does not include evidence of stroke observed on MRI in the absence of clinical deficit. CT and MRI assist in determining the stroke type. All validated cases of acute ischemic stroke (AIS) in the BASIC project between January 1, 2000, and June 30, 2012, were included in the current analysis.
tPA and covariate collection.
During abstraction of cases meeting inclusion criteria, tPA administration was abstracted directly from the medical record. In addition, demographics (age, sex, race-ethnicity) and initial stroke severity were collected from the medical record. Stroke severity was assessed by the NIH Stroke Scale (NIHSS), which was abstracted directly from the medical record or calculated based on a previously validated method.20 We have previously demonstrated high agreement between race-ethnicity from the medical record and self-report (κ = 0.94).17
Statistical analysis.
Demographic characteristics and other variables were summarized for the overall study population as well as aggregated for the first 3 years (2000–2002) and the last 3 years (2010–2012) of the study period. To calculate the mean change of each variable from 2000 to 2012, we fitted generalized estimating equations (GEE) to account for repeated observations among participants. For the primary analysis, we first examined the unadjusted temporal trend in tPA use using the yearly data. We explored the potential for nonlinear temporal trends in tPA use by fitting generalized additive models (GAM) with a smooth term for calendar year. Visual inspection of the fitted trend and the degrees of freedom of the smooth term indicated a quartic (fourth degree) polynomial would sufficiently describe the temporal trend. We then fit a model using GEE, where the temporal trend was modeled using a fourth-degree polynomial; GEE allowed us to account for repeated observations among participants. A binomial distribution and log link were used in both the GEE and GAM models. We then fit an adjusted model additionally including age, sex, ethnicity, and NIHSS. Functional forms of continuous covariates (age and NIHSS) were also examined in GAMs, which indicated a linear term for age and a quadratic term for NIHSS would adequately