Abstract
Over the past few decades, several cardiac autoantibodies have been reported in sera from patients with dilated cardiomyopathy (DCM). Immunoadsorption (IA) therapy is one of the therapeutic tools to remove such autoantibodies. The objective of this study was to investigate functional effects of IA therapy using a tryptophan column in severe DCM patients. Of 49 patients enrolled, 44 were randomized from 10 sites in Japan. IA therapy was conducted in 40 patients with DCM (refractory to standard therapy for heart failure, New York Heart Association [NYHA] class III/IV, left ventricular ejection fraction [LVEF] <30%). Mean echocardiographic LVEF was significantly improved (23.8 ± 1.3% to 25.9 ± 1.3%, P = 0.0015). However, mean radionuclide LVEF over 3 months of IA therapy was not significantly improved (20.8 ± 1.1% to 21.9 ± 1%, P = 0.0605). The cardiothoracic ratio was also significantly decreased (P = 0.0010). NYHA functional class (P < 0.0001), subjective symptoms assessed by a quality of life questionnaire (P = 0.0022), maximum oxygen consumption (P = 0.0074), and 6‐minute walk distance (P = 0.0050) were improved after IA therapy. Subgroup analysis revealed improvement of echocardiographic LVEF in patients with higher baseline autoantibody scores but not in those with lower scores. IA therapy improved subjective symptoms and exercise capacity in patients with refractory heart failure resulting from DCM. Favorable effect on cardiac function was noted in patients with higher autoantibody scores. J. Clin. Apheresis 31:535–544, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: dilated cardiomyopathy, antibodies, heart failure, immune system
INTRODUCTION
Autoimmunity is one of the pivotal mechanisms, along with viral infection and genetic predisposition, underlying the pathophysiology of dilated cardiomyopathy (DCM). Several autoantibodies found in sera from patients with DCM 1, 2, 3, 4, 5 play a role in mediating cardiac damage 6 and provide arrhythmic substrates 7, resulting in sudden cardiac death 8. Immunoapheresis removing these autoantibodies has been developed to treat DCM patients with refractory heart failure 9, 10, 11, 12. In these preliminary trials except for Müller, et al. 9, intravenous immunoglobulin was replaced after each session of apheresis, because the columns used in these trials nonspecifically removed immunoglobulin. Historical studies have shown that clinically relevant autoantibodies are found in various IgG subclasses in sera from patients with DCM, but those belonging to IgG class 3 play a pivotal role in mediating efficacy of IA therapy 13, 14. Although protein A column under the modification may remove IgG3 subclass 15, tryptophan columns treating as low as 1.5 L plasma had a relatively high specificity for IgG3 than other IgG subclasses, and safely removed pathophysiologically relevant autoantibodies without immunoglobulin substitution in our proof‐of‐concept study 16. We therefore conducted a multicenter trial on immunoapheresis using tryptophan columns in patients with advanced heart failure resulting from DCM.
METHODS
The trial was carried out in accordance with the principles of good clinical practice and the Declaration of Helsinki; all patients provided written informed consent before any study‐related procedure. The protocol was approved by the institutional review boards of all participating centers. The study was registered with the University Hospital Medical Information Network Clinical Trials Registry (trial number UMIN000003106).
Study Participants
Eligible patients had severe idiopathic DCM 17 resistant to standard therapy including angiotensin‐converting enzyme inhibitor (ACE‐I)/angiotensin‐receptor blocker (ARB), β‐blocker, and aldosterone antagonist for heart failure for at least 3 months. Other inclusion criteria were age over 18 years, New York Heart Association (NYHA) functional class III/IV, left ventricular (LV) ejection fraction (LVEF) measured by radionuclide ventriculography <30% or LVEF measured by echocardiography <35% in the past 3 months. Patients were excluded if they had a secondary DCM resulting from other diseases, were implanted with an LV assist device, received cardiac resynchronization therapy in the past 6 months, or underwent heart transplantation. Coronary arteriography was performed in all patients, but endomyocardial biopsy was not necessarily performed in all patients.
Study Design
This was a prospective, multicenter, randomized, within‐patient and parallel‐group comparative study. Eligible patients were randomized in a 1:1 ratio to the immunoadsorption (IA) and delay groups. In the IA group, after the baseline test was completed, five sessions of IA therapy over the course of 2 weeks (first treatment period) were performed using the IMMUSORBA TR‐350(L) (Asahi Kasei Medical, Tokyo, Japan), followed, at 3 months after the start of the trial, by five more sessions of IA therapy, again administered over the course of 2 weeks (second treatment period). In total, 10 sessions were performed for patients in the IA group. In the delay group, after the baseline test was completed, patients were hospitalized for 2 weeks without IA therapy (nontreatment period), followed by five sessions of IA therapy over the course of 2 weeks (first treatment period) at 3 months after the start of the trial. In total, five sessions were performed for patients in the delay group. Endpoints were assessed at 3 months after IA therapy in the IA group (A period), 3 months after observation in the delay group (C period), and 3 months after IA therapy in the delay group (D period). Figure 1 shows an overview of the study design. We noted +3.1 ± 5.9% increase of LVEF in the preliminary study 16, and we calculated sample size as 31, assuming α = 0.05 and β = 0.20, according to the following formula:
where Δ is the assumed difference/standard deviation. We decided to collect 40 cases, considering 20% will be dropped out.
Figure 1.
