Abstract
Behavior analytic approaches and techniques have much to offer to the study of remembering. There is currently great interest in the development of animal models of human memory processes in order to enhance understanding of the neurobiology of memory and treatment of dementia and related disorders. Because rodent models are so important in contemporary neuroscience and genetics, development of procedures to study various forms of memory in rodents is a point of emphasis. The sense of smell plays an important role in rodent behavior and use of olfactory stimuli has permitted demonstrations of complex forms of stimulus control that have also served as baselines for studying drug effects on remembering. This article focuses on the effects of drugs on behavior maintained by two related procedures: delayed matching-to-sample with odors and the Odor Span Task. These types of procedures provide an opportunity to explore drug effects on behavior maintained by multiple stimuli and across a range of delay intervals with potential to advance analysis of the behavioral pharmacology of remembering.
The study of drug effects on behavior generated by procedures thought to reflect learning and memory processes is a highly active research field. There is interest both in cognitive enhancing drugs with potential to treat dementia, as well as in the analysis of amnestic compounds that may provide insight about receptor sites and neural pathways important to remembering (Insel, Ehlers, & Krystal, 2013; Talpos, Aerts, Fellini, & Steckler, 2014). While the study of memory is often viewed as the exclusive domain of cognitive psychology, behavior analytic approaches have made important contributions (e.g., see White, 2013). Research from the cognitive neuroscience perspective tends to categorize tasks in terms of the extent to which they are thought to reflect specific memory processes (e.g., working vs. reference memory). Behavioral approaches generally place more emphasis on identifying changes in stimulus control of behavior over time as a function of variables in effect during stimulus presentation, the delay interval and following the behavior. Remembering is defined by retention of stimulus control across a temporal gap between initial learning and testing; forgetting by a loss of such control (Catania, 2013; White, 2013). Although the traditions of the experimental analysis of behavior and behavioral pharmacology emphasize the importance of stable, steady-state behavioral baselines, adaptations that permit the study of transition states related to learning and remembering have been developed (e.g., repeated acquisitions, delayed matching-to-sample). The present paper reviews research based on recent developments that permit the study of complex stimulus control in rodents using olfactory stimuli and suggests that these procedures offer an opportunity to contribute to the study of the behavioral pharmacology of remembering.
Delayed Matching-to-Sample (DMTS) and Non-Matching to Sample (DNMTS)
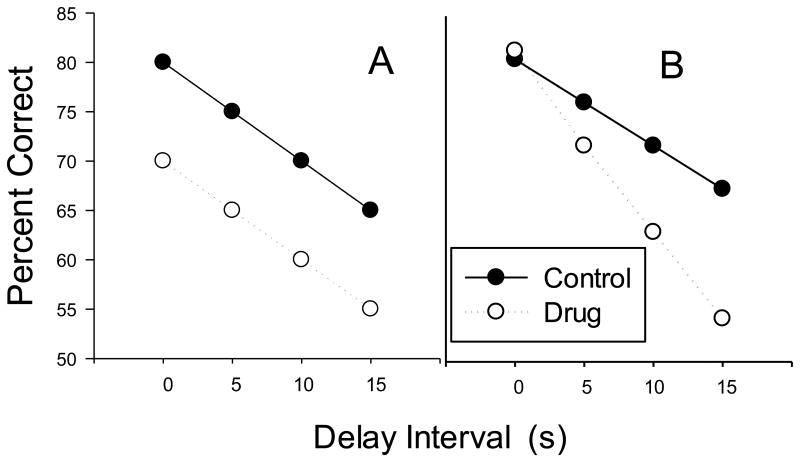
The DMTS and DNMTS tasks are the most widely used procedures in the experimental analysis of short-term remembering and forgetting (White, 2013). Both procedures involve insertion of a variable delay between the presentation of a sample stimulus and two or more comparison stimuli. Accuracy of selection of the matching (or non-matching) stimulus is generally inversely related to the length of the delay interval: the familiar forgetting function (cf. Blough, 1959). A useful feature of this function is that it permits separation of drug effects on initial discriminability (or other features of performance) based on differences in the intercept from effects on rate of forgetting based on differences in the slope (cf. White, 1985; 2013). For example, if a drug impairs matching accuracy at a zero or very short delay by the same magnitude as at longer delays, the intercept of the forgetting function under drug would be lower than that of control conditions, but the slope would be the same. Such an effect is said to be delay-independent and an idealized illustration is shown in Figure 1 (Panel A). Delay-independent drug effects might be due to impaired discrimination, reduced motivation, or other actions unrelated to remembering. On the other hand if the effects are delay-dependent (e.g., impairment of accuracy emerges only with longer delay), the slope of the forgetting function would be steeper under drug conditions (see Figure 1, Panel B for an illustration). When drug effects depend on the delay value, it is reasonable to argue that the drug is altering rate of forgetting during the delay interval.
