Abstract
Objective:
To determine the outcome of patients with psychogenic pseudosyncope (PPS) after communication of the diagnosis.
Methods:
This was a retrospective cohort study of patients with PPS referred in 2007 to 2015 to a tertiary referral center for syncope. We reviewed patient records and studied attack frequency, factors affecting attack frequency, health care use, and quality of life using a questionnaire. We explored influences on attack freedom and attack frequency in the 6 months before follow-up for age, sex, education level, duration until diagnosis, probability of diagnosis, additional syncope, and acceptance of diagnosis.
Results:
Forty-seven of 57 patients with PPS could be traced, of whom 35 (74%) participated. Twelve (34%) were attack-free for at least 6 months. The median time from diagnosis to follow-up was 50 months (range 6–103 months). Communicating and explaining the diagnosis resulted in immediate reduction of attack frequency (p = 0.007) from the month before diagnosis (median one attack, range 0–156) to the month after (median one attack, range 0–16). In the 6 months before follow-up, the number of admissions decreased from 19 of 35 to 0 of 35 (p = 0.002). The use of somatic and mental health care shifted toward the latter (p < 0.0001). Quality of life at follow-up (Short Form Health Survey 36) showed lower scores for 7 of 8 domains compared to matched Dutch control values; quality of life was not influenced by attack freedom.
Conclusions:
After communication of the diagnosis in PPS, attack frequency decreased and health care use shifted toward mental care. Low quality of life underlines that PPS is a serious condition.
The 3 main groups of apparent transient loss of consciousness are syncope, epileptic seizures, and psychogenic attacks.1 Psychogenic transient loss of consciousness consists of psychogenic nonepileptic seizures (PNES), resembling epileptic seizures, and psychogenic pseudosyncope (PPS), resembling syncope. The prevalence of PNES is 2 to 33 per 100,000 people.2 In tertiary epilepsy clinics, PNES accounts for 20% to 30% of patients.3–5 While patients with PNES and PPS probably have the same psychiatric disorder,6,7 they are seen by different specialties, which may affect diagnosis, therapy, and prognosis.6
Patients with PPS typically lie immobile and unresponsive with closed eyes during attacks. Attacks last longer and are more frequent than in syncope.8,9 The diagnosis rests on history taking from patients and eyewitnesses and on documenting an event with a tilt table test8,9 or home video recording.7 Such documented attacks must be recognized by patients and eyewitnesses as the same as habitual ones.5 The diagnosis of both PPS and PNES must rest not on exclusion but on positive evidence.5,6 The absence of PPS in large syncope series7 suggests that PPS is not always recognized, as does the paucity of PPS research compared to PNES.8,10
The prognosis of PNES is generally better for those who are young, are more highly educated, and have a short delay between the first event and the diagnosis.11–13 A comprehensive PNES study showed that 26% of patients receiving psychotherapy were attack-free after 42 months, with another 40% having a 50% reduction of attack frequency.14 Follow-up studies in PNES have shown that not only attack frequency but also quality of life, use of health care facilities, and employment are important indicators.14–17 We did not find any studies on the prognosis of PPS and therefore studied the prognosis in a cohort of patients with PPS, taking these aspects into account.
METHODS
Patients.
We searched the database of the tertiary syncope outpatient department of the Leiden University Medical Center from 2007 to 2015 for possible patients with PPS who were at least 18 years old at follow-up. Patients were seen by a neurologist (J.G.v.D.) experienced in syncope and PPS. The diagnosis rested on history taking of patients and eyewitnesses and on event documentation with tilt table testing, home video recording or, rarely, home blood pressure recording. The explanation of the nature of PPS conformed to PNES procedures,8,9 stressing that attacks happen involuntarily, that patients were taken seriously and were not “mad,” and that the attacks signaled an underlying psychological problem. Terms such as psychological were used and not avoided. Patients were seen after 1 or 2 weeks to repeat the explanation, to address questions, and to discuss therapeutic options. If attacks did not resolve, patients received advice to seek psychiatric or psychological help. Because patients came from a wide area, the choice of a suitable therapist was left to their general practitioner or the referring specialist. In earlier years, contact was ended after psychiatric therapy was advocated, but later, contact was maintained until psychotherapy was underway.18
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the local institutional review ethics board. We sent potential participants an informational letter and asked them to fill in a questionnaire on paper or online. Two authors (D.P.S. and M.J.O.) contacted patients by telephone to provide additional information and to encourage a response until patients completed the questionnaire or further attempts seemed futile. All participants gave informed consent.