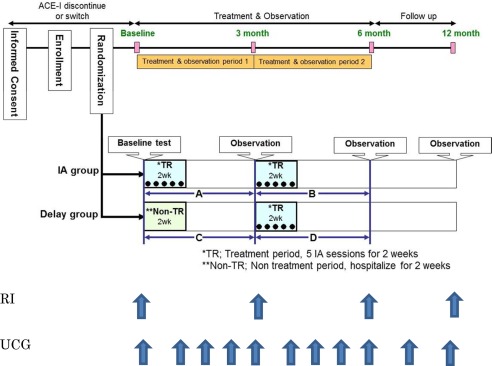
Study protocol. RI, radionuclide ventriculography; UCG, ultrasonic echocardiography
IA Procedure
ACE‐I was discontinued or switched to ARB at least 2 weeks before the first IA therapy, because use with the IMMUSORBA TR column is contraindicated in the presence of ACE‐I with potential bradykinin production 18. The patients were admitted to the hospital for the treatment period to avoid the risk of infection and bleeding. IA therapy was achieved with IMMUSORBA TR and Plasmaflo OP‐05W(L) (Asahi Kasei Medical), as described previously 16. The patients were treated with a plasma throughput of 1.5 L in each session of IA therapy, according to our preliminary study, in which 1.5 L treatment was sufficient to eliminate IgG3 antibodies without losing immunoglobulin specificity 16. None received intravenous immunoglobulin treatment.
Efficacy and Safety Assessment
The primary endpoint was radionuclide LVEF change before and 3 months after IA therapy (A and D periods). Secondary endpoints included change of echocardiographic LVEF, 6‐minute walk test, cardiothoracic ratio on chest radiograph, maximal oxygen consumption (VO2 max) measured by cardiopulmonary exercise testing, brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) levels, NYHA functional class, specific activity scale (SAS) determined by a quality of life questionnaire 19, and hospitalization for heart failure during the 12 months of the study. Cardiac events were defined as all‐cause death; hospitalization for heart failure or arrhythmias; new addition or uptitration of cardiovascular medications because of worsening symptoms; and worsening by at least 1 grade of SAS score or NYHA class.
Assessment of Cardiac Function and Exercise Tolerance
After intravenous injection of technetium‐99m‐labeled human serum albumin, multigated radionuclide ventriculography was performed. Radionuclide LVEF was calculated by dividing the background‐corrected difference between end‐systolic (minimum) and end‐diastolic (maximum) counts by the end‐diastolic counts. Two‐dimensional echocardiography was done by experienced sonographers. Echocardiographic LVEF was evaluated by a modified Simpson's method with manual planimetry of the endocardial border at end‐diastole and end‐systole. To assess the trend of LVEF change during the 3 months after observation began, a regression line was created using five LVEF values during the 3 months. LVEF was judged as improvement when the slope of the regression line was more than +5% for 12 weeks and as aggravation when the slope was less than −5% 20. Independent experienced physicians who were blinded to patient allocation judged the overall change of echocardiographic findings. Symptom‐limited ergometer exercise test was performed to determine maximal oxygen consumption according to the previously reported method 21.
Autoantibody Measurement
Autoantibodies (total IgG and IgG3 subclass) directed against each antigen (β1‐adrenergic receptor, muscarinic M2‐receptor, Na‐K‐ATPase, troponin I, and myosin) were measured using enzyme‐linked immunosorbent assay (ELISA) according to previously described methods 14. The concentration of the target antibody was calculated from the standard curve using a known standard antibody. Total antibody score was calculated as log10 X 0(1) + log10 X 0(2) + log10 X 0(3) + log10 X 0(4) + log10 X 0(5), where X 0(1) through X 0(5) represent each autoantibody. Using the total antibody score provided by five different IgG3 subclass autoantibodies, we divided patients into two groups according to the median value.
Statistical Analysis
All values are expressed as the mean ± standard deviation. The baseline characteristics of the two groups were compared with the use of Student's t‐tests, Fisher's exact test, or the Wilcoxon rank‐sum test. Statistical significance of baseline was defined as P < 0.05. The change of parameters before and after IA therapy was analyzed using paired t test (except for NYHA class, which was assessed by Wilcoxon signed‐rank test). The relationship between response to IA therapy and baseline autoantibody profiles was evaluated using Spearman's rank correlation coefficient. The difference between groups was analyzed by Student's t test and Wilcoxon rank‐sum test. Statistical significance was defined as P < 0.05. Analyses were conducted with the use of SAS software version 9.1.3 (SAS Institute, Cary, NC, USA).
RESULTS
Patient Disposition and Baseline Characteristics
Patient enrollment was started in March 2010 and completed in November 2011. Of 49 patients enrolled from 10 sites in Japan, 44 patients were randomized (IA group, n = 22; delay group, n = 22). Patient disposition is summarized in Figure 2. Overall, 49 patients registered were included in the safety analysis, and 43 patients who completed baseline testing were eligible for efficacy analysis.
Figure 2.
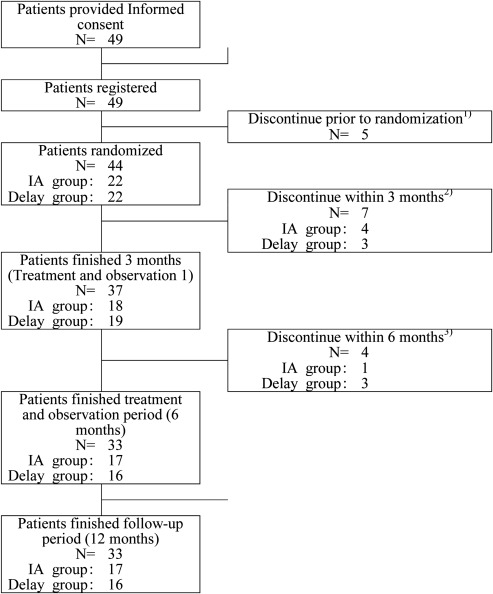
Randomization and allocation of the study subjects.