Figure 1.
Illustrations of hypothetical forgetting functions. Both panels show a forgetting function under control conditions (black circles). Panel A illustrates a delay-independent drug effect and Panel B, a delay-dependent effect (white circles).
There are many studies of drug effects on DMTS accuracy, but perhaps surprisingly, most have obtained delay-independent effects (Steckler, Sahgal, Aggleton, & Drinkenburg, 1998; White, 2013). That is, the drugs failed to affect the forgetting function (although there are interesting exceptions, e.g., Lane, Cherek, Lieving, & Tcheremissine, 2005). A complete review of this literature is beyond the scope of the present review, but it is worth noting that the delay-independent drug effects often found with DMTS procedures may reflect limitations of the procedure rather than an absence of drug effects on forgetting. For example, the nature of the forgetting function and related sensitivity to drug effects may depend on selection of a sufficient range of delay values (Kangas, Vaidya, & Branch, 2010; Sargisson & White, 2003). Additionally, ceiling effects are often observed in DMTS that may limit detection of enhancement of remembering. Both of the above limitations can be addressed through the use of a titrating DMTS procedure in which the value of the delay is adjusted based on the accuracy of responding within the session (Kangas et al., 2010) and indeed titrating DMTS procedures may be more sensitive to the effects of drugs on forgetting (e.g., Wenger, Hudzik, & Wright, 1993; Wenger & Wright, 1990).
Much of the DMTS literature uses visual stimuli with pigeons or non-human primates as subjects. Rodents are slow to learn matching to sample procedures using visual stimuli (Iversen, 1993; 1997; Slotnick, 2001), and these training difficulties have hindered research in this area. One solution has been the use of a range of mazes (e.g., Radial Arm Maze and Morris Water Task) in the cognitive neuroscience of learning and memory. These procedures opened the door to the analysis of drug effects on spatial learning and memory which has become an important field in its own right. However, the kind of forgetting functions noted above are not often studied with these procedures, and controls for drug effects unrelated to learning and memory are often lacking (although see Galizio et al., 2014; Kay, Harper, & Hunt, 2010). Another solution has been to use delayed-matching to position procedures with rodents in which insertion of the left or right lever into an operant chamber serves as the sample and insertion of both levers presents the comparison stimuli (Steckler, Drinkenburg, Sahgal, & Aggleton, 1998). The difficulty in preventing the rat from engaging in postural behavior that might mediate the delay has limited interpretation of data from this procedure (Dudchenko, 2004). The focus of the present paper is on the potential for using olfactory stimuli as an alternative method to study remembering of non-spatial stimuli that is more readily learned by rodents.
Rats and mice have highly developed olfactory systems and it appears that odor stimuli play a dominant role in the natural stimulus control of rodents (Slotnick, 2001). In contrast with their relatively poor performances with visual stimuli, rats show rapid learning and even learning set development with odor stimuli (Slotnick, 2001). The exceptional olfactory discrimination abilities of rodents have led to their use in applied settings to detect explosives and diagnose tuberculosis (Poling et al., 2011). Rats can also learn conditional discriminations fairly rapidly with odor stimuli (Lu, Slotnick, & Silberberg, 1993) and generalized identity and oddity have been demonstrated as well (April, Bruce, & Galizio, 2011; Pena, Pitts, & Galizio, 2006; Prichard, Panoz-Brown, Bruce, & Galizio, 2015).
DMTS and DNMTS procedures with odor stimuli have also been studied with both rats and mice; above chance accuracy is evident even with relatively long delays (Bodyak & Slotnick, 1999; Lu et al., 1993; Otto & Eichenbaum, 1992; Roddick, Schellinck, & Brown, 2014). Of course, a potential concern here is the possibility that the odorant might linger during the delay period. This has been addressed by introduction of a masking odor presented during the delay, which did not change the forgetting function (Lu et al., 1993).
