SECTION 1
A 64-year-old right-handed man presented to the emergency department with a 4-week history of bilateral blurred and distorted vision. He reported that objects in his central visual field appeared to change shape and were disproportionately large or small. He also reported intermittent flashes of light with altered color perception. His medical history was notable for prostate cancer treated with prostatectomy, Bell palsy at age 25, gastroesophageal reflux disease, and bilateral refractive errors. His family had also noted intermittent episodes of confusion and anger over the preceding weeks along with difficulty recognizing family members. He endorsed minimal right-sided periorbital headache but reported no diplopia, weakness, paresthesiae, numbness, speech changes, or history of similar symptoms. He had no recent history of fever, illness, or infectious exposures. He had no history of seizures or migraine. He was taking omeprazole and did not smoke or use alcohol or illicit drugs.
Question for consideration:
What is the differential diagnosis and localization of the patient's symptoms?
SECTION 2
The differential diagnosis for distortions of vision in terms of size (micropsia or macropsia), shape (metamorphopsia), and color (dyschromatopsia) is broad and can localize to the orbit itself or alternatively to the occipital cortex. Binocular visual disturbances are more likely to localize to the occipital cortex than the bilateral orbits; possible disease processes affecting the occipital cortex in this case include occipital seizures, ischemic lesions, primary brain tumors/metastatic lesions, migraine variants, or Alzheimer disease (posterior cortical atrophy variant). Posterior cortical atrophy can present with progressive cortical visual impairment with features of the Balint syndrome (simultanagnosia, optic ataxia, and ocular apraxia), visual agnosia, prosopagnosia, visual hallucinations, and visual field defects, often with some degree of memory impairment. The differential diagnosis of ophthalmologic processes that may present in this manner include pathology at the fovea such as subretinal hemorrhage in macular degeneration, retinal trauma, or inflammatory processes such as chorioretinitis or vasculitis.
The patient's description of the visual misperceptions in terms of both size and shape are suggestive of Alice in Wonderland syndrome and localize to the right (nondominant) parieto-occipital lobes. Figure e-1 at Neurology.org illustrates his subjective interpretation of the appearance of the visual distortions.
Examination revealed intact mental status including orientation, attention, language, 3-word recall at 5 minutes, long-term memory, calculations, abstract reasoning, and praxis. The patient had equal and reactive pupils, visual acuity without correction of 20/30 right eye and 20/40 left eye, severe impairment on Ishihara color plate testing (0/8 in both eyes), binocular central and pericentral distortion of vertical lines on Amsler grid testing, left hemifield defect in the right eye and left inferonasal defect in the left eye on Humphrey visual field testing, sharp optic discs with unremarkable funduscopic examination, intact cranial nerves, and otherwise normal neurologic examination.
Question for consideration:
What additional workup is indicated?
SECTION 3
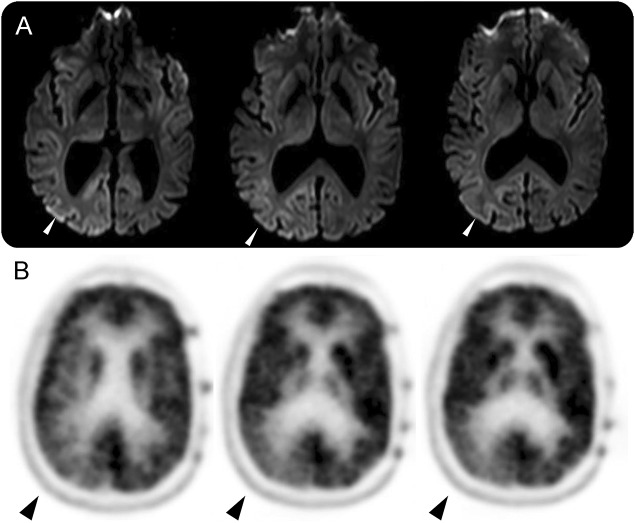
The patient's examination objectively confirmed dyschromatopsia, metamorphopsia, micropsia, and macropsia as well as an incongruous left homonymous hemianopia, localizing to the right parieto-occipital lobe. Laboratory workup included a normal complete blood count, negative serum and urine toxicology screens, erythrocyte sedimentation rate 2 mm/hour, C-reactive protein 2.3 mg/L, and prostate-specific antigen < 0.10 ng/mL. Initial MRI brain without contrast and magnetic resonance angiography head and neck were reported as normal. Ocular coherence tomography and fundus autofluorescence were unremarkable. A spot EEG revealed continuous, generalized periodic discharges with occipital predominance at 1–2 Hz (figure e-2). MRI brain with contrast was repeated a few days later and showed restricted diffusion in a cortical ribboning pattern predominantly involving the right parieto-occipital lobe (figure 1A).