1) Patients withdrew consent, n = 1; physicians judged to exclude, n = 4.
2) Physicians discontinued because of adverse events, n = 3; patients withdrew consent, n = 2; physicians judged to discontinue IA, n = 1; withdrew because of cardiac event, n = 1.
3) Patients discontinued IA, n = 2; Patients withdrew consent, n = 2.
Demographic and baseline characteristics are summarized in Table 1. Of the 43 patients, 37 were men. Mean patient age was 56 years old, and mean duration of heart failure was 93 months. The LVEF of all patients was severely reduced (mean radionuclide LVEF value, 19.1%). Almost all patients were in NYHA class III except for two patients who were in class IV. The baseline clinical characteristics of the patients were similar in the two groups. There were five patients in the IA group and eight patients in the delay group, who withdrew ACE‐I and switched to ARB.
Table 1.
Demographic and Baseline Characteristics
| Total (N = 43) | IA group (N = 21) | Delay group (N = 22) | |
|---|---|---|---|
| Age (yrs) | 56 ± 13 | 56 ± 12 | 56 ± 13 |
| Weight (kg) | 60.0 ± 10.8 | 61.6 ± 11.9 | 58.5 ± 9.6 |
| Duration of heart failure (months) | 93 ± 57 | 115 ± 61 | 73 ± 47 |
| Gender (Male/Female) | 37/6 | 19/2 | 18/4 |
| Atrial fibrillation (%) | 16/43 (37) | 10 (47) | 6 (27) |
| Echocardiography LVEF (%) | 24.5 ± 6.7a | 23.1 ± 6.3c | 25.7 ± 7.0 |
| Radionuclide LVEF (%) | 19.1 ± 6.7a | 18.6 ± 6.7c | 19.5 ± 6.7 |
| NYHA class (III/IV) | 41/2 | 19/2 | 22/0 |
| SAS (METs) | 3.3 ± 1.3 | 3.5 ± 1.3 | 3.1 ± 1.4 |
| Cardiothoracic ratio (%) | 57.8 ± 6.8 | 59.9 ± 6.1 | 55.9 ± 7.1 |
| Six‐minute walk distance (m) | 347 ± 127a | 353 ± 128 | 340 ± 128c |
| VO2 max (mL/kg/min) | 12.1 ± 4.8b | 11.2 ± 5.6d | 12.7 ± 4.6d |
| BNP (pg/mL) | 471 ± 452 | 509 ± 523 | 435 ± 382 |
| ANP (pg/mL) | 187 ± 158 | 187 ± 153 | 187 ± 166 |
Data for one patient were excluded.
For VO2 max, only patients who were able to undergo cardiopulmonary exercise testing (total, n = 16; IA group, n = 6; delay group, n = 10).
Two‐tailed Student's t test.
Fisher's exact test.
ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; METs, metabolic equivalents; NYHA, New York Heart Association; SAS, specific activity scale; VO2 max, maximal oxygen consumption.
Echocardiographic data in each group over the 12 months were shown in Table II. LVEF as well as end‐diastolic volumes decreased 4 months after IA in the IA group (P < 0.05). End‐systolic volume decreased 3 months and just after 2nd treatment in addition to 4 months after IA. In the delay group these parameters improved only after IA. We did not see additional increase in LVEF after the 2nd treatment in the IA group. There was no significant change in the stroke volume.
Table 2.
Echocardiographic Data Over the 12 Months
| IA group | LVEF (%) | LVEDV (ml) | LVESV (ml) | SV (ml) | |
|---|---|---|---|---|---|
| Before | 23.1 ± 6.3 | 245 ± 105 | 192 ± 93 | 53 ± 18 | |
| 1st TR 2 weeks | 23.2 ± 6.0 | 237 ± 97 | 185 ± 86 | 51 ± 17 | |
| 1 month | 23.3 ± 6.7 | 247 ± 114 | 193 ± 102 | 53 ± 18 | |
| 2 months | 23.6 ± 5.6 | 240 ± 120 | 186 ± 102 | 53 ± 22 | |
| 3 months | 24.4 ± 5.7 | 237 ± 96 | 182 ± 85* | 54 ± 14 | |
| 2nd TR 2 weeks | 25.3 ± 6.2 | 230 ± 97 | 175 ± 87* | 54 ± 16 | |
| 4 months | 25.8 ± 7.4* | 233 ± 97* | 176 ± 86* | 56 ± 18 | |
| 5 months | 25.7 ± 7.3 | 241 ± 102 | 183 ± 94 | 57 ± 17 | |
| 6 months | 24.1 ± 7.1 | 237 ± 108 | 183 ± 92 | 53 ± 21 | |
| 9 months | 24.4 ± 5.0 | 250 ± 110 | 191 ± 93 | 58 ± 19 | |
| 12 months | 25.6 ± 5.7 | 249 ± 106 | 188 ± 91 | 60 ± 18 | |
| Delay group | Before | 25.7 ± 7.0 | 213 ± 56 | 159 ± 49 | 53 ± 15 |
| Non‐TR 2 weeks | 25.4 ± 9.8 | 204 ± 50 | 154 ± 49 | 49 ± 18 | |
| 1 months | 24.4 ± 10.1 | 201 ± 51 | 156 ± 53 | 44 ± 24* | |
| 2 months | 25.3 ± 9.3 | 204 ± 54 | 154 ± 49 | 49 ± 18 | |
| 3 months | 25.3 ± 8.5 | 206 ± 50 | 156 ± 48 | 50 ± 17 | |
| TR 2 weeks | 25.8 ± 9.0 | 193 ± 53* | 145 ± 50* | 47 ± 15 | |
| 4 month | 26.5 ± 9.5 | 193 ± 51* | 144 ± 50* | 48 ± 15 | |
| 5 months | 26.9 ± 9.3 | 193 ± 58* | 144 ± 53* | 49 ± 19 | |
| 6 months | 27.5 ± 9.6 | 201 ± 61 | 148 ± 54 | 52 ± 20 | |
| 9 months | 27.1 ± 9.2 | 206 ± 66 | 153 ± 57 | 53 ± 22 | |
| 12 months | 26.9 ± 10.1 | 201 ± 57 | 149 ± 53 | 51 ± 19 |
LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; SV, stroke volume.