It seems surprising that DMTS procedures with odor stimuli have not been used more frequently in behavioral pharmacology, but there appears to be only one such report in the literature. Ravel, Vigouroux, Elaagouby, and Gervais (1992) studied the effects of scopolamine on DMTS accuracy in rats using a T-maze apparatus. An olfactometer infused one of three odors into the sample compartment and exposure to the sample odor was followed by delays ranging from 4 s through 3 min. After the delay, the rat was allowed access to a choice compartment with two odor ports and selection of the odor that matched the sample was reinforced. Ravel et al. observed a forgetting function with performances showing a slight decline at 1 min and finally dropping to chance levels only after a 3-min delay. The effects of scopolamine were studied at the 4-s and 30-s delay conditions and accuracy declined in both in a dose-dependent fashion. Importantly, accuracy at the 30-s delay was impaired at a dose of scopolamine (0.125 mg/kg) that had no effect on performance at the 4-s delay. In short, the effects of scopolamine appeared to depend on the delay. Because such delay-dependent effects are relatively uncommon in the behavioral pharmacology literature, it would be of considerable interest to assess the effects of scopolamine across the entire delay gradient to determine whether the rate of forgetting was altered by scopolamine. Further, it might be possible to train rodents under titrating DMTS contingencies using odor stimuli to take advantage of the sensitivity to drug effects that may be afforded using the titrating procedure (Kangas, et al., 2010). Certainly the results of Ravel et al. suggest that DMTS using odor stimuli has considerable potential to permit researchers to examine drug effects on forgetting of non-spatial stimuli across a range of delays in rats and mice.
Control by Multiple Stimuli: the Odor Span Task
The forgetting functions produced in DMTS experiments provide some validity for the procedure as an animal model of human short-term or working memory. However, in addition to its short duration, human working memory is often characterized by limited capacity. Thus, DMTS studies may be limited in that they test remembering of a single stimulus. Indeed as Wright (2007) noted: “Traditional single-item tests of animal memory perhaps miss the most important aspect of memory. Events in the real world are virtually never encountered in isolation. Any single event is part of an ongoing stream of events. Memory for any particular event is influenced by the events that surround it, which in turn can radically alter memory for any single event” (p. 405). Wright's comment comes at the beginning of a review of list learning experiments in which monkeys were exposed to a series of items to remember. Such studies can generate capacity and serial position effects similar to those observed in humans, but they generally use visual stimuli with non-human primates or pigeons as subjects (Wright, 2007). Despite the potential for translational significance, there are only a few studies of drug effects on remembering multiple stimuli using these and related procedures (e.g., Aigner, Walker, & Mishkin, 1991; Soto et al., 2013).
As with DMTS, the use of odor stimuli opens the door to studying control by multiple stimuli in rodents and the procedure that has been most successfully used is the Odor Span Task (OST) developed by Dudchenko, Wood, and Eichenbaum (2000). The OST may be characterized as an incrementing non-matching-to-sample task. The apparatus is generally a large open field arena, and Dudchenko et al. presented stimuli in the form of cups filled with sand mixed with common household spices or other odorants. On the first trial of a session, a single cup was placed in the arena scented with the first odor (Odor A) and digging in the sand produced food reinforcement. On Trial 2, two cups were presented: Odor A and a new odor (B) with selection of B reinforced. On Trial 3, both A and B odors served as negative comparisons and once again, selection of a new odor (C) was reinforced. Dudchenko et al. continued the procedure for up to 24 trials with each stimulus serving as S+ on the trial it was first presented, and as an S- on each subsequent trial. Dudchenko et al. found that rats averaged about eight trials before making their first error (span length) and accuracy decreased through the session as the number of odors to remember increased. They viewed the procedure as a model for the study of working memory capacity in rodents and it has been used as such in a number of neurobiological studies in both rats (Davies, Greba, & Howland, 2013; Davies, Molder, Greba, & Howland, 2013; Turchi & Sarter, 2000) and mice (Young et al., 2007). Indeed, the CNTRICS (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia) group, charged with identifying animal models with translational validity for drug development, nominated the OST as a benchmark task to assess working memory capacity (Dudchenko, Talpos, Young, & Baxter, 2013).
At present, only a few studies have examined the effects of drugs on performance in the OST. Our UNC Wilmington laboratory has adapted the procedure to make it more suitable for behavioral pharmacology research (Galizio, Deal, Hawkey, & April, 2013; Hawkey, April, & Galizio, 2014; MacQueen, Bullard, & Galizio, 2011). First, we use scented plastic lids to deliver odor stimuli. The operant response is to remove the lid to access a sucrose pellet located in the cup below. We have found response definition to be better with this methodology, compared to the digging procedure used by Dudchenko et al. (2000), and have obtained high inter-rater reliability. The procedure requires control trials in which none of the cups contains a sugar pellet to assure that the scent of the stimulus lid (and not the pellet) is controlling behavior. On these trials the pellet is delivered manually following a correct response and we consistently confirm that performance is equivalent on these control (unbaited) trials. Further, in most OST studies, the number of stimuli to remember and the number of comparison stimuli in the arena are confounded. In order to separate these variables, in our version of the OST the number of cups in the arena is permitted to increase only up to five comparisons. Thereafter, each trial consists of one new odor (S+) and four odors randomly selected from among those previously presented.