Patient inclusion.
Three authors (D.P.S., R.D.T., and J.G.v.D.) studied case records using diagnostic criteria adapted from PNES criteria6; the adaptation meant that we ignored interictal EEG findings and in their place stressed ictal heart rate and blood pressure. The history had to contain positive features of PPS such as closed eyes during attacks, a long duration, and high frequency.7 Attack documentation required recording an event recognized as typical by patients or relatives during a tilt table test, comprising continuous blood pressure, ECG, EEG, and video,8,9,19 or ictal home video or blood pressure recording. Patients with a positive history and attack documentation were classified as definite; those with a positive history without documentation were classified as probable. The final inclusion criterion was that the diagnosis had been explained as stated above.
Study data.
The following information was noted: age, sex, frequency of events in 1 month both before and after diagnosis, duration from the first PPS attack until diagnosis, the additional presence of syncope, earlier consultation of medical specialists and psychologists, and hospital admission or emergency department visits for these attacks.
The questionnaire asked for duration since last attack and attack frequency in the last week, month, and 6 months. We defined attack-free as no attack in the last 6 months. Questions addressing health care use included frequency of visits to general practitioners, emergency departments, medical specialists, and psychologists and the number of admissions for PPS in the last 6 months before follow-up.
We investigated patients’ reception of the diagnosis, asking whether patients had felt that they were treated with respect, whether they had felt offended by the psychological nature of attacks, and whether they agreed with the explanation at the time of diagnosis and at follow-up, all of which were noted as “agree,” “no opinion,” and “disagree.”
Other questions concerned marital status, housing situation, education level, and employment. We used the Dutch version of the Short Form Health Survey 36 (SF-36) to assess quality of life.20
Data analysis.
We compared baseline variables between participants, i.e., those who completed the questionnaire, and nonparticipants, who did not. Because attack frequency and various other variables had skewed distributions, we favored nonparametric analyses. We examined an immediate effect of diagnosing PPS on attack frequency by comparing attack frequency in the months before and after explanation of the diagnosis. The main analysis of attack frequency concerned a comparison between the number of attacks in the month before diagnosis and that in the month before follow-up using the Wilcoxon paired signed-rank test and between the proportions of participants who were attack-free and those who were not attack-free using the Fisher exact test.
We explored influences on attack frequency in the 6 months before follow-up and on attack freedom for age, sex, education level, and duration until diagnosis; probability of diagnosis; the presence of syncope; and the reception of the diagnosis. To analyze effects on attack frequency, we used the Spearman ρ for quantitative variables (e.g., age, duration until diagnosis) and the Mann-Whitney test for dichotomous variables (e.g., sex). To analyze effects on attack freedom (yes/no), we used the Mann-Whitney, χ2, and Fisher exact tests.
Answers concerning reception of the diagnosis were dichotomized into disagree and agree, ignoring the category no opinion.
We compared somatic and mental health care use before and 6 months after diagnosis. We compared the subset of patients of working age (25–65 years) to 2015 employment data from Statistics Netherlands (www.CBS.nl). We compared the expected number of patients not working to the actual number not working, taking into account the patient group’s age and sex composition (Fisher exact test).
We compared the 8 domains of the SF-36 to published Dutch control values, taking age and sex into account.20 We calculated individual patient z scores per domain by first calculating the difference between a patient value and the control group mean for the correct sex and age group and then dividing that by the control group’s SD. We used the Student t test to investigate whether mean patient z scores differed from zero, i.e., whether their mean scores differed from age- and sex-corrected control values. We also compared mean z scores per domain between the groups that were attack-free and not attack-free.
No attempts were made to impute any missing data. We used SPSS-20 and Matlab for statistical analysis. A significance threshold of p < 0.01 was used.
RESULTS
Baseline data.
Participants and nonparticipants.