Figure 1. MRI brain.
(A) DWI sequences with restricted diffusion in a cortical ribboning pattern in the right parietal and occipital lobes and (B) PET-CT showing hypometabolism in the right occipital lobe.
Questions for consideration:
What other diagnosis should be considered?
What are the next steps in workup and management?
SECTION 4
An important diagnosis to consider at this point is Creutzfeldt-Jacob disease (CJD) given the rapid onset of symptoms, cortical ribboning pattern on MRI, and abnormal EEG.
A lumbar puncture was completed and CSF analysis revealed colorless fluid, protein 38 mg/dL, glucose 62 mg/dL, 0 total nucleated cells and 8 erythrocytes, negative cultures, varicella-zoster virus (VZV) immunoglobulin M <1.1, negative VZV PCR, negative paraneoplastic antibody panel, negative 14-3-3 protein, and negative cytology. PET scan of the brain revealed hypometabolism in the right occipital lobe (figure 1B). Repeat neurologic examination did not reveal myoclonus or alterations in mental status or memory. There was no known family history of prion disease. He was clinically diagnosed with probable sporadic CJD (sCJD), Heidenhain variant. He was trialed on levetiracetam and clonazepam, with minimal clinical or electrographic improvement, and both were discontinued. He continued to report ongoing visual distortions. CSF sent to the National Prion Disease Surveillance Center subsequently returned with positive 14-3-3 protein, elevated t-tau protein 6301 pg/mL (consistent with >75% probability of prion disease), and positive real-time quaking-induced conversion test (RTQuIC).
The patient rapidly declined and died 4 months after initial presentation. An autopsy was performed, sequencing of the prion protein gene did not reveal a pathogenic mutation. Abnormal prion protein was characterized by Western blot and confirmed with histopathologic and immunohistochemical examinations by the National Prion Disease Surveillance Center, confirming the diagnosis of prion disease characteristic of sCJD.
DISCUSSION
The Alice in Wonderland syndrome is a perceptual disorder that includes somesthetic or visual misperceptions of size (micropsia or macropsia), shape (metamorphopsia), or distance (pelopsia or telopsia) of the patient's own self or the external environment.1 The term was first coined in 1955 by John Todd, and was named after Alice's own misperception of body image in Alice's Adventures in Wonderland (1865), believed to be based on the author's (Lewis Carroll) own experience of migraines.1 Reported causes of this syndrome include infections, (including Epstein-Barr virus, VZV, influenza, scarlet fever, and coxsackie B1), migraines, seizures, stroke, TIAs, and toxic encephalopthy.2 The symptoms are thought to localize to the nondominant posterior parietal and anterior occipital lobes.3 In this patient's case, predominant involvement of the right parieto-occipital lobe in CJD explained his symptoms.
