Abstract
Background
Although the exact pathophysiology of preeclampsia is not completely understood, the utility of different platelets indices can be utilized to predict preeclampsia.
Objective
To compare platelet indices, namely platelet count (PC), mean platelet volume (MPV), platelet distribution width (PDW), and PC to MPV ratio in women with preeclampsia compared with healthy controls.
Setting
Qassim Hospital, Kingdom of Saudi Arabia.
Design
A case–control study. Sixty preeclamptic women were the cases and an equal number of healthy pregnant women were the controls.
Results
There was no significant difference in age, parity, and body mass index between the study groups. Sixteen and 44 of the cases were severe and mild preeclampsia, respectively. There was no significant difference in PDW and MPV between the preeclamptic and control women. Both PC and PC to MPV ratios were significantly lower in the women with preeclampsia compared with the controls. There was no significant difference in the PC, PDW, MPV, and PC to MPV ratio when women with mild and severe preeclampsia were compared. Using receiver operating characteristic (ROC) curves, the PC cutoff was 248.0×103/µL for diagnosis of pre-eclampsia (P=0.019; the area under the ROC curve was 62.4%). Binary regression suggests that women with PC <248.010×103/µL were at higher risk of preeclampsia (odds ratio =2.2, 95% confidence interval =1.08–4.6, P=0.03). The PC/MPV cutoff was 31.2 for diagnosis of preeclampsia (P=0.035, the area under the ROC curve was 62.2%).
Conclusion
PC <248.010×103/µL and PC to MPV ratio 31.2 are valid predictors of preeclampsia.
Keywords: preeclampsia, platelets, PDW, mean platelet volume
Background
Preeclampsia is one of the major health problems during pregnancy. It complicates 3%–8% of pregnancies and causes marked increase in perinatal, maternal morbidity, and mortality.1–4 Although the exact pathophysiology of preeclampsia is not completely understood, certain factors have been attributed to it, which include deficient trophoblastic invasion of the maternal vascular bed with subsequent reduction of placental blood flow.5,6 Placental under perfusion initiates widespread systemic, maternal endothelial dysfunction, and increased vascular permeability.7 Coagulation system is activated by the contact of platelets with the injured endothelium leading to increase in consumption as well as bone marrow production of platelets.8 Various indices are used to measure platelet functions, for example, the platelet count (PC), mean platelet volume (MPV), the PC to MPV ratio, and platelet distribution width (PDW); PDW measures platelet size distribution.9 The utility of different platelets indices as predictors of preeclampsia has been studied previously;10–21 however, reports in this regard are controversial. The current study was conducted to evaluate platelets indices in women with preeclampsia.
Methods
A retrospective case–control study was conducted at Maternity and Children’s Hospital, Buraidah, Kingdom of Saudi Arabia (KSA) during the period of February–July 2016. Maternity and Children’s Hospital is a tertiary care center for the Qassim region. Cases were women with preeclampsia, which was defined as “occurrence of hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg) in the second half of pregnancy (after 20 weeks of gestation) and proteinuria (presence of 300 mg or more of protein in 24 h urine sample or ≥2+ on dipstick)”.22 Pre-eclampsia cases were considered mild or severe according to the diastolic blood pressure of <110 or ≥110 mmHg, respectively.23 Healthy pregnant women attended for antenatal care were taken as controls. Women with thyroid disease, hypertension, renal disease, diabetes mellitus, and liver diseases were excluded from the study groups.
The medical records of both cases and controls were reviewed for obstetrics history (age, parity, and gestational age). Data were collected using predesigned questionnaire. Maternal weight and height were recorded, and body mass index (BMI) was calculated as kg/m2. Data of maternal hemogram taken at presentation were recorded. The hemogram included hemoglobin level, white blood cell (WBC) count, and platelet indices, namely PC, MPV, PDW, and PC to MPV ratio. The hemogram was performed using an automated hematology analyzer (Sysmex XN-9000; Sysmex Corporation, Hyogo, Japan).
A total sample size of 60 participants in each arm of the study groups was recorded to investigate the mean difference of the PC and MPV that would provide 80% power to detect a 5% difference at a=0.05, with an assumption that complete data might not be available for 10% of participants.
Ethics
This study was approved by Regional Research Ethical Committee, Ministry of Health, Qassim, KSA who deemed written consent was not required due to the retrospective nature of the study.
Statistics
SPSS for Windows (version 20.0) was used for the data analyses. Continuous variables were checked for normality and their differences were compared between the cases and the controls using Student’s t-test and Mann–Whitney U-test, when the data were normally and abnormally distributed, respectively. Binary regression was conducted, where pre-eclampsia was the dependent variable and medical, obstetrics, and platelets indices were the independent variable. Diagnostic screening tests were used to determine the diagnostic cutoffs of various parameters (based on test sensitivity and specificity) using receiver operating characteristic (ROC) curve. P<0.05 was considered statistically significant.
Results
While there was no significant difference in the basic characteristics (age, parity, and BMI) between the two study groups (60 women in each arm), gestational age was significantly lower in women with preeclampsia (Table 1). Sixteen and 44 of the cases were suffering from severe and mild preeclampsia, respectively. There was no case with HELLP syndrome.
Table 1.
Characteristics of the cases and controls
| Variable | Cases (n=60) | Controls (n=60) | P-value |
|---|---|---|---|
| Mean (SD) | |||
| Age, years | 30.6 (6.0) | 30.01(6.2) | 0.504 |
| Parity | 2.3(1.8) | 2..6(1.4) | 0.521 |
| Gestational age, weeks | 33.8(4.3) | 32.9(4.0) | 0.001 |
| Body mass index, kg/m2 | 31.0 (6.3) | 29.7(6.1) | 0.833 |
Abbreviation: SD, standard deviation.