Fifty-seven potential participants fulfilled PPS criteria (48 definite, 9 probable). Ten patients were lost to follow-up. Of the remaining 47 patients, 12 (25.5%) did not reply (figure 1), leaving 35 participants. The participant and nonparticipant groups did not differ in age, sex, and attack frequency in the month before diagnosis; duration until diagnosis; and probability of the diagnosis (table 1).
Figure 1. Flowchart explaining the number of subjects.
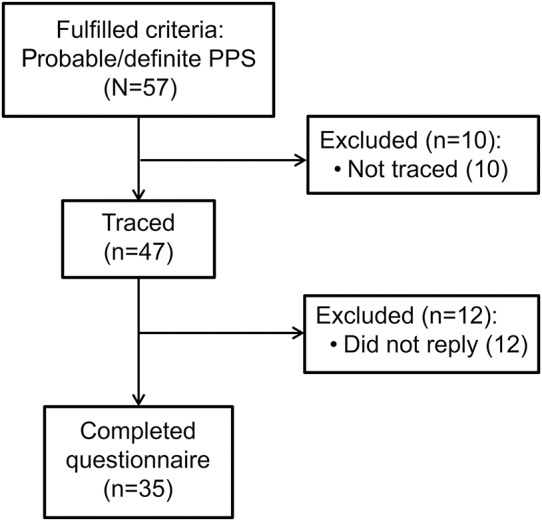
PPS = psychogenic pseudosyncope.
Table 1.
Comparison of participants and nonparticipants
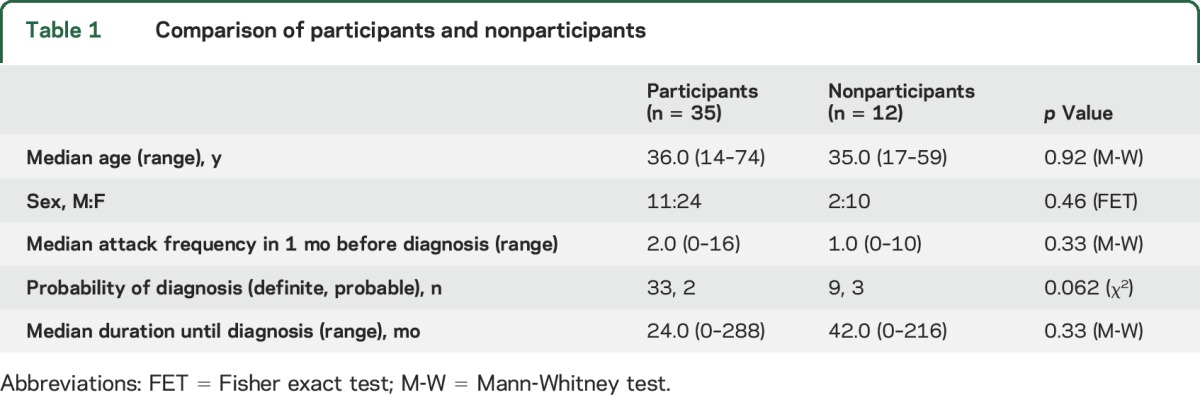
Participant baseline data.
Most participants were female (24 of 35, 69%). The median age was 36 years (range 14–74 years). The median delay to diagnosis was 24 months (range 0–288). Almost all patients (33 of 35, 94%) had a definite diagnosis of PPS; 2 had probable PPS. The median attack frequency in the month before diagnosis was 2 (range 0–16).
Health care use.
Patients had on average each seen 1.49 somatic specialties and 0.14 mental health care providers (i.e., a psychologist or psychiatrist) in the 6 months before diagnosis. The number of previous admissions for PPS was unknown in 5 patients; of the remaining 30 patients, 19 (54%) had been admitted in the year before diagnosis.
Follow-up analysis.
Attack frequency.
Data on attack frequency in the first month after diagnosis were available for 21 patients. Their median frequency had decreased (p = 0.007) from the month before diagnosis (median 1, range 0–156; mean 4.0 + 4.9 attacks) to the month after (median 1, range 0–16; mean 1.7 + 3.5).
The median time from diagnosis to follow-up was 50 months (range 6–103 months). The median attack frequency in the last month of follow-up was 0 attacks per month (range 0–35), which was lower than in the month before diagnosis (p = 0.006). At follow-up, 12 patients were attack-free.
Factors affecting attack frequency.