*P < 0.05 vs. before.
The combined analysis of A and D periods is shown in Table 3. Echocardiographic LVEF was significantly increased from 23.8 ± 1.3% to 25.9 ± 1.3% (P = 0.0015). In the trend analysis of LVEF using multiple data during the IA therapy, eight patients exhibited improvement (22%). The cardiothoracic ratio was also significantly decreased. There was a significant increase in VO2 max determined by cardiopulmonary testing. NYHA class was improved by at least 1 grade in 21 patients (52%). However, there was no improvement in the remaining 19 patients (48%), of whom one patient exhibited worsening of heart failure. The SAS score was increased from 3.4 ± 0.2 to 3.9 ± 0.2 metabolic equivalents (METs), and the 6‐minute walk distance was increased from 359 ± 20 to 390 ± 19 m. Plasma BNP and ANP levels tended to be decreased, but the difference was not statistically significant.
Table 3.
Primary and Secondary Endpoints Changes Between Before and 3 Months After IA Therapy
| Parameters | Combined A+D | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Change for 3 months after IA | P values | |||||||||
| Echocardiographic LVEF (%)a | 39 | 23.8 ± 1.3 to 25.9 ± 1.3 | 0.0015 | ||||||||
| Trend analysis of Echocardiographic LVEFb | 36 | Improvement: eight patients (22%), | – | ||||||||
| Unchanged:27 patients (75%), | |||||||||||
| Aggravation:one patient (3%) | |||||||||||
| Radionuclide LVEF (%)c | 39 | 20.8 ± 1.1 to 21.9 ± 1.3 | 0.0605 | ||||||||
| Cardiothoracic ratio (%) | 40 | 57.3 ± 1.2 to 55.9 ± 1.2 | 0.001 | ||||||||
| VO2max (mL/min/kg)d | 15 | 12.3 ± 1.2 → 16.0 ± 1.8 | 0.0064 | ||||||||
| NYHA class N (%) | 40 | IV | : | 2 | → | 1 | −3% | <0.0001 | |||
| III | : | 33 | → | 18 | −45% | Improvement: | 21 | −52% | |||
| II | : | 4 | → | 15 | −37% | unchanged: | 18 | −45% | |||
| II | : | 1 | → | 5 | −12% | Aggravation: | 1 | −3% | |||
| I | : | 0 | → | 1 | −3% | ||||||
| SAS (METs) | 40 | 3.4 ± 0.2 → 3.9 ± 0.2 | 0.0022 | ||||||||
| 6‐minute walk distance (m) | 40 | 359 ± 20 to 390 ± 19 | 0.005 | ||||||||
| BNP (pg/mL) | 40 | 426 ± 84 to 393 ± 82 | 0.4759 | ||||||||
| ANP (pg/mL) | 40 | 172 ± 24 to 163 ± 27 | 0.5261 | ||||||||
The data for changes in the 3 months after IA therapy in the IA group (A period) and the delay group (D period) were combined.
aData were not obtained in one patient.
bThe regression line was constructed using five LVEF values during the 3 months, and the slope of the regression line more than +5% or less than −5% for the period was defined as improvement or aggravation, respectively. Data missing in three patients.
cData missing in one patient.
dOnly patients who were able to undergo cardiopulmonary exercise testing.
ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; METs, metabolic equivalents; NYHA, New York Heart Association; SAS, specific activity scale; VO2 max, maximal oxygen consumption.
Mean radionuclide LVEF 3 months after IA therapy improved nonsignificantly in the IA group (from 18.6% to 19.5%) and in the delay group (from 22.3% to 23.5%). However, there was a statistically significant increase in radionuclide LVEF during the 3‐month observation period in the delay group without IA (from 19.5% to 22.3%, P = 0.0343). LVEF tended to be increased in the combined data from the A and D periods when IA therapy was performed.
Cardiac Events
Comparisons between experimental periods with and without IA therapy are shown in Supporting Information Tables S1 and S2. In the IA group, there were three cardiac events (two hospitalizations and one death) during the 3 months with IA therapy (A period), whereas in the delay group, there were six cardiac events (four hospitalizations, one death, and one treatment modification) during the 3 months without IA therapy (C period) (P = 0.4566). Furthermore, in the delay group, compared with the six cardiac events during the C period, there was only one event (hospitalization for heart failure) during the 3 months with IA therapy (D period) (P = 0.0994). From 6 months to 12 months, there were additional three cardiac events in the IA group and two cardiac events in the delay group.