describe the associations. We then assessed for effect modification of the temporal trend in tPA administration by age, sex, ethnicity, and NIHSS score by including interaction terms between these covariates and time in separate models using GEE. The final step was to fit a model including covariates and any significant interactions identified in the previous step. For the interaction terms, a significance level of 0.10 was used. Statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC) and R 3.1.1 (R Development Core Team, 2014).
RESULTS
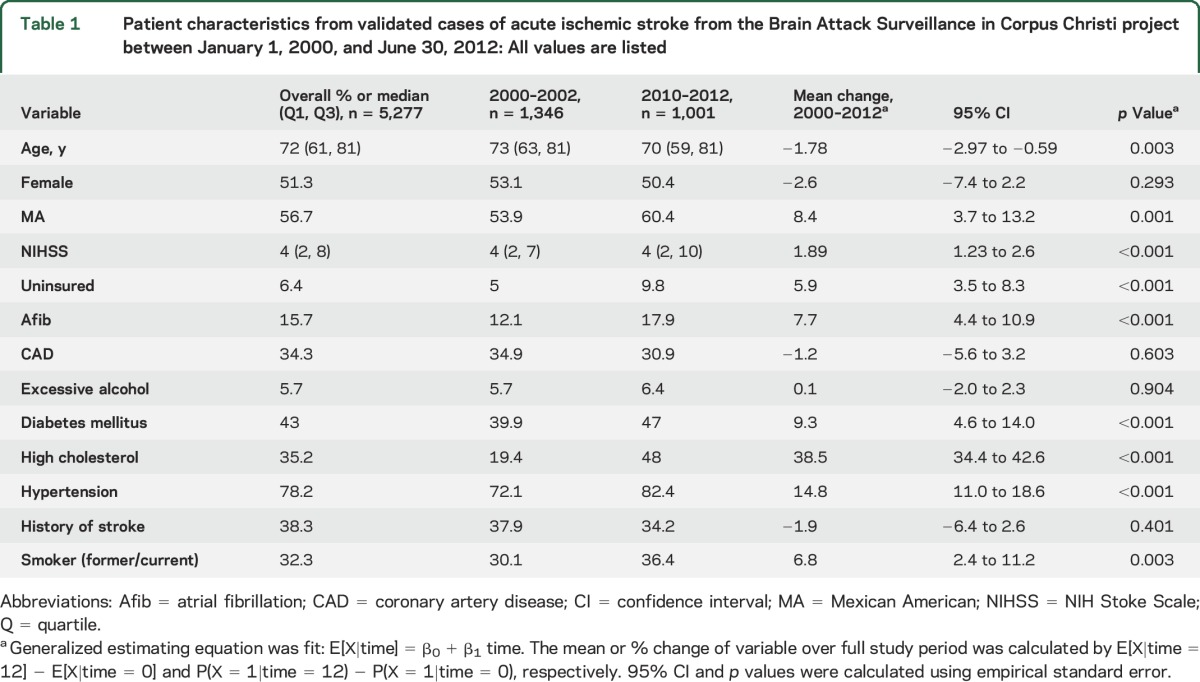
There were 5,661 validated cases of AIS recorded in the BASIC project between January 1, 2000, and June 30, 2012. Of these cases, 384 were excluded due to race/ethnicity other than NHW or MA. In addition, 3 cases were missing an NIHSS score and were thus excluded. Patient characteristics are shown in table 1, displayed for the overall study population, as well as an early (2000–2002) and late (2010–2012) time period in order to compare changes over time. The median age of the study population was 72 (interquartile range [IQR] 61–81). Fifty-one percent were female and 56.7% were MA. Overall, the cases of stroke were mild to moderate in nature, with median NIHSS score of 4 (IQR 2–8). The risk factors for stroke present among the cases of AIS showed variation between the early time period and the late time period. There was an increase in the prevalence of uninsured, atrial fibrillation, diabetes mellitus, high cholesterol, hypertension, and former/current smoker status.
Table 1.
Patient characteristics from validated cases of acute ischemic stroke from the Brain Attack Surveillance in Corpus Christi project between January 1, 2000, and June 30, 2012: All values are listed
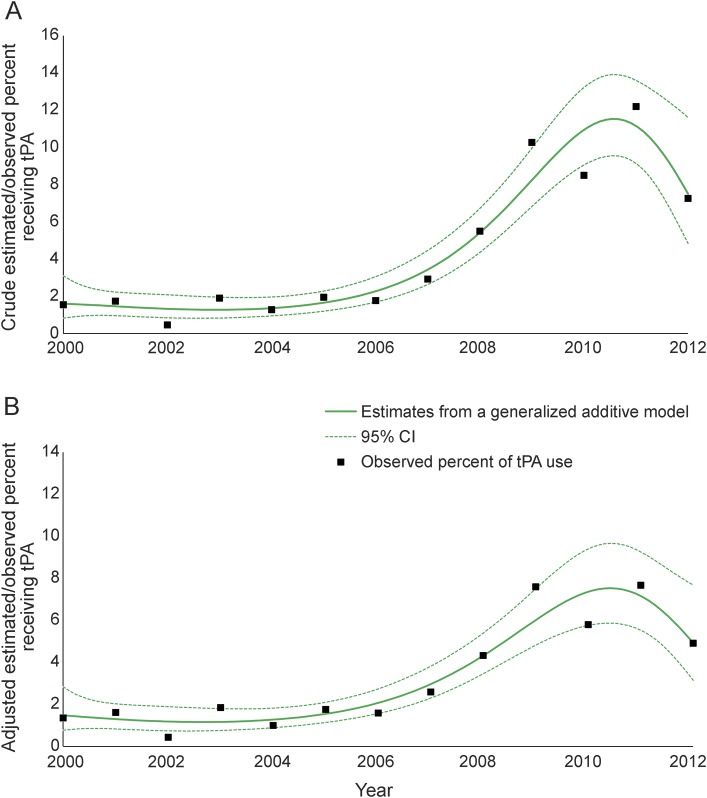
The analysis included 5,277 cases, from 4,589 unique individuals, and in 219 of these cases, representing 212 unique individuals, tPA was administered. The crude/adjusted proportion of receiving tPA each year was steady at 2% until around 2006 and then increased substantially (figure 1), reaching 7%–11% in 2011.
Figure 1. Crude and adjusted proportion receiving tissue plasminogen activator (tPA).