Age did not influence attack frequency in the last 6 months of follow-up (p = 0.9) and did not differ between those who were attack-free and those who were not (p = 0.25). Likewise, sex affected neither attack frequency (p = 0.33) nor attack freedom (p = 1.0). Education level did not affect attack frequency (p = 0.9) or attack freedom (p = 0.14). The duration between the first attack and diagnosis did not affect attack frequency (p = 0.74) or attack freedom (p = 0.33). This also held for the presence of syncope, both for attack frequency (p = 0.66) and for attack freedom (p = 1.0).
Reception of the diagnosis.
Most patients felt that they had been treated respectfully: 29 agreed (83%), 3 disagreed, and 3 had no opinion. Eight had felt offended, 22 had not, while 5 had no opinion. Fifteen agreed with the psychological explanation at the time of diagnosis, while 13 did not, with 7 having no opinion. At follow-up, 18 agreed, 11 did not, while 6 neither agreed nor disagreed. We used the dichotomized form of these answers to explore a relation with attack frequency and attack freedom and found no effects. For example, of the 18 who agreed at follow-up that their attacks had a psychological explanation, 11 were not attack-free, compared with 8 of 11 who did not agree (p = 0.4).
Health care use.
No patient had visited an emergency department or had been admitted for PPS in the 6 months before follow-up. This differed from the year before diagnosis (19 of 35 admissions) after correction for the difference in duration of the periods (Fisher exact test, p = 0.002).