Adverse Events
Adverse events were observed in 42 patients and severe adverse events in 19 patients, but most of the adverse events appeared to be attributed to the underlying heart failure. Hypotension occurred in 14 patients during the IA procedure, but symptoms resolved without any treatment or immediately after intravenous fluid administration. Most of the adverse events that occurred during the IA procedure, including hypotension, were mild in nature, easily managed, and similar to those observed in other extracorporeal circulation procedures.
Autoantibody Profiles
Total IgG was decreased from 1348 ± 449 mg/dL to 821 ± 269 mg/dL over the 1st set of five sessions of IA, and from 1397 ± 344 mg/dL to 925 ± 288 mg/dL during the 2nd set of IA in the IA group. IgG3 concentration decreased from 32 ± 22 mg/dL to 25 ± 17 mg/dL after single session of IA. Elimination rate of each IgG subclass and each autoantibody during the each single session of IA was presented in Table 4, showing IgG3 as well as five putative autoantibodies directed against specific epitopes were efficiently removed by 1.5 L plasma treatment. Full autoantibody profile is shown in Figure 3. Unfortunately, we were unable to find significant correlation between individual autoantibodies and LVEF response to IA therapy (data not shown). However, echocardiographic LVEF was significantly increased in patients with a higher autoantibody score but not in those with a lower score. Similarly, LV end‐systolic volume (LVESV) was decreased in patients with a higher baseline autoantibody score but not in those with a lower score (Fig. 4).
Table 4.
Elimination Rate of IgG Subclasses and Each Autoantibody During the Single Session of IA
| 0.5L treatment (n) | 1.0L treatment (n) | 1.5L treatment (n) | |
|---|---|---|---|
| IgG1 | 99.8 ± 0.7 (39) | 23.8 ± 15.0 (40) | 1.5 ± 11.8 (39) |
| IgG2 | 99.1 ± 2.3 (39) | −13.8 ± 11.7 (40) | −11.4 ± 9.1 (39) |
| IgG3 | 98.0 ± 9.1 (38) | 80.6 ± 22.4 (38) | 62.1 ± 29.8 (38) |
| IgG4 | 98.9 ± 3.2 (36) | −26.9 ± 23.5 (37) | −9.2 ± 11.1 (36) |
| β1‐antibody | 92.9 ± 15.7 (27) | 68.6 ± 40.1 (23) | 61.9 ± 43.1 (22) |
| M2 antibody | 100.0 ± 0.0 (5) | 62.5 ± 51.3 (5) | 54.1 ± 51.3 (6) |
| Na‐K‐ATPase antibody | 93.6 ± 22.1 (12) | 80.4 ± 40.9 (14) | 75.9 ± 43.8 (12) |
| Troponin I antibody | 100.0 ± 0.0 (17) | 56.6 ± 44.0 (14) | 51.8 ± 45.7 (14) |
| Myosin antibody | 100.0 ± 0.0 (8) | 100.0 ± 0.0 (10) | 85.7 ± 40.4 (8) |
Elimination rate (%) = (precolumn value − postcolumn value)/precolumn value × 100.
Figure 3.
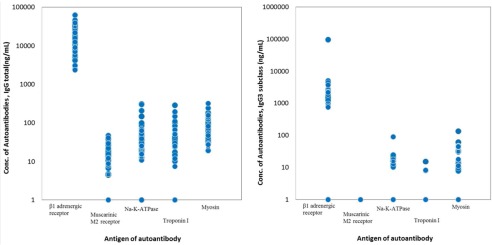
Baseline autoantibody concentrations. Left panel, total IgG antibodies; right panel, IgG3 subclass antibodies.
Figure 4.
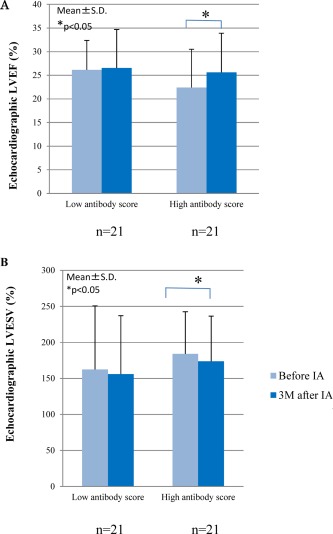
Changes in cardiac function before and 3 months (3M) after immunoadsorption (IA) therapy according to the baseline autoantibody score. *P < 0.05. (A) Left ventricular ejection fraction (LVEF); (B) left ventricular end‐systolic volume (LVESV).
DISCUSSION
We demonstrated that IA therapy using a tryptophan column could be safely conducted in patients with refractory heart failure resulting from DCM. Although we were not able to detect significant improvement of LVEF measured by radionuclide ventriculography, echocardiographic LVEF, NYHA functional class, cardiothoracic ratio, VO2 max, SAS score, and 6‐minute walk distance were significantly improved. Echocardiographic LVEF and LVESV were improved in patients with higher baseline autoantibody scores compared with those with lower scores.
Primary Endpoint
We defined a change in LVEF measured by radionuclide ventriculography 3 months after IA therapy by combining the A and D periods and failed to detect a significant change. We adopted this endpoint because radionuclide ventriculography is a highly objective tool to assess cardiac function. However, it is sometimes difficult to select the region of interest 22. In addition, measurement is also affected by arrhythmias such as atrial fibrillation, commonly observed in patients with DCM 23. We cannot completely exclude the possibility that LVEF spontaneously recovered during the 3 months without IA in the delay group.