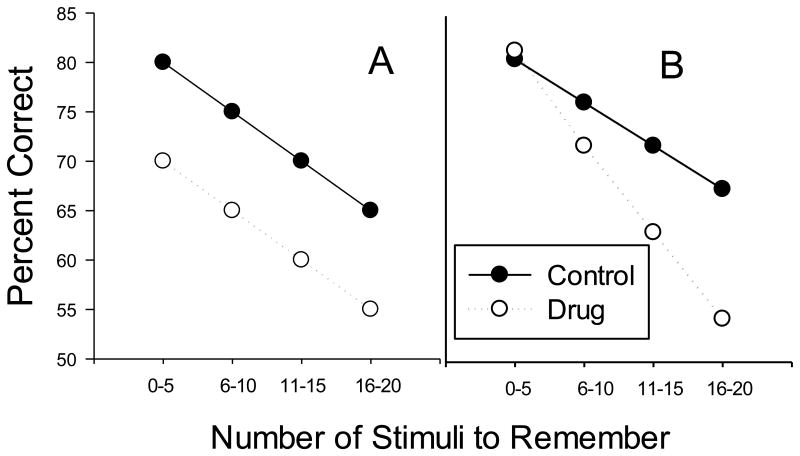
Drugs produce multiple actions not specific to remembering that might influence performance on the OST and it is critical to develop controls that permit separation of these various effects. For example, a drug that affected detection of olfactory stimuli, motivation for food, or motor control might impair OST accuracy, and in the absence of appropriate controls, might be misinterpreted as an effect on remembering. Our laboratory addresses this issue through the inclusion of a simple odor discrimination task. On simple discrimination trials, five odor stimuli are presented in the arena with one odor serving as S+ and four others as S- throughout the experiment. These odors are never used in the OST and simple discrimination trials are interspersed with OST trials throughout the session. The logic is essentially the same as that the performance control conditions developed by Thompson and Moerschbaecher (1979) for use in the repeated acquisition procedure. Simple discrimination trials place the same task demands on the rat as do OST trials in terms of performance (lid removal, odor discrimination, motivation), except that they do not require remembering the stimuli presented within the session. Thus, if a drug dose results in impairment of OST performance, but not simple discrimination, it seems reasonable to infer that the drug is affecting some aspect of within-session stimulus control (often used as the definition of working memory in non-humans, Dudchenko, 2004). Within-session analysis of OST performance permits a more direct assessment of the extent to which the drug effects depend on the number of stimuli to remember. Figure 2 shows an idealized illustration with the control data (black circles) showing a decrease in accuracy as the number of stimuli to remember increases. Panel A (white circles) illustrates a hypothetical outcome of a drug that impairs responding independently of the “memory load” and note that intercept, but not the slope of the function is affected. Panel B (white circles) illustrates an outcome in which the drug effect depends on the number of stimuli to remember: the slope of the function is steeper under drug.
Figure 2.
Illustrations of hypothetical functions between accuracy and the number of odors to remember. Both panels show decreasing accuracy as the number of stimuli to remember increases under control conditions (black circles). Panel A illustrates a drug effect that is independent of “memory load”, and Panel B, shows an effect that depends on the number of stimuli to remember (white circles).
The first drug we studied using these procedures was the non-competitive NMDA antagonist, MK-801 or dizocilpine (DZP). NMDA antagonists are thought to produce amnestic effects by virtue of their action in blocking long-term potentiation; further, the demonstration by Morris (1989) that NMDA antagonists also impaired spatial learning in the water maze is frequently cited in support of the importance of NMDA receptor activation in memory. However, NMDA antagonists also can produce a host of performance effects unrelated to learning and memory and when these are controlled for experimental support has been mixed (Bannerman, Rawlins, & Good, 2006; Cain, Saucier, Hall, Hargreaves, & Boon, 1996; Keith & Galizio, 1997). The Bannerman et al. (2006) review of this literature emphasized that NMDA antagonists were most likely to produce selective impairment of remembering under conditions requiring within-session memory when task demands are high, and would thus predict that NMDA antagonists would produce selective impairments on OST performance. Indeed, MacQueen et al. (2011) found that DZP produced dose-dependent decreases in span length, OST accuracy and simple discrimination. Importantly though, moderate doses of DZP impaired OST performance while completely sparing simple discrimination. These effects were subsequently replicated by Galizio et al. (2013) and in both studies the effects of DZP were shown to depend on the number of stimuli to remember. That is, the 0.1 mg/kg dose of DZP had no effect on accuracy early in the session when there were only a few stimuli to remember, but accuracy declined (relative to saline controls) as the number of stimuli to remember incremented during the course of the session (much like Panel B of Figure 2). Simple discrimination accuracy was not affected at any point in the session at this dose. Thus, DZP did not impair non-matching-to-sample per se, but rather seemed to impair control only when multiple sample stimuli controlled behavior (when the “memory load” was relatively high). The nature and generality of these effects require much further analysis, but on the face of it, they seem consistent with the Bannerman et al. hypothesis.