In the year before diagnosis, patients had together seen 52 somatic specialists and 5 mental health care providers (psychologists or psychiatrists), 57 health care providers in all. Corresponding numbers in the 6 months before follow-up were 6 and 14, i.e., 20 health care providers in all. After correction for the difference in duration, the proportion of somatic to mental health care providers had shifted over time toward more mental health care use (p < 0.0001).
Social status and employment.
The number of patients who were married or having a relationship was the same before diagnosis and at follow-up (24 of 35, 69%). At follow-up, 9 of 24 patients of working age did not work. The expected unemployment rate for a group with this age and sex composition was 7.0%, leading to an expected number of patients not working of 1.67. After this number was rounded to 2, the actual proportion of those not working and working (9:15) was compared to the expected proportion (2:22). Although more patients did not work than expected, this difference was not significant (p = 0.036).
Quality of life (SF-36).
Because of an entry error, the last 4 questions concerning the general health domain were missing from the online version (n = 19) of the questionnaire. Quality of life is shown in tables 2 and 3. Patient scores differed for 7 of the 8 domains from Dutch normal values corrected for age and sex. Mean domain scores did not differ between patients who were attack-free and those who were not attack-free, although values were worse for those who were not attack-free for all 8 domains (figure 2).
Table 2.
Quality of life (SF-36)
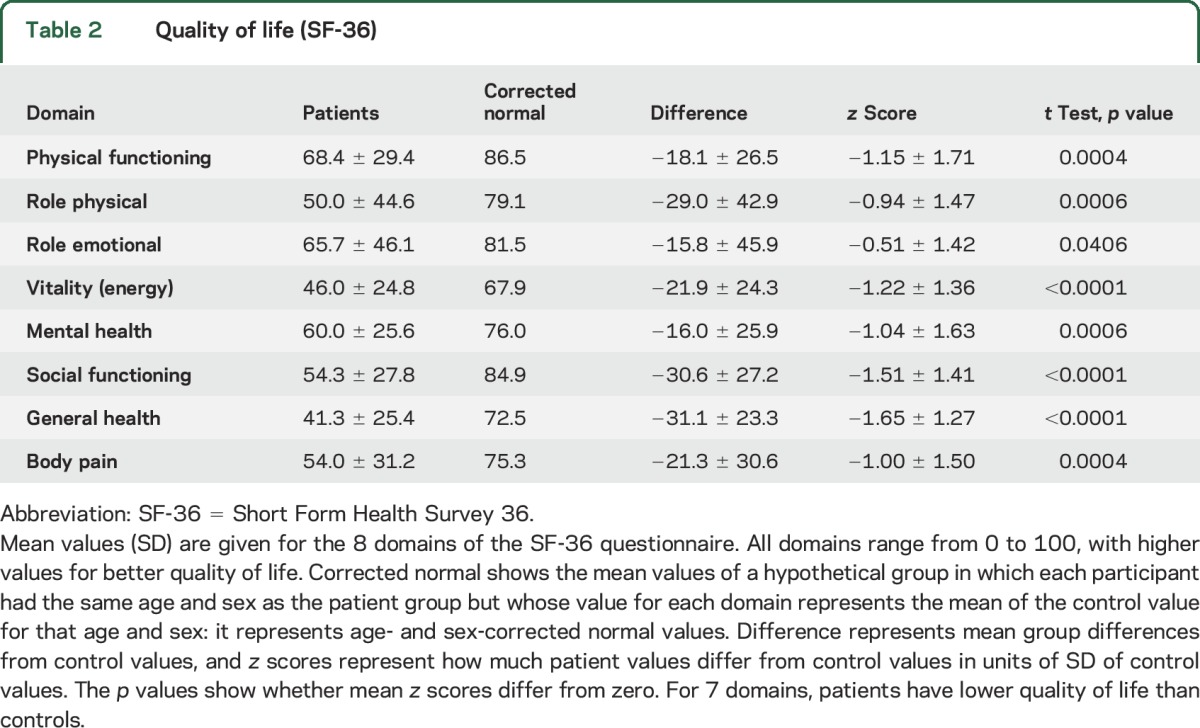
Table 3.
Quality of life as a function of attack freedom
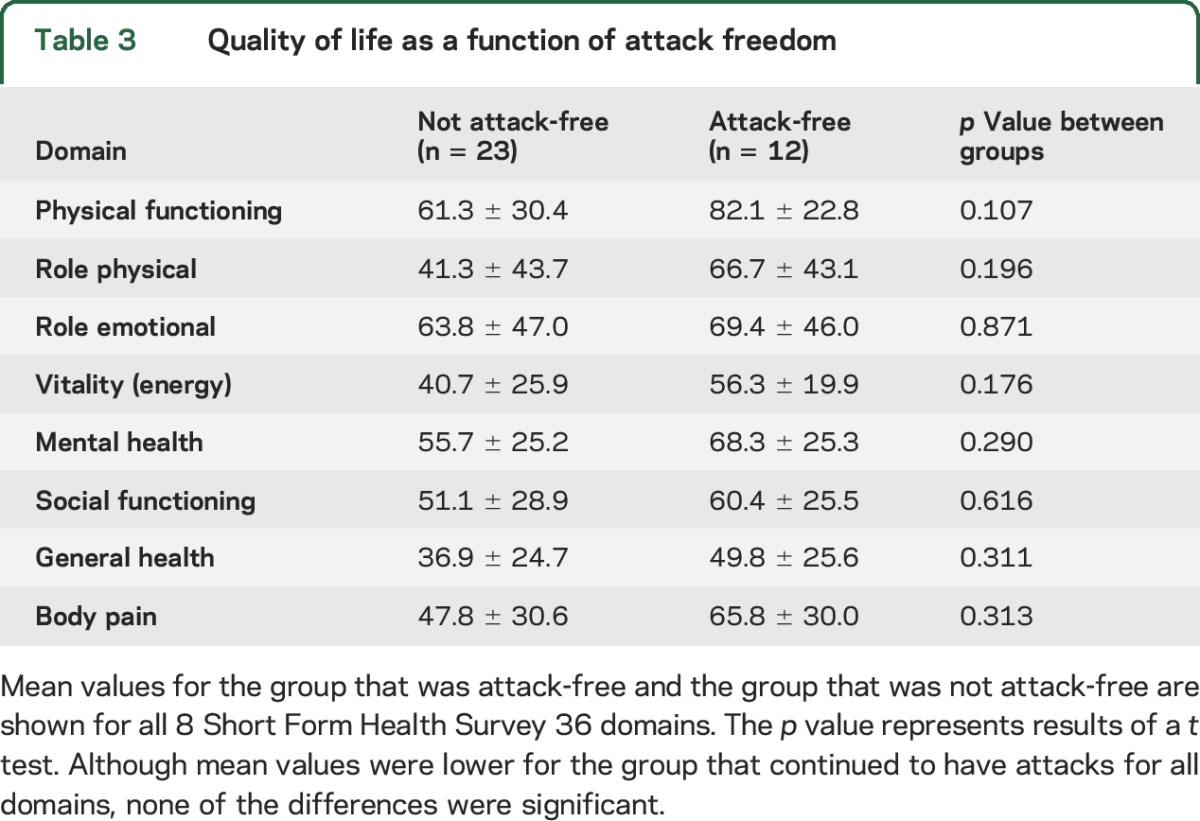
Figure 2. Attack freedom and quality of life.