We decided to enroll 40 patients in total assuming radionuclide LVEF was increased by 3.1% according to our preliminary finding 16. Although previous report defined 5% or more increase in LVEF as a responder 12, we did not perform responder/nonresponder analysis.
Echocardiographic Assessment
Echocardiography has limitations in terms of objectivity but is superior to radionuclide ventriculography in terms of repeatability. Echocardiographic assessment is also able to avoid potential errors caused by atrial fibrillation by averaging multiple beats. We have shown that echocardiographic LVEF was significantly increased 3 months after IA. For comparison between A and C periods, the change in LVEF tended to be greater in A than C, although this difference was statistically insignificant. For comparison between C and D periods, LVEF was increased during IA therapy but not during the observation period without IA therapy. Trend analysis of echocardiography also showed that improvement was more common during IA therapy than during the observation period.
Other Secondary Endpoints
VO2 max, NYHA functional class, SAS score, and 6‐minute walk distance were improved 3 months after IA therapy in the combined data of A and D periods. For comparison between A and C periods, the change in VO2 max was significantly greater in A than C. The change in NYHA functional class also tended to be better in A than C. The change in VO2 max was also better in D than C for the delay group. These findings suggest IA therapy improved maximal and submaximal exercise capacity. Plasma concentrations of BNP and ANP did not change significantly after IA. The tryptophan column may absorb natriuretic peptides 24, confounding the levels after IA. It may be difficult to estimate the severity of heart failure from the levels of natriuretic peptides, which are accepted biomarkers to estimate cardiac function.
Cardiac Events
Hospitalizations for heart failure tended to be less common during the 3 months after IA therapy than during the observation period. IA therapy may be useful to prevent such major cardiac events, thus ameliorating the quality of life in patients with heart failure.
In addition, our preliminary study showed that 1.5 L plasma treatment was sufficient to remove IgG3 subclass specifically 16. A tryptophan column with high specificity for IgG3 may be superior to previously available columns such as anti‐IgG and protein A columns, because removal of IgG is minimized to avoid substitution of high‐dose immunoglobulin after the procedure.
Significance of Baseline IgG3 Autoantibody Score
We measured five different kinds of autoantibodies that are well known to play a major role in the pathophysiology of DCM 1, 3, 4, 5, 25 and failed to demonstrate that individual autoantibodies predict the LVEF response to IA therapy. Previous findings regarding the presence of autoantibodies in predicting efficacy of IA therapy have been inconsistent 9, 26. We previously showed that IA therapy was efficacious in patients who were positive for either autoantibody directed against β1‐adrenergic receptor or muscarinic M2‐receptor 16. In contrast, some reports have shown that autoantibodies belonging to IgG subclass 3 have a pivotal role in mediating the pathophysiology of various autoimmune disorders 27, 28. As expected, removal of autoantibodies belonging to IgG3 was associated with clinical efficacy in patients who received IA therapy 13, 14. Dandel, et al. reported that heart transplantation or left ventricular assist device‐free survival was better in patients with positive autoantibody for β1‐receptors than those without autoantibody, who received IA therapy 10. In the present study, we demonstrated that the total score for the five putative autoantibodies belonging to IgG3 subclass was associated with a clinical response as defined by echocardiographically estimated LVEF and LVESV. Therapeutic efficacy appears to be more prominent in the study by Dandel, et al. as well as our preliminary experience than the present study. Inclusion criteria for autoantibody, study design (randomized or observational study), and plasma treatment are all different in these studies, accounting for the differential results.
Limitations
There was a discrepancy between radionuclide LVEF and echocardiographic LVEF. Unfortunately, we failed to show significant improvement for radionuclide LVEF. Instead, we attempted to minimize the time‐to‐time variation by multiple measurements during the 3 months of each observation using echocardiography. We added trend analysis by correlating the obtained data, and successfully validated the working hypothesis that IA therapy could improve cardiac function in patients with severe heart failure because of DCM. LVEF response was smaller than expected in this study. This may be because of the fact that the presence of β1‐autonatibody was not a pre‐requisite in this study. Cardiac events including heart failure hospitalization did not reach statistical significance. A larger sample size would successfully demonstrate clinical efficacy in addition to cardiac function.
CONCLUSIONS
We have demonstrated that IA therapy using a novel tryptophan column ameliorated subjective symptoms and exercise capacity in patients with refractory heart failure resulting from DCM. Assessment of a baseline autoantibody profile showed that this type of therapy was especially useful in patients who had a high autoantibody score. This type of therapy may be also useful to reduce medical expenditure who are awaiting cardiac transplantation.