Estimated and observed proportion receiving tPA among cases of validated acute ischemic stroke from the Brain Attack Surveillance in Corpus Christi project between January 1, 2000, and June 30, 2012. (A) Crude estimated/observed proportion of receiving tPA. (B) Adjusted estimated/observed proportion of receiving tPA. Note that year was modeled as a categorical variable for observed percent in tPA use. CI = confidence interval.
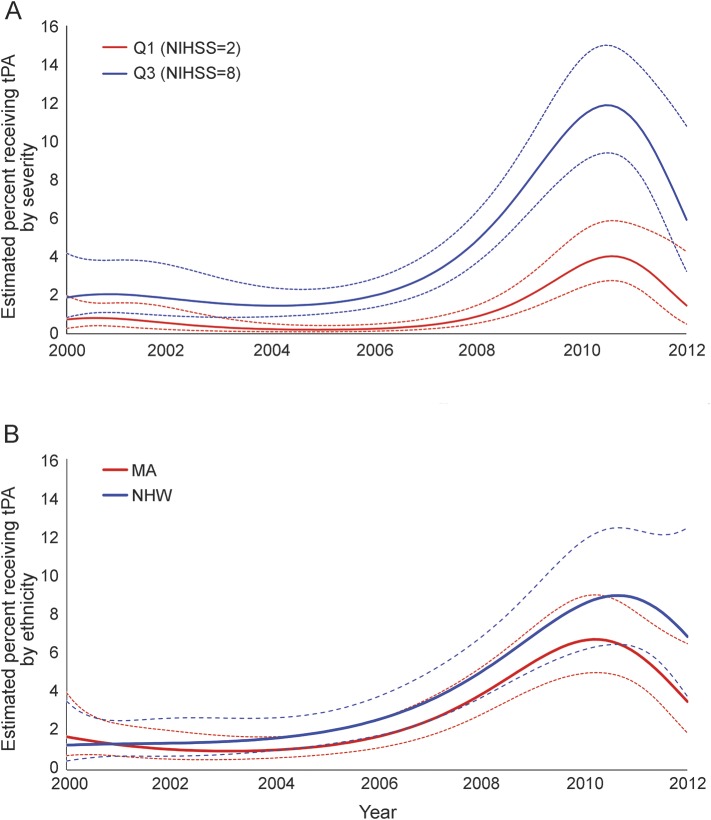
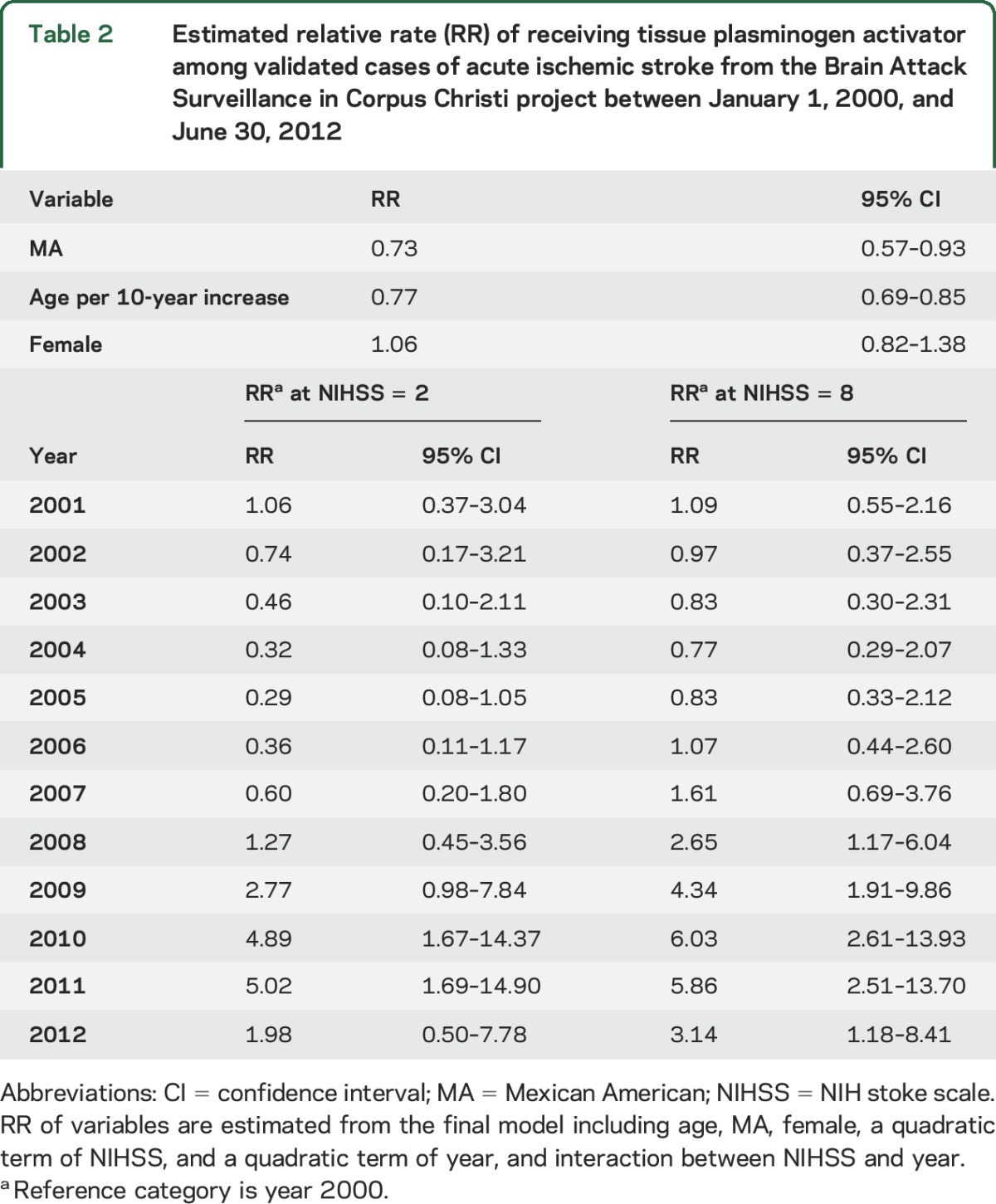
Effect modification of the temporal trend in tPA use was present for NIHSS (p = 0.003). Larger increases in tPA use were observed for patients with higher NIHSS than for those with lower NIHSS (figure 2A). With adjustment for other covariates at their means, the estimated proportion of patients receiving tPA with a lower NIHSS (evaluated at 25th percentile) increased from 0.74% (95% CI 0.28–1.98) in 2000 to 1.47% (95% CI 0.50–4.26) in 2012. The rapidity of this increase was substantially lower than the rise observed in the patients with higher NIHSS (evaluated at 75th percentile), in whom the estimated percent of receiving tPA increased from 1.88% (95% CI 0.85–4.18) in 2000 to 5.92% (95% CI 3.25–10.79) in 2012. Results for other covariates in the final multivariable model are summarized in table 2. Greater age was associated with reduced tPA use (relative rate [RR] 0.77 for 10-year increase in age [95% CI 0.69–0.85]). MAs were less likely to receive tPA (RR 0.73; 95% CI 0.57–0.93) compared to NHWs although eligibility criteria were not available for assessment. Although ethnicity did not modify the temporal trend, there was a widening difference in tPA use between NHWs and MAs in the years since 2006 (figure 2B). Sex and age did not modify the effect of time on tPA administration.
Figure 2. Estimated proportion receiving tissue plasminogen activator (tPA) by severity and ethnicity.
(A) Estimated percent (adjusted) receiving tPA use at NIH Stroke Scale (NIHSS) Q1 (NIHSS = 2) and NIHSS Q3 (NIHSS = 8) in 2000–2012. (B) Estimated percent (adjusted) receiving tPA use by ethnicity. MA = Mexican American; NHW = non-Hispanic white.
Table 2.