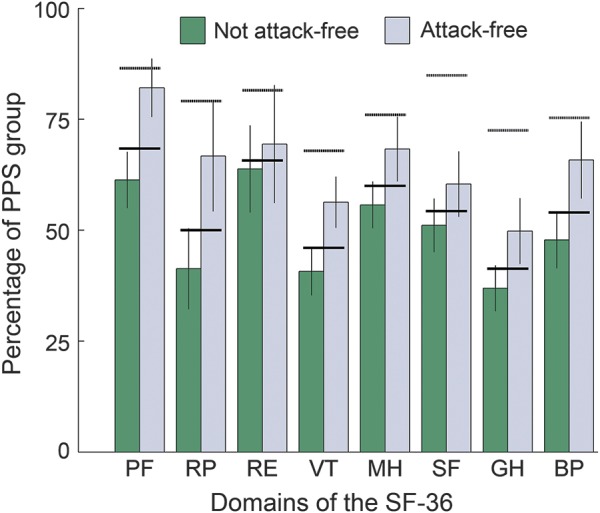
The 8 domains of the Short Form Health Survey 36 (SF-36) are shown for those who were and who were not attack-free. The horizontal dotted lines above the bars indicate normal values per domain corrected for age and sex. Vertical bars indicate mean values for those who were not attack-free (n = 23) and those who were attack-free (n = 12). Vertical lines show standard errors. Horizontal bold lines indicate the mean value per domain for the entire psychogenic pseudosyncope (PPS) group (n = 35). BP = body pain; GH = general health; MH = mental health (emotional wellbeing); PF = physical functioning; RE = role emotional; RP = role physical; SF = social functioning; VT = vitality (energy).
DISCUSSION
The main result of this first PPS follow-up study was a reduction in the number of attacks, expressed both as attack frequency and as attack freedom: at the follow-up of >4 years, one-third (12 of 35) were attack-free. Other important findings were that conveying the diagnosis resulted in an immediate decrease in attacks in the first month after diagnosis. At follow-up, the number of hospital admissions had decreased and the nature of health care use had shifted from somatic to mental health care. Patients tended to have a higher-than-expected unemployment rate. Quality of life was low for both those who were attack-free and those who were not.
The positive effects in our study are largely in line with PNES studies showing that a clear explanation may lead to a reduction of attacks13,21–23 and a reduction of emergency service use.13 We confirm that communication of the diagnosis can cause an immediate reduction of attack frequency.13,24,25 Fifty-seven percent had syncope besides PPS, conforming to PNES results, in which 10% to 30% of patients also have epilepsy. The incidence of syncope among patients with PPS seems higher than that of epilepsy among patients with PNES, possibly because syncope is much more prevalent than epilepsy.
The analysis did not reveal some relations reported for PNES such as a poor prognosis for those with a long duration between the first attack and diagnosis.11,24,26 This might be due to the relatively short duration in our PPS group: the median duration was 24 months (mean 46 months), contrasting with the typically longer mean duration for PNES of 6 to 7 years.13 We did not find a worse prognosis for higher age11,27 or for women.13 We suspect that the lack of these relations represents sampling effects because such relations are not universally found in PNES either. We also did not find a relation between how well the diagnosis was received and attack frequency. Intriguingly, this also held for the question of whether patients accepted the psychological nature of PPS. Hence, acceptance of the psychological nature does not seem to be a prerequisite for attack freedom, nor does its absence imply that attacks must continue.
The number of admissions decreased, and health care use shifted from somatic to psychiatric care. The rate of unemployment did not differ from Dutch values corrected for age and sex but was higher (37.5%) than expected (7%). The lack of significance is probably due to the restriction to those of working age, reducing the sample size of the remaining group (n = 24).