However, using these same procedures, we have also found that several putative amnestic drugs did not produce selective effects on OST performance. For example, both scopolamine and MDMA produced dose-dependent decreases in OST accuracy and span length, but significant impairment was only noted at doses that also impaired simple discrimination accuracy (Galizio et al., 2013; Hawkey et al., 2014). Thus, neither drug appeared to act in any selective fashion on within session remembering or to affect the number of stimuli rats could remember. The scopolamine findings were of particular interest given the delay-dependent effects of this drug in DMTS discussed above (Ravel et al., 1992). Morphine and the benzodiazepine, chlordiazepoxide, also failed to produce selective effects on overall OST accuracy, although chlordiazepoxide did reduce span length at a dose that was without effect on simple discrimination (Galizio, et al., 2013).
It should also be noted that the OST may be useful in the search for drugs that may enhance remembering. For example, Rushforth and colleagues (Rushforth, Allison, Wonnacut, & Shoaib, 2010; Rushforth, Steckler, & Shoaib, 2011) have shown that nicotine increased span length suggesting that it may increase the number of stimuli rats can remember within a session. More research with additional classes of drugs is needed to develop the behavioral pharmacology of the OST and to determine the extent to which effects of drugs on OST performance converge with those obtained using other procedures.
At this point it is also premature to speculate about the mechanisms through which drugs may affect OST performance because so little is known about the determinants of this complex stimulus control procedure. For example, drug effects might be altered by variations in OST parameters such as the number of comparison stimuli in the arena or the delay interval separating stimulus presentations. Additionally, the extent to which the procedure actually measures rodent memory capacity has been challenged. Although several studies have shown a decrease in OST accuracy as the number of stimuli to remember, the slope of this function is fairly shallow and accuracy is well above chance even with more than 20 odors to remember (Dudchenko et al., 2000; Galizio et al., 2013; MacQueen et al., 2011). We wondered how many odors rats could remember in this procedure and therefore arranged conditions with 36, 48 and 72 novel odorants presented in a single sessions. Rats showed highly accurate performances even with 72 stimuli to remember (April, Bruce, & Galizio, 2013). These results suggest that the type of stimulus control established in OST may not translate in any simple way to the kinds of procedures used to study human working memory in which performance drops to chance with four to seven items to remember. Rather, rodent performance in the OST seems to be much like that seen in human recognition memory tasks for meaningful pictures which appears to be virtually unlimited in terms of the number of items remembered (Standing, 1973). Thus, the interpretation and translational significance of the behavioral pharmacological data will require further exploration.
Although many questions remain about the theoretical interpretation of OST performances, the procedure remains of considerable potential translational value in that it permits analysis of drug effects on rodent behavior maintained by multiple stimuli. Indeed, variations of these procedures could be developed which allow study of the interaction of the number of odors to remember and the duration of the delay interval (cf. Aigner et al., 1991; Wright, 2007). A factor likely to limit enthusiasm of many operant researchers for the OST and related variations is the reliance on manual arena-based methodologies with the attendant problems of control for experimenter effects and unauthorized odor cues, all of which contribute to a very labor-intensive set of procedures. However, in principle, OST and list learning procedures could be automated using olfactometer technology (cf. Otto & Eichenbaum, 1992; Prichard et al., 2015). Development of automated versions of these procedures would require multiple olfactometer channels, but inexpensive commercial olfactometers are now available that make this feasible.