Estimated relative rate (RR) of receiving tissue plasminogen activator among validated cases of acute ischemic stroke from the Brain Attack Surveillance in Corpus Christi project between January 1, 2000, and June 30, 2012
DISCUSSION
The dramatic increase in tPA administration observed in this study is greater than previously reported temporal trends over shorter time periods from national samples, which represent primarily academic medical centers.8,10,21 Previously published evidence suggests that of all the patients who present to the ED with stroke, the proportion arriving within 3 hours from symptom onset is less than 50% and within 4 hours is approximately 55%.22 It is possible that the increase in tPA use over time reflects a higher proportion of patients presenting within the appropriate treatment window. We did not have reliable data concerning time from symptom onset to ED presentation to test this hypothesis; however, previous work suggests that the proportion of patients arriving within 2 hours has not changed significantly over time.22 This leads us to believe that temporal trends in arrival time may be playing a small role and the more likely explanation is that clinical decision-making and in-hospital systems are leading to more patients receiving tPA.
The observed ethnic difference appears to be driven primarily by differences in the recent years of the project when tPA use was increasing dramatically and more so in NHWs. This contradicts findings from other studies of temporal trends, which indicate racial-ethnic disparities in tPA use are narrowing, though these studies did not specifically examine MAs.8–10 This is of particular importance due to the recent finding that functional outcomes following stroke are worse for MAs as compared with NHWs.14 Previous work in BASIC demonstrated that MAs were less likely to arrive to the ED by emergency medical services (EMS) than NHWs.23 Arrival by EMS has been associated with greater likelihood of receiving tPA, which could be driving some of the observed ethnic difference in tPA use.24–26 Of note, the prevalence of uninsured status among the MAs in the study was 1.65 times higher than among NHWs. While there was variation over the study period, including an increase in the proportion of uninsured patients, the ratio between MAs and NHWs remained stable. It is unlikely that the small differences in insurance status could be a contributing factor to MAs receiving tPA less often, especially because of the emerging divergence in treatment rates during later years of the study. There were 2 primary risk factors that showed ethnic differences. Prevalence of atrial fibrillation was higher in NHWs and prevalence of diabetes was higher in MAs. It is important to note that these both may affect eligibility for tPA administration to a varying degree. Patients with atrial fibrillation are often on anticoagulation medication and an international normalized ratio >1.7, usually exceeded by those on anticoagulation medication, is an exclusion criterion for tPA administration. This would be expected to result in more NHWs being ineligible to receive tPA. Diabetes combined with a history of prior ischemic stroke is a relative exclusion criterion in the extended window (3–4.5 hours), which would have a lesser impact on administration rates compared to atrial fibrillation. These differences in primary risk factors are unlikely to explain the observed temporal trends and may in fact lead to the ineligibility of more NHWs than MAs due to propensity for hemorrhagic complications. Future research should consider the causes of lower tPA use in MAs to identify possible intervention targets such as increased symptom recognition and use of EMS.
Findings suggest that the population of patients with more severe strokes based on NIHSS saw greater increases in tPA administration as compared to those with more mild strokes. Recently there has been more attention paid to the potential benefits for mild stroke as well, which has manifested as greater tPA use over time for mild stroke in other studies.10 In the current study, mild strokes did show greater likelihood of receiving tPA over time; however, as mentioned above, it was much less than the rise among more severe strokes.
As stated in the Methods, 2 of the participating hospitals achieved certification as PSCs during the study period. This certification administered by the Joint Commission requires health centers to meet certain quality of care metrics including the use of thrombolytics in eligible patients with AIS. One center was certified in 2009 and the other in 2010, and both have maintained certification. Those hospitals achieving PSC certification have been shown to have significant increases in IV thrombolysis rates as well as improved 30-day mortality and readmission outcomes.27–33 Thus, the efforts by these 2 hospitals to achieve and maintain PSC status likely contributed to the abrupt rise in tPA use observed in the community.
In previous studies, there have been conflicting data concerning sex differences in tPA use. A large meta-analysis found that women were consistently less likely to receive tPA than men across studies, while a nationally representative sample found no difference.7,34 The evidence in the current study suggests no sex differences, and trends did not differ by sex. Similarly, previous research has shown that older stroke patients receive less care than younger patients, including fewer recommended diagnostic tests and treatments.35–37 In our analysis, we found that as age increases, the likelihood of receiving tPA decreases, which is consistent with previous work.