Quality of life was remarkably poor compared to sex- and age-matched Dutch control data. Quality of life was not higher in those who were attack-free than in those who were not. The overall poor quality of life and lack of a clear relation with attack freedom suggest that the underlying psychological problems impair quality of life more than the mere presence of attacks. This emphasizes the opinion derived from PNES studies that attack frequency should not be the sole parameter of follow-up; quality of life may well be the most relevant outcome parameter.
The study contained no control group and took place in a tertiary referral center, with a likely bias toward difficult cases and, presumably for that reason, a high rate of PPS (8%–10% of cases). We had made no attempt to standardize psychological treatment because patients came from across the Netherlands, making long-term treatment in our hospital impractical. Hence, only the diagnostic process, explanation of the diagnosis, and early follow-up were standardized. The lack of a standardized treatment may have impaired treatment efficacy.
The low quality of life emphasizes that PPS represents a considerable burden to patients. Although we did not aim to calculate the burden to society, our results concerning employment and medical consumption suggest that that burden also is considerable. At follow-up, attack frequency was reduced, and there was a shift away from somatic to psychiatric health care use. The outcome may well be worse for patients with PPS who remain undiagnosed or who are not directed to psychiatric health care.
We feel that all those diagnosing syncope should be aware of PPS, in particular those working in dedicated syncope units.28 Much like specialized epilepsy units attract a concentration of patients with PNES, so a high rate of PPS is a corollary of tertiary syncope care. Neurologists and cardiologists who see patients with PPS may tend to shy away from communicating a psychiatric diagnosis out of fear of offending patients. In this study, words such as psychological were consistently used, yet the vast majority of patients (83%) felt that they were treated with respect. This suggests that this fear is not warranted, provided that adequate time is taken to communicate the diagnosis. Hence, we urge somatic specialists seeing patients with PPS to overcome their reticence in communicating the diagnosis because otherwise they are likely to do their patients a disservice.
GLOSSARY
- PNES
psychogenic nonepileptic seizures
- PPS
psychogenic pseudosyncope
- SF-36
Short Form Health Survey 36
AUTHORS CONTRIBUTIONS
Dirk P. Saal: study concept and design, acquisition of data. M. Jolein Overdijk: acquisition of data, critical revision. Roland D. Thijs: study concept and design, interpretation of data, critical revision. Irene M. van Vliet: study concept and design, interpretation of data, critical revision. J. Gert van Dijk: study concept and design, analysis, critical revision, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.van Dijk JG, Thijs RD, Benditt DG, Wieling W. A guide to disorders causing transient loss of consciousness: focus on syncope. Nat Rev Neurol 2009;5:438–448. [DOI] [PubMed] [Google Scholar]
- 2.Benbadis SR, Allen Hauser W. An estimate of the prevalence of psychogenic non-epileptic seizures. Seizure 2000;9:280–281. [DOI] [PubMed] [Google Scholar]
- 3.Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci 2011;18:1602–1607. [DOI] [PubMed] [Google Scholar]
- 4.Lesser RP. Psychogenic seizures. Neurology 1996;46:1499–1507. [DOI] [PubMed] [Google Scholar]
- 5.Benbadis SR, Chichkova R. Psychogenic pseudosyncope: an underestimated and provable diagnosis. Epilepsy Behav 2006;9:106–110. [DOI] [PubMed] [Google Scholar]
- 6.LaFrance WC Jr. Psychogenic nonepileptic seizures. Curr Opin Neurol 2008;21:195–201. [DOI] [PubMed] [Google Scholar]
- 7.Raj V, Rowe AA, Fleisch SB, Paranjape SY, Arain AM, Nicolson SE. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci 2014;184:66–72. [DOI] [PubMed] [Google Scholar]