Summary and Conclusions
The search for drugs that might improve cognitive performance in patients with dementia as well as for cognitive enhancers (drugs that improve learning and memory in normal participants) has been a highly active research area, but outcomes have been disappointing (Farah, 2015). This has led to concerns about the translational validity of current animal models and calls for improved techniques (Keeler & Robbins, 2011; Sarter, 2004; Stensbol & Kapur, 2015). Thus, there is an opportunity for behavioral pharmacologists to make important contributions to the cognitive neuroscience of learning and memory. Use of olfactory stimuli makes it possible to study complex stimulus control in rodents with techniques that resemble methodologies used in the human memory literature and may enhance translational validity. Preliminary results with these tasks are promising, but it remains to be seen whether they truly provide advantages over more traditional procedures used to study remembering in rodents. At the least, the behavioral pharmacology of olfactory stimulus control complements the current literature on remembering in rodents which mostly involves spatial tasks. The extent to which findings using these complex olfactory procedures in rats converge with those obtained from the more standard rodent techniques as well as from research using pigeon and non-human primates is of critical interest in this regard.
More generally, it should be noted that sophisticated quantitative methods for analysis of remembering that may further enhance interpretation of behavioral pharmacological studies are being developed (Nevin, Davison, Odum, & Shahan, 2007; White, 2013). Thus, research on drug effects on remembering has the potential to enhance the field by permitting new ways of conceptualizing behavioral mechanisms of drug action (see Pitts, 2014). A first step in such an analysis is to demonstrate that a drug effect is modulated by an environmental variable such as response rate (rate-dependency) or the nature of the reinforcer (event-dependency). As we come to understand the conditions under which the effects of certain drugs are dependent on variables such as the delay interval or the number of stimuli controlling behavior, new analyses of behavioral mechanisms of drug action may emerge.
Acknowledgments
The development of this article was supported in part by NIH Grant DA 029252. Thanks to Katherine Bruce, Angela Goolsby, Michael Mathews, Madeleine Mason, and Raymond Pitts for providing helpful comments on an earlier version of the manuscript and for many discussions about olfactory stimulus control.
References
- Aigner TG, Walker DL, Mishkin M. Comparison of the effects of scopolamine administered before and after acquisition in a test of visual recognition memory in monkeys. Behavioral and Neural Biology. 1991;55:61–67. doi: 10.1016/0163-1047(91)80127-z. [DOI] [PubMed] [Google Scholar]
- April LB, Bruce K, Galizio M. Matching- and non-matching-to-sample concept learning in rats using olfactory stimuli. Journal of the Experimental Analysis of Behavior. 2011;96:123–138. doi: 10.1901/jeab.2011.96-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April LB, Bruce K, Galizio M. The magic number 70 (plus or minus 20): Variables determining performance in the rodent Odor Span Task. Learning and Motivation. 2013;44:143–158. doi: 10.1016/j.lmot.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, Good MA. The drugs don't work-or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A containing AMPA receptors to hippocampal-dependent memory. Psychopharmacology. 2006;188:552–566. doi: 10.1007/s00213-006-0403-6. [DOI] [PubMed] [Google Scholar]
- Blough DS. Delayed matching in the pigeon. Journal of the Experimental Analysis of Behavior. 1959;2:151–160. doi: 10.1901/jeab.1959.2-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodyak N, Slotnick BM. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chemical Senses. 1999;24:637–645. doi: 10.1093/chemse/24.6.637. [DOI] [PubMed] [Google Scholar]
- Cain DP, Saucier D, Hall J, Hargreaves EL, Boon F. Detailed behavioral analysis of water maze acquisition under APV or CNQX: Contribution of sensorimotor disturbances to drug-induced acquisition deficits. Behavioral Neuroscience. 1996;110:86–102. doi: 10.1037//0735-7044.110.1.86. [DOI] [PubMed] [Google Scholar]
- Catania AC. Learning. 5th. Cornwall on Hudson, NY: Sloan Publishing; 2013. [Google Scholar]