A major strength of our study worth emphasizing is the population-based setting. By conducting the BASIC surveillance project as a community-wide, population-based study, we are able to reflect the health care experience of a previously understudied large proportion of the US population. The characteristics of the BASIC project make it the ideal setting to study the stroke management practices of general nonacademic community hospitals, which may have different practice patterns than the more often studied large academic medical centers.11 This includes studies on the effect of PSC certification, which have been focused on academic medical centers; the few that examine community hospitals are small and lack sufficient follow-up. Our study fills this gap by providing evidence from multiple facilities with a long duration of data collection.
In addition to providing valuable information concerning care in the community hospital setting, the BASIC project provides high-quality information for the MA population, one not well-represented in previous work. The 2010 Census showed that the United States has a Hispanic population of 50.5 million, which comprises 16% of the total population. Of the 50.5 million Hispanics, 63% are MAs, with a growth of 54.1% since 2000.38 The increased stroke burden in MAs along with the knowledge that MAs comprise a rapidly growing group of the US population underlies the need for further research surrounding stroke management in this population.
Some limitations warrant discussion. The current study was unable to explore, and adjust for, hospital arrival and door-to-needle times due to a lack of reliable arrival time data across the entire study period. We focused our analysis on the trend of patients receiving treatment with IV tPA. It would be advantageous to consider arrival within the treatment window and IV thrombolytic eligibility to better understand the observed trends. A lack of prognostic and clinical outcome data associated with the increasing use of tPA means that we are unable to comment on this relationship. It would be an important next step to explore if the increase in tPA use is improving outcomes. The generalizability of the results may be limited by the demographic makeup of the region, which differs from other areas of the country. Previously established regional variation in thrombolytic therapy means that the well-defined geographic region may also limit generalizability.39 However, this is balanced with the ability of the study design to capture nearly all cases of stroke in which the patient presented to a medical center.
Rapid increases in tPA use were apparent in this community with the years since 2006 showing an exponential increase. This is likely a result of the success of initiatives, such as PSC certification, designed to improve the use of IV thrombolytics in those patients in whom it has been proven effective. Varying temporal trends indicate that severe strokes have seen greater increases in tPA use, and a gap in tPA use between MAs and NHWs may be emerging. As physician experience with tPA and its use in community settings increases, follow-up studies should continue to explore temporal trends in tPA as well as identify possible strategies to improve tPA use in MAs.
Supplementary Material
GLOSSARY
- AIS
acute ischemic stroke
- BASIC
Brain Attack Surveillance in Corpus Christi
- ED
emergency department
- EMS
emergency medical services
- GAM
generalized additive models
- GEE
generalized estimating equations
- ICD-9
International Classification of Diseases–9
- IQR
interquartile range
- IRB
internal review board
- MA
Mexican American
- NHW
non-Hispanic white
- NIHSS
NIH Stroke Scale
- PSC
primary stroke centers
- RR
relative rate
- tPA
tissue plasminogen activator
Footnotes
Editorial, page 2178
AUTHOR CONTRIBUTIONS
L.D.L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: J.S.D., L.D.L. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: J.S.D., Y.B., L.D.L. Critical revision of the manuscript intellectual content: All authors. Statistical analysis: Y.B., J.S.D., L.D.L. Obtained funding: L.D.L., L.B.M. Administrative, technical, or material support: L.D.L., L.B.M. Study supervision: L.D.L., L.B.M.
STUDY FUNDING
Supported by NIH (R01 NS38916).