Supporting information
Supporting Information
Executive Committee Members: Masafumi Kitakaze, National Cardiovascular Research Center; Tohru Izumi, Kitasato University School of Medicine; Tsutomu Yoshikawa, Sakakibara Heart Institute; Toshiaki Monkawa, Keio University School of Medicine; Makoto Akaishi, Kitasato Institute Hospital; Akiyasu Baba, Kitasato Institute Hospital; Yasuhisa Wakabayashi, Kitasato Institute Hospital
Endpoint Subcommittee Members: Junichi Yamazaki, Toho University Omori Medical Center; Makoto Suzuki, Toho University Ohashi Medical Center; Shohei Yamashina, Toho University Ohashi Medical Center; Michihiro Yoshimura, The Jikei University School of Medicine
Data and Safety Monitoring Committee: Yoshiki Sawa, Osaka University School of Medicine; Kazuhiro Sase, Juntendo University School of Medicine; Kenichi Matsuda, Yamanashi University School of Medicine; Hideki Origasa, Toyama University School of Medicine
Statistics and Data Management: Masahiro Takeuchi, Kitasato Univeristy
Protocol Committee: Hitonobu Tomoike, Sakakibara Heart Institute; Masafumi Kitakaze, National Cardiovascular Research Center; Kazuhiko Hashimura, National Cardiovascular Research Center; Haruko Yamamoto, National Cardiovascular Research Center; Tsutomu Yoshikawa, Sakakibara Heart Institute; Hiroshi Sato, Keio University School of Medicine; Toshiaki Monkawa, Keio University School of Medicine; Tohru Izumi, Kitasato University School of Medicine; Takayuki Inomata, Kitasato University School of Medicine; Masahiro Takeuchi, Kitasato University; Toshihiko Sato, Kitasato University; Makoto Akaishi, Kitasato Institute Hospital; Akiyasu Baba, Kitasato Institute Hospital; Yasuhisa Wakabayashi, Kitasato Institute Hospital
Investigators and Enrolling Sites: Hideaki Kanzaki, National Cardiovascular Research Center; Tsutomu Yoshikawa, Keio Univeristy School of Medicine; Tohru Izumi, Kitasato University School of Medicine; Makoto Akaishi, Kitasato Institute Hospital; Shinichi Momomura, Jichi University Saitama Medical Center; Yoshio Yasumura, National Hospital Organization Osaka National Hospital; Haruo Hirayama, Nagoya 2nd Red Cross Hospital; Koichiro Kinugawa, University of Tokyo; Minoru Ono, Toranomon Hospital; Nobuo Iguchi, Sakakibara Heart Institute
REFERENCES
- 1. Limas CJ, Goldenberg IF, Limas C. Autoantibodies against beta‐adrenoceptors in human idiopathic dilated cardiomyopathy. Circ Res 1989; 64:97–103. [DOI] [PubMed] [Google Scholar]
- 2. Baba A, Yoshikawa T, Chino M, Murayama A, Mitani K, Nakagawa S, Fujii I, Shimada M, Akaishi M, Iwanaga S, Asakura Y, Fukuda K, Mitamura H, Ogawa S. Characterization of anti‐myocardial autoantibodies in Japanese patients with dilated cardiomyopathy. Jpn Circ J 2001; 65:867–873. [DOI] [PubMed] [Google Scholar]
- 3. Lauer B, Schanwell M, Kühl U, Strauer BE, Schultheiss HP. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol 2000; 35:11–18. [DOI] [PubMed] [Google Scholar]
- 4. Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD‐1‐deficient mice. Nat Med 2003; 9:1477–1483. [DOI] [PubMed] [Google Scholar]
- 5. Baba A, Yoshikawa T, Fukuda Y, Sugiyama T, Shimada M, Akaishi M, Tsuchimoto K, Ogawa S, Fu M. Autoantibodies against M2‐muscarinic acetylcholine receptors: new upstream targets in atrial fibrillation in patients with dilated cardiomyopathy. Eur Heart J 2004; 25:1108–1115. [DOI] [PubMed] [Google Scholar]
- 6. Iwata M, Yoshikawa T, Baba A, Anzai T, Nakamura I, Wainai Y, Takahashi T, Ogawa S. Autoimmunity against the second extracellular loop of beta(1)‐adrenergic receptors induces beta‐adrenergic receptor desensitization and myocardial hypertrophy in vivo. Circ Res 2001; 88:578–586. [DOI] [PubMed] [Google Scholar]
- 7. Fukuda Y, Miyoshi S, Tanimoto K, Oota K, Fujikura K, Iwata M, Baba A, Hagiwara Y, Yoshikawa T, Mitamura H, Ogawa S. Autoimmunity against the second extracellular loop of beta(1)‐adrenergic receptors induces early afterdepolarization and decreases in K‐channel density in rabbits. J Am Coll Cardiol 2004; 43:1090–1100. [DOI] [PubMed] [Google Scholar]
- 8. Iwata M, Yoshikawa T, Baba A, Anzai T, Mitamura H, Ogawa S. Autoantibodies against the second extracellular loop of beta1‐adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2001; 37:418–424. [DOI] [PubMed] [Google Scholar]
- 9. Müller J, Wallukat G, Dandel M, Bieda H, Brandes K, Spiegelsberger S, Nissen E, Kunze R, Hetzer R. Immunoglobulin adsorption in patients with idiopathic dilated cardiomyopathy. Circulation 2000; 101:385–391. [DOI] [PubMed] [Google Scholar]
- 10. Dandel M, Wallukat G, Englert A, Lehmkuhl HB, Knosalla C, Hetzer R. Long‐term benefits of immunoadsorption in β(1)‐adrenoceptor autoantibody‐positive transplant candidates with dilated cardiomyopathy. Eur J Heart Fail 2012; 14:1374–1388. [DOI] [PubMed] [Google Scholar]