The Heidenhain variant of CJD is a prion disease characterized by predominant visual symptoms with mild to absent cognitive symptoms early on in the disease course, followed by rapidly progressive cognitive decline and short disease duration.4 Visual disturbances can include visual misperceptions in terms of size, shape, and color, visual agnosia, visual field defects, cortical blindness with visual anosognosia (Anton syndrome), and visual hallucinations.5 The histopathologic findings of spongiform degeneration, neuronal loss, and reactive gliosis are most prominent in the occipital lobes.5
According to the Centers for Disease Control and Prevention diagnostic criteria for CJD, definite sporadic CJD is diagnosed through autopsy. Detection of protease-resistant PrPSc in brain tissue is the gold standard for diagnosis of prion disease. Probable sporadic CJD requires (1) rapidly progressive dementia; (2) at least 2 of the following 4 clinical features: myoclonus, visual or cerebellar signs, pyramidal/extrapyramidal signs, and akinetic mutism; (3) a positive result on one of the following tests: typical EEG (periodic sharp wave complexes), positive 14-3-3 CSF assay and disease duration of less than 2 years, MRI brain with high signal abnormalities in caudate nucleus/putamen on diffusion-weighted imaging (DWI) or fluid-attenuated inversion recovery sequences; and (4) routine investigations excluding alternative diagnoses.6
The 14-3-3 protein is considered an adjunct, rather than confirmatory, test for the diagnosis of prion diseases. The sensitivity has been reported to be 92% with a specificity of 80% for diagnosis of sporadic CJD,7 although sensitivity rates as low as 43% have been reported.8 It is believed to be a marker of neuronal injury and positive tests can occur in nonprion diseases, including paraneoplastic disease, brain metastases, metabolic and viral encephalopathies, and neurodegenerative diseases including Alzheimer disease.9 Thus, a negative 14-3-3 test does not exclude CJD, particularly in patients where there is a strong clinical suspicion for prion disease. The recently developed RTQuIC prion protein conversion assay detects disease-associated prion protein in the CSF, with a reported sensitivity of 87% and specificity of 99% for sCJD, offering a more specific premortem diagnostic test for CJD.10
Periodic sharp wave complexes, which can be generalized or localized to the bilateral occipital lobes, were reported in 79% of cases of Heidenhain variant CJD in one case series. In this case series, increased signal intensity in the basal ganglia was reported in 64% of cases, with increased signal intensity confined to the occipital lobes in the remaining 36%.5 DWI is the most sensitive technique for detecting CJD-related changes, particularly cortical changes. PET-CT, SPECT, and MRI spectroscopy can also be used to confirm occipital lobe involvement, although their diagnostic utility has not been well-established.
Patients with the Heidenhain variant of CJD often present initially to an ophthalmologist with visual symptoms and a normal ophthalmologic examination. A diagnosis of Heidenhain variant of CJD should be considered in the differential diagnosis of patients presenting with subacute onset of visual symptoms including visual misperception, visual agnosia, or visual field defects in the presence of a normal ophthalmologic examination, particularly in those who have, or subsequently develop, cognitive symptoms.
Supplementary Material
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
E.R.M. was responsible for drafting/revising the manuscript for content, study concept and design, and interpretation of data. A.B. was responsible for drafting/revising the manuscript for content, study concept and design, and interpretation of data. A.D.L. was responsible for revising the manuscript. J.F.R. was responsible for revising the manuscript. A.J.C. was responsible for revising the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Todd J. The syndrome of Alice in Wonderland. Can Med Assoc J 1955;73:701–704. [PMC free article] [PubMed] [Google Scholar]
- 2.Lanska JR, Lanska DJ. Alice in Wonderland syndrome: somesthetic vs visual perceptual disturbance. Neurology 2013;80:1262–1264. [DOI] [PubMed] [Google Scholar]
- 3.Rolak LA. Literary neurologic syndromes: Alice in Wonderland. Arch Neurol 1991;48:649–651. [DOI] [PubMed] [Google Scholar]
- 4.Meyer A, Leigh D, Bagg CE. A rare presenile dementia associated with cortical blindness (Heidenhain's syndrome). J Neurol Neurosurg Psychiatry 1954;17:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol 1999;56:55–61. [DOI] [PubMed] [Google Scholar]
- 6.Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009;132:2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muayqil T, Gronseth G, Camicioli R. Evidence-based guideline: diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2012;79:1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamlin C, Puoti G, Berri S, et al. A comparison of tau and 14-3-3 protein in the diagnosis of Creutzfeldt-Jakob disease. Neurology 2012;79:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol 2003;60:813–816. [DOI] [PubMed] [Google Scholar]
- 10.McGuire LI, Peden AH, Orru CD, et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol 2012;72:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.