DISCLOSURE
J. Domino, J. Baek, W. Meurer, N. Garcia, L. Morgenstern, and M. Campbell report no disclosures relevant to the manuscript. L. Lisabeth is funded by R01 NS062675, R01 HL098065, R21 NS086144, R01 MD008879, R01 NS070941, R01 HL07175909, R01 NS38916, R01 HL123379, and PCORIS grant IHIS-1310-07421. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics: 2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Prevalence and most common causes of disability among adults: United States, 2005. MMWR Morb Mortal Wkly Rep 2009;58:421–426. [PubMed] [Google Scholar]
- 3.Cruz-Flores S, Rabinstein A, Biller J, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2091–2116. [DOI] [PubMed] [Google Scholar]
- 4.Hsia AW, Edwards DF, Morgenstern LB, et al. Racial disparities in tissue plasminogen activator treatment rate for stroke: a population-based study. Stroke 2011;42:2217–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston SC, Fung LH, Gillum LA, et al. Utilization of intravenous tissue-type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity. Stroke 2001;32:1061–1068. [DOI] [PubMed] [Google Scholar]
- 6.Nasr DM, Brinjikji W, Cloft HJ, Rabinstein AA. Racial and ethnic disparities in the use of intravenous recombinant tissue plasminogen activator and outcomes for acute ischemic stroke. J Stroke Cerebrovasc Dis 2013;22:154–160. [DOI] [PubMed] [Google Scholar]
- 7.Reeves M, Bhatt A, Jajou P, Brown M, Lisabeth L. Sex differences in the use of intravenous rt-PA thrombolysis treatment for acute ischemic stroke: a meta-analysis. Stroke 2009;40:1743–1749. [DOI] [PubMed] [Google Scholar]
- 8.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke 2011;42:1952–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonarow GC, Reeves MJ, Zhao X, et al. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation 2010;121:879–891. [DOI] [PubMed] [Google Scholar]
- 10.Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at get with the guidelines-stroke hospitals. Circ Cardiovasc Qual Outcomes 2013;6:543–549. [DOI] [PubMed] [Google Scholar]
- 11.Caveney AF, Silbergleit R, Frederiksen S, et al. Resource utilization and outcome at a university versus a community teaching hospital in tPA treated stroke patients: a retrospective cohort study. BMC Health Serv Res 2010;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgenstern LB, Smith MA, Sanchez BN, et al. Persistent ischemic stroke disparities despite declining incidence in Mexican Americans. Ann Neurol 2013;74:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgenstern LB, Smith MA, Lisabeth LD, et al. Excess stroke in Mexican Americans compared with non-Hispanic Whites: the Brain Attack Surveillance in Corpus Christi project. Am J Epidemiol 2004;160:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lisabeth LD, Sanchez BN, Baek J, et al. Neurological, functional, and cognitive stroke outcomes in Mexican Americans. Stroke 2014;45:1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Census Bureau, Nueces County, TX Census Data. 2015. Available at: quickfacts.census.gov/qfd/states/48/48355.html. Accessed April 14, 2015.
- 16.Piriyawat P, Smajsova M, Smith MA, et al. Comparison of active and passive surveillance for cerebrovascular disease: the Brain Attack Surveillance in Corpus Christi (BASIC) project. Am J Epidemiol 2002;156:1062–1069. [DOI] [PubMed] [Google Scholar]
- 17.Smith MA, Risser JM, Moye LA, et al. Designing multi-ethnic stroke studies: the Brain Attack Surveillance in Corpus Christi (BASIC) project. Ethn Dis 2004;14:520–526. [PubMed] [Google Scholar]
- 18.Morgenstern LB, Wein TH, Smith MA, Moye LA, Pandey DK, Labarthe DR. Comparison of stroke hospitalization rates among Mexican-Americans and non-Hispanic whites. Neurology 2000;54:2000–2002. [DOI] [PubMed] [Google Scholar]
- 19.Asplund K, Tuomilehto J, Stegmayr B, Wester PO, Tunstall-Pedoe H. Diagnostic criteria and quality control of the registration of stroke events in the MONICA project. Acta Med Scand Suppl 1988;728:26–39. [DOI] [PubMed] [Google Scholar]