- 11. Ameling S, Herda LR, Hammer E, Steil L, Teumer A, Trimpert C, Dörr M, Kroemer HK, Klingel K, Kandolf R, Völker U, Felix SB. Myocardial gene expression profiles and cardiodepressant autoantibodies predict response of patients with dilated cardiomyopathy to immunoadsorption therapy. Eur Heart J 2013; 34:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reinthaler M, Empen K, Herda LR, Schwabe A, Rühl M, Dörr M, Felix SB. The effect of a repeated immunoadsorption in patients with dilated cardiomyopathy after recurrence of severe heart failure symptoms. J Clin Apher 2015; 30:217–223. [DOI] [PubMed] [Google Scholar]
- 13. Staudt A, Böhm M, Knebel F, Grosse Y, Bischoff C, Hummel A, Dahm JB, Borges A, Jochmann N, Wernecke KD, Wallukat G, Baumann G, Felix SB. Potential role of autoantibodies belonging to the immunoglobulin G‐3 subclass in cardiac dysfunction among patients with dilated cardiomyopathy. Circulation 2002; 106:2448–2453. [DOI] [PubMed] [Google Scholar]
- 14. Baba A, Akaishi M, Shimada M, Monkawa T, Wakabayashi Y, Takahashi M, Nagatomo Y, Yoshikawa T. Complete elimination of cardiodepressant IgG3 autoantibodies by immunoadsorption in patients with severe heart failure. Circ J 2010; 74:1372–1377. [DOI] [PubMed] [Google Scholar]
- 15. Staudt A, Dörr M, Staudt Y, Böhm M, Probst M, Empen K, Plötz S, Maschke HE, Hummel A, Baumann G, Felix SB. Role of immunoglobulin G3 subclass in dilated cardiomyopathy: Results from protein A immunoadsorption. Am Heart J 2005; 150:729–736. [DOI] [PubMed] [Google Scholar]
- 16. Nagatomo Y, Baba A, Ito H, Naito K, Yoshizawa A, Kurita Y, Nakamura I, Matsubara T, Wakabayashi Y, Ogawa S, Akaishi M, Yoshikawa T. Specific immunoadsorption therapy using a tryptophan column in patients with refractory heart failure due to dilated cardiomyopathy. J Clin Apher 2011; 26:1–8. [DOI] [PubMed] [Google Scholar]
- 17. JCS Joint Working Group. Guidelines for diagnosis and treatment of myocarditis (JCS 2009): digest version. Circ J 2011; 75:734–743. [DOI] [PubMed] [Google Scholar]
- 18. Tsuboi Y, Takahashi M, Ishikawa Y, Okada H, Yamada T. Elevated bradykinin and decreased carboxypeptidase R as a cause of hypotension during tryptophan column immunoabsorption therapy. Ther Apher 1998; 2:297–299. [DOI] [PubMed] [Google Scholar]
- 19. Sasayama S, Asanoi H, Ishizaka S. Evaluation of functional capacity of patients with congestive heart failure Yasuda H, Kawaguchi H, editors. New Aspects in the Treatment of Failing Heart. Berlin: Springer, 1992; 113–117. [Google Scholar]
- 20. de Groote P, Delour P, Mouquet F, Lamblin N, Dagorn J, Hennebert O, Le Tourneau T, Foucher‐Hossein C, Verkindére C, Bautners C. The effects of beta‐blockers in patients with stable chronic heart failure. Predictors of left ventricular ejection fraction improvement and impact on prognosis. Am Heart J 2007; 154:589–595. [DOI] [PubMed] [Google Scholar]
- 21. Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr , Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991; 83:778–786. [DOI] [PubMed] [Google Scholar]
- 22. Williams KA, Bryant TA, Taillon LA. First‐pass radionuclide angiographic analysis with two regions of interest to improve left ventricular ejection fraction accuracy. J Nucl Med 1998; 39:1857–1861. [PubMed] [Google Scholar]
- 23. Grönefeld GC, Hohnloser SH. Heart failure complicated by atrial fibrillation: mechanistic, prognostic, and therapeutic implications. J Cardiovasc Pharmacol Ther 2003; 8:107–113. [DOI] [PubMed] [Google Scholar]
- 24. Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamura M, Ikeda H, Sakai M. Cytokine adsorptive property of various adsorbents in immunoadsorption columns and a newly developed adsorbent: an in vitro study. Blood Purif 2004; 22:530–536. [DOI] [PubMed] [Google Scholar]
- 25. Baba A, Yoshikawa T, Ogawa S. Autoantibodies produced against sarcolemmal Na‐K‐ATPase: possible upstream targets of arrhythmias and sudden death in patients with dilated cardiomyopathy. J Am Coll Cardiol 2002; 40:1153–1159. [DOI] [PubMed] [Google Scholar]
- 26. Mobini R, Staudt A, Felix SB, Baumann G, Wallukat G, Deinum J, Svensson H, Hjalmarson A, Fu M. Hemodynamic improvement and removal of autoantibodies against β1‐adrenergic receptor by immunoadsorption therapy in dilated cardiomyopathy. J Autoimmun 2003; 20:345–350. [DOI] [PubMed] [Google Scholar]
- 27. Mulder AH, Stegeman CA, Kallenberg CG. Activation of granulocytes by anti‐neutrophil cytoplasmic antibodies (ANCA) in Wegener's granulomatosis: A predominant role for the IgG3 subclass of ANCA. Clin Exp Immunol 1995; 101:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warraich RS, Noutsias M, Kazak I, Seeberg B, Dunn MJ, Schultheiss HP, Yacoub MH, Kuhl U. Immunoglobulin G3 cardiac myosin autoantibodies correlate with left ventricular dysfunction in patients with dilated cardiomyopathy: Immunoglobulin G3 and clinical correlates. Am Heart J 2002; 143:1076–1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