- Davies DA, Greba Q, Howland JG. GluN2B-containing NMDA receptors and AMPA receptors in medial prefrontal cortex are necessary for odor span in rats. Frontiers in Behavioral Neuroscience. 2013;7:1–8. doi: 10.3389/fnbeh.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DA, Molder JJ, Greba Q, Howland JG. Inactivation of medial prefrontal cortex or acute stress impairs odor span in rats. Learning & Memory. 2013;20:665–669. doi: 10.1101/lm.032243.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neuroscience and Biobehavioral Reviews. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. Animal models of working memory: a review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neuroscience and Biobehavioral Reviews. 2013;37:2111–2124. doi: 10.1016/j.neubiorev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. The Journal of Neuroscience. 2000;20:2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ. The unknowns of cognitive enhancement: Can science and policy catch up with practice. Science. 2015;350:379–380. doi: 10.1126/science.aad5893. [DOI] [PubMed] [Google Scholar]
- Galizio M, Byrd BD, Robinson AM, Hawkey A, Rayburn-Reeves R, April LB. Repeated acquisition in the Morris Swim Task: Effects of methylenedioxymethamphetamine, methamphetamine, and methylphenidate. Psychological Record. 2014;64:143–150. doi: 10.1007/s40732-014-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, Deal M, Hawkey A, April LB. Working memory in the odor span task: effects of chlordiazepoxide, dizocilpine (MK801), morphine, and scopolamine. Psychopharmacology. 2013;225:397–406. doi: 10.1007/s00213-012-2825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey A, April LB, Galizio M. Effects of MDMA on olfactory memory and reversal learning in rats. Neurobiology of Learning and Memory. 2014;114:209–216. doi: 10.1016/j.nlm.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Ehlers MD, Krystal JH. New drug development for cognitive enhancement in mental health: Challenges and opportunities. Neuropharmacology. 2013;64:2–7. doi: 10.1016/j.neuropharm.2012.07.041. [DOI] [PubMed] [Google Scholar]
- Iversen IH. Acquisition of matching-to-sample performance in rats using visual stimuli on nose keys. Journal of the Experimental Analysis of Behavior. 1993;59:471–482. doi: 10.1901/jeab.1993.59-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen IH. Matching-to-sample performance in rats: A case of mistaken identity? Journal of the Experimental Analysis of Behavior. 1997;68:27–45. doi: 10.1901/jeab.1997.68-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Vaidya M, Branch MN. Titrating-delay matching-to-sample in the pigeon. Journal of the Experimental Analysis of Behavior. 2010;94:69–82. doi: 10.1901/jeab.2010.94-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay C, Harper DN, Hunt M. Differential effects of MDMA and scopolamine on working versus reference memory in the radial arm maze task. Neurobiology of Learning and Memory. 2010;93:151–156. doi: 10.1016/j.nlm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Keeler JF, Robbins TW. Translating cognition from animals to humans. Biochemical Pharmacology. 2011;81:1356–1366. doi: 10.1016/j.bcp.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Keith JR, Galizio M. Acquisition in the Morris swim task is impaired by a benzodiazepine but not an NMDA antagonist: a new procedure for distinguishing acquisition and performance effects. Psychobiology. 1997;25:217–228. [Google Scholar]
- Lane SD, Cherek DR, Lieving LM, Tcheremissine OV. Marijuana effects on human forgetting functions. Journal of the Experimental Analysis of Behavior. 2005;83:67–84. doi: 10.1901/jeab.2005.22-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XM, Slotnick BM, Silberberg AM. Odor matching and odor memory in the rat. Physiology & Behavior. 1993;53:795–804. doi: 10.1016/0031-9384(93)90191-h. [DOI] [PubMed] [Google Scholar]
- MacQueen DA, Bullard L, Galizio M. Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiology of Learning and Memory. 2011;95:57–63. doi: 10.1016/j.nlm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: selective impairment of learning in rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. Journal of Neuroscience. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, Davison M, Odum AL, Shahan TA. A theory of attending, remembering and reinforcement in delayed matching to sample. Journal of the Experimental Analysis of Behavior. 2007;88:285–317. doi: 10.1901/jeab.2007.88-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behavioral Neuroscience. 1992;106:7. doi: 10.1037//0735-7044.106.5.762. 62-775. [DOI] [PubMed] [Google Scholar]
- Pena T, Pitts RC, Galizio M. Identity matching with olfactory stimuli in rats. Journal of the Experimental Analysis of Behavior. 2006;85:203–222. doi: 10.1901/jeab.2006.111-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RC. Reconsidering the concept of behavioral mechanisms of drug action. Journal of the Experimental Analysis of Behavior. 2014;101:422–441. doi: 10.1002/jeab.80. [DOI] [PubMed] [Google Scholar]