- 20.Williams LS, Engin YY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with NIH Stroke Scale. Stroke 2000;31:858–862. [DOI] [PubMed] [Google Scholar]
- 21.Kleindorfer D, de los Rios La Rosa F, Khatri P, Kissela B, Mackey J, Adeoye O. Temporal trends in acute stroke management. Stroke 2013;44(6 suppl 1):S129–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtman JH, Watanabe E, Allen NB, Jones SB, Dostal J, Goldstein LB. Hospital arrival time and intravenous t-PA use in US academic medical centers, 2001–2004. Stroke 2009;40:3845–3850. [DOI] [PubMed] [Google Scholar]
- 23.Smith MA, Lisabeth LD, Bonikowski F, Morgenstern LB. The role of ethnicity, sex, and language on delay to hospital arrival for acute ischemic stroke. Stroke 2010;41:905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng YZ, Reeves MJ, Jacobs BS, et al. IV tissue plasminogen activator use in acute stroke: experience from a statewide registry. Neurology 2006;66:306–312. [DOI] [PubMed] [Google Scholar]
- 25.Menon SC, Pandey DK, Morgenstern LB. Critical factors determining access to acute stroke care. Neurology 1998;51:427–432. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder EB, Rosamond WD, Morris DL, Everson KR, Hinn AR. Determinants of use of emergency medical services in a population with stroke symptoms: the Second Delay in Accessing Stroke Healthcare (DASH II) Study. Stroke 2000;31:2591–2596. [DOI] [PubMed] [Google Scholar]
- 27.Douglas VC, Tong DC, Gillum LA, et al. Do the Brain Attack Coalition's criteria for stroke centers improve care for ischemic stroke? Neurology 2005;64:422–427. [DOI] [PubMed] [Google Scholar]
- 28.Gropen TI, Gagliano PJ, Blake CA, et al. Quality improvement in acute stroke: the New York State Stroke Center Designation Project. Neurology 2006;67:88–93. [DOI] [PubMed] [Google Scholar]
- 29.Lattimore SU, Chalela J, Davis L, et al. Impact of establishing a primary stroke center at a community hospital on the use of thrombolytic therapy: the NINDS Suburban Hospital Stroke Center experience. Stroke 2003;34:e55–e57. [DOI] [PubMed] [Google Scholar]
- 30.Lichtman JH, Jones SB, Wang Y, Watanabe E, Leifhert-Limson E, Goldstein LB. Outcomes after ischemic stroke for hospitals with and without joint commission-certified primary stroke centers. Neurology 2011;76:1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhakaran S, McNulty M, O'Neill K, Ouyang B. Intravenous thrombolysis for stroke increases over time at primary stroke centers. Stroke 2012;43:875–877. [DOI] [PubMed] [Google Scholar]
- 32.Rajamani K, Millis S, Watson S, et al. Thrombolysis for acute ischemic stroke in joint commission-certified and -noncertified hospitals in Michigan. J Stroke Cerebrovasc Dis 2013;22:49–54. [DOI] [PubMed] [Google Scholar]
- 33.Stradling D, Yu W, Langdorf ML, et al. Stroke care delivery before vs after JCAHO stroke center certification. Neurology 2007;68:469–470. [DOI] [PubMed] [Google Scholar]
- 34.Allen NB, Myers D, Watanabe E, et al. Utilization of intravenous tissue plasminogen activator for ischemic stroke: are there sex differences? Cerebrovasc Dis 2009;27:254–258. [DOI] [PubMed] [Google Scholar]
- 35.Bhalla A, Grieve R, Tilling K, Rudd AG, Wolfe CDA. Older stroke patients in Europe: stroke care and determinants of outcome. Age Ageing 2004;33:618–624. [DOI] [PubMed] [Google Scholar]
- 36.Di Carlo A, Lamassa M, Baldereschi M, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke 2003;34:1114–1119. [DOI] [PubMed] [Google Scholar]
- 37.Palnum KD, Petersen P, Sorensen HT, et al. Older patients with acute stroke in Denmark: quality of care and short-term mortality. A nationwide follow-up study. Age Ageing 2008;37:90–95. [DOI] [PubMed] [Google Scholar]
- 38.Ennis SR, Rios-Vargas M, Albert NG. The Hispanic population: 2010. In: 2010 Census Briefs. Washington, DC: United States Census Bureau; 2011. [Google Scholar]
- 39.Skolarus LE, Meurer WJ, Shanmugasundaram K, et al. Marked regional variation in acute stroke treatment among Medicare beneficiaries. Stroke 2015;46:1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.