- 8.Tannemaat MR, van Niekerk J, Reijntjes RH, Thijs RD, Sutton R, van Dijk JG. The semiology of tilt-induced psychogenic pseudosyncope. Neurology 2013;81:752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tannemaat MR, Thijs RD, van Dijk JG. Managing psychogenic pseudosyncope: facts and experiences. Cardiol J 2014;21:658–664. [DOI] [PubMed] [Google Scholar]
- 10.van Dijk JG, Wieling W. Pathophysiological basis of syncope and neurological conditions that mimic syncope. Prog Cardiovasc Dis 2013;55:345–356. [DOI] [PubMed] [Google Scholar]
- 11.Reuber M, Pukrop R, Bauer J, Helmstaedter C, Tessendorf N, Elger CE. Outcome in psychogenic nonepileptic seizures: 1 to 10-year follow-up in 164 patients. Ann Neurol 2003;53:305–311. [DOI] [PubMed] [Google Scholar]
- 12.Carton S, Thompson PJ, Duncan JS. Non-epileptic seizures: patients’ understanding and reaction to the diagnosis and impact on outcome. Seizure 2003;12:287–294. [DOI] [PubMed] [Google Scholar]
- 13.McKenzie P, Oto M, Russell A, Pelosi A, Duncan R. Early outcomes and predictors in 260 patients with psychogenic nonepileptic attacks. Neurology 2010;74:64–69. [DOI] [PubMed] [Google Scholar]
- 14.Mayor R, Howlett S, Grunewald R, Reuber M. Long-term outcome of brief augmented psychodynamic interpersonal therapy for psychogenic nonepileptic seizures: seizure control and health care utilization. Epilepsia 2010;51:1169–1176. [DOI] [PubMed] [Google Scholar]
- 15.Bodde NM, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures: definition, etiology, treatment and prognostic issues: a critical review. Seizure 2009;18:543–553. [DOI] [PubMed] [Google Scholar]
- 16.Reuber M, House AO. Treating patients with psychogenic non-epileptic seizures. Curr Opin Neurol 2002;15:207–211. [DOI] [PubMed] [Google Scholar]
- 17.Mayor R, Brown RJ, Cock H, et al. A feasibility study of a brief psycho-educational intervention for psychogenic nonepileptic seizures. Seizure 2013;22:760–765. [DOI] [PubMed] [Google Scholar]
- 18.Drane DL, LaRoche SM, Ganesh GA, Teagarden D, Loring DW. A standardized diagnostic approach and ongoing feedback improves outcome in psychogenic nonepileptic seizures. Epilepsy Behav 2016;54:34–39. [DOI] [PubMed] [Google Scholar]
- 19.Blad H, Lamberts RJ, Van Dijk JG, Thijs RD. Tilt-induced vasovagal syncope and psychogenic pseudosyncope: overlapping clinical entities. Neurology 2015;85:2006–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 1998;51:1055–1068. [DOI] [PubMed] [Google Scholar]
- 21.Mayor R, Brown RJ, Cock H, et al. Short-term outcome of psychogenic non-epileptic seizures after communication of the diagnosis. Epilepsy Behav 2012;25:676–681. [DOI] [PubMed] [Google Scholar]
- 22.Gambini O, Demartini B, Chiesa V, Turner K, Barbieri V, Canevini MP. Long-term outcome of psychogenic nonepileptic seizures: the role of induction by suggestion. Epilepsy Behav 2014;41:140–143. [DOI] [PubMed] [Google Scholar]
- 23.Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia 2010;51:70–78. [DOI] [PubMed] [Google Scholar]
- 24.Buchanan N, Snars J. Pseudoseizures (non epileptic attack disorder): clinical management and outcome in 50 patients. Seizure 1993;2:141–146. [DOI] [PubMed] [Google Scholar]
- 25.Aboukasm A, Mahr G, Gahry BR, Thomas A, Barkley GL. Retrospective analysis of the effects of psychotherapeutic interventions on outcomes of psychogenic nonepileptic seizures. Epilepsia 1998;39:470–473. [DOI] [PubMed] [Google Scholar]
- 26.Walczak TS, Papacostas S, Williams DT, Scheuer ML, Lebowitz N, Notarfrancesco A. Outcome after diagnosis of psychogenic nonepileptic seizures. Epilepsia 1995;36:1131–1137. [DOI] [PubMed] [Google Scholar]
- 27.Wyllie E, Friedman D, Luders H, Morris H, Rothner D, Turnbull J. Outcome of psychogenic seizures in children and adolescents compared with adults. Neurology 1991;41:742–744. [DOI] [PubMed] [Google Scholar]
- 28.Kenny RA, Brignole M, Dan GA, et al. Syncope unit: rationale and requirement: the European Heart Rhythm Association position statement endorsed by the Heart Rhythm Society. Europace 2015;17:1325–1340. [DOI] [PubMed] [Google Scholar]