- Poling A, Weetjens B, Cox C, Beyene NW, Bach H, Sully A. Using trained pouched rats to detect land mines: Another victory for operant conditioning. Journal of Applied Behavior Analysis. 2011;44:351–355. doi: 10.1901/jaba.2011.44-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard A, Panoz-Brown D, Bruce K, Galizio M. Emergent identity but not symmetry following successive olfactory discrimination training in rats. Journal of the Experimental Analysis of Behavior. 2015;104:133–145. doi: 10.1002/jeab.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel N, Vigouroux M, Elaagouby A, Gervais R. Scopolamine impairs delayed matching in an olfactory task in rats. Psychopharmacology. 1992;109:439–443. doi: 10.1007/BF02247720. [DOI] [PubMed] [Google Scholar]
- Roddick KM, Schellinck HM, Brown RE. Olfactory delayed matching to sample performance in mice: Sex differences in the 5XFAD mouse model of Alzheimer's disease. Behavioural Brain Research. 2014;270:165–170. doi: 10.1016/j.bbr.2014.04.038. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacut S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neuroscience Letters. 2010;471:114–118. doi: 10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Steckler T, Shoaib M. Nicotine improves working memory span capacity in rats following sub-chronic ketamine exposure. Neuropsychopharmacology. 2011;36:2774–2781. doi: 10.1038/npp.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargisson RJ, White KG. On the form of the forgetting function: The effects of arithmetic and logarithmic distribution of delays. Journal of the Experimental Analysis of Behavior. 2003;80:295–309. doi: 10.1901/jeab.2003.80-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M. Animal cognition: defining the issues. Neuroscience and Biobehavioral Reviews. 2004;28:645–650. doi: 10.1016/j.neubiorev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Slotnick B. Animal cognition and the rat olfactory system. Trends in Cognitive Sciences. 2001;5:216–222. doi: 10.1016/s1364-6613(00)01625-9. [DOI] [PubMed] [Google Scholar]
- Soto PL, Ator NA, Rallapalli SK, Biawat P, Clayton T, Cook JM, Weed MR. Allosteric modulation of GABAA receptor subtypes: Effects on visual recognition and visuospatial working memory in Rhesus monkeys. Neuropsychopharmacology. 2013;38:2315–2325. doi: 10.1038/npp.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standing L. Learning 10,000 pictures. Quarterly Journal of Experimental Psychology. 1973;25:207–222. doi: 10.1080/14640747308400340. [DOI] [PubMed] [Google Scholar]
- Steckler T, Drinkenburg WHIM, Sahgal A, Aggleton JP. Recognition memory in rats—I. Concepts and classification. Progress in Neurobiology. 1998;54:289–311. doi: 10.1016/s0301-0082(97)00060-9. [DOI] [PubMed] [Google Scholar]
- Steckler T, Sahgal A, Aggleton JP, Drinkenburg WHIM. Recognition memory in rats—III. Neurochemical substrates. Progress in Neurobiology. 1998;54:333–348. doi: 10.1016/s0301-0082(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Stensbol TB, Kapur S. NEWMEDS special issue commentary. Psychopharmacology. 2015;232:3849–3851. doi: 10.1007/s00213-015-4083-y. [DOI] [PubMed] [Google Scholar]
- Talpos JC, Aerts N, Fellini L, Steckler T. A touch-screen based paired-associates learning (PAL) task for the rat may provide a translatable pharmacological model of human cognitive impairment. Pharmacology, Biochemistry & Behavior. 2014;122:97–106. doi: 10.1016/j.pbb.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Moerschbaecher JM. Drug effects on repeated acquisition. In: Thompson T, Dews PB, editors. Advances in behavioral pharmacology. Vol. 2. Academic Press; New York: 1979. pp. 229–259. [Google Scholar]
- Turchi J, Sarter M. Cortical cholinergic inputs mediate processing capacity: Effects of 192 IgG-saporin-induced lesions on olfactory span performance. European Journal of Neuroscience. 2000;12:4505–4514. [PubMed] [Google Scholar]
- Wenger GR, Hudzik TJ, Wright DW. Titrating matching-to-sample performance in pigeons: effects of diazepam, morphine, and cholinergic agents. Pharmacology Biochemistry & Behavior. 1993;46:435–443. doi: 10.1016/0091-3057(93)90376-5. [DOI] [PubMed] [Google Scholar]
- Wenger GR, Wright DW. Disruption of performance under a titrating matching-to-sample schedule of reinforcement by drugs of abuse. Journal of Pharmacology and Experimental Therapeutics. 1990;254:258–269. [PubMed] [Google Scholar]
- White KG. Characteristics of forgetting functions in delayed matching to sample. Journal of the Experimental Analysis of Behavior. 1985;44:15–34. doi: 10.1901/jeab.1985.44-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KG. Remembering and forgetting. In: Madden GJ, editor. APA handbook of behavior analysis, Volume 1: Methods and principles. Washington DC: American Psychological Association; 2013. pp. 411–439. [Google Scholar]
- Wright AA. An experimental analysis of memory processing. Journal of the Experimental Analysis of Behavior. 2007;88:405–433. doi: 10.1901/jeab.2007.88-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kerr LE, Kelly JS, Marston HM, Spratt C, Finlayson K, Sharkey J. The odour span task: A novel paradigm for assessing working memory in mice. Neuropharmacology. 2007;52:634–645. doi: 10.1016/j.neuropharm.2006.09.006. [DOI] [PubMed] [Google Scholar]