There was no significant difference in WBC, hematocrit (HCT), red cell distribution width (RDW), PDW, and MPV between the two groups. Both PC and PC to MPV ratio values were significantly lower in the cases compared with the controls (Table 2).
Table 2.
Median (interquartile range) of hematological values in preeclamptic and control women
| Variable | Cases (n=60) |
Controls (n=60) |
P-value |
|---|---|---|---|
| White blood cells, × 103/µL | 8.1 (6.7–9.9) | 7.9 (5.8–9.9) | 0.319 |
| Hematocrit, % | 35.4 (33.32–37.32) | 34.3 (32.7–36.3) | 0.3471 |
| Red cell distribution width, % | 14.5 (13.4–17.2) | 14.1 (13.4–15.0) | 0.172 |
| Platelets count, × 103/µL | 236.5 (176.0–278.25) | 259.0 (215.7–322.7) | 0.019 |
| Mean platelet volume, fL | 10.0 (9.3–10.8) | 10.1 (9.4–10.8) | 0.799 |
| Platelet distribution width, % | 12.7 (11.4–15.8) | 13.2 (11.4–14.9) | 0.877 |
| Platelets count/mean platelet volume | 22.2 (16.8–29.7) | 26.1 (20.5–32.0) | 0.035 |
WBC, HCT, RDW, PC, PDW, MPV, and PC to MPV ratio were not significantly different when mild and severe preeclampsia women were compared (Table 3).
Table 3.
Median (interquartile range) of hematological values in the women with mild and severe preeclampsia
| Variable | Mild preeclampsia (n=16) |
Severe preeclampsia (n=44) |
P-value |
|---|---|---|---|
| White blood cells, × 103/µL | 8.2 (6.0–9.5) | 8.1 (6.7–10.2) | 0.519 |
| Hematocrit, % | 35.7 (33.7–37.1) | 35.1 (33.0–37.3) | 0.564 |
| Red cell distribution width, % | 13.6 (12.8–15.8) | 14.9 (13.7–17.4) | 0.069 |
| Platelet count, ×103/µL | 249.0 (185.2–277.7) | 219.0 (164.7–286.5) | 0.300 |
| Mean platelet volume, fL | 10.1 (9.3–10.6) | 9.9 (9.3–11.4) | 0.575 |
| Platelet distribution width, % | 12.5 (11.4–14.1) | 12.8 (11.4–16.0) | 0.688 |
| Platelet count/mean platelet volume | 24.2 (18.7–29.0) | 20.6 (15.4–30.1) | 0.380 |
ROC curves were used to derive cutoffs for PC and the PC/MPV ratio. The PC cutoff was 248.0×103/µL for diagnosis of preeclampsia (P=0.19). The area under the ROC curve was 62.4% (standard error =5.1%), which suggest poor predictability.
Based on binary regression analysis, women with PC <248.010×103/µL were at higher risk to have preeclampsia (odds ratio =2.2, 95% confidence interval =1.08–4.6, P=0.03). The PC/MPV cutoff was 31.2 for diagnosis of preeclampsia (P=0.035). The area under the ROC curve was 62.2%, and the standard error was 5.2% (Figure 1).
Figure 1.
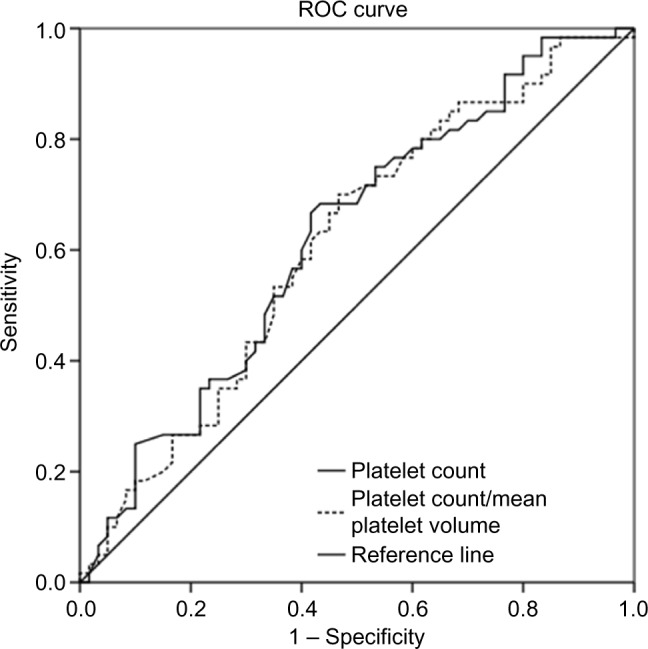
ROC curve for platelets, platelet count, and mean platelet volume ratio.
Note: Diagonal segments are produced by ties.
Abbreviation: ROC, receiver operating characteristic.
Discussion
The current study demonstrated significantly lower PC and PC to MPV ratio in patients with preeclampsia compared with the normal controls but failed to show similar trend when MPV and PDW were evaluated in the same study groups. Recently, Doğan et al14 observed significantly lower PC and PC/MPV in preeclamptic women compared with the controls. The same study documented significantly higher MPV in preeclamptic women than the control group. Likewise, Freitas et al,12 reported lower PC in women with preeclampsia.
There was no significant difference in PDW in the current study. This goes with the previous findings, where Doğan et al14 found no significant difference in PDW among women with severe preeclampsia, mild preeclampsia, and healthy controls. Nonetheless, a significantly higher level of PDW has recently been observed among women with preeclampsia.12,17,24
Although, the current study showed no difference in the MPV between the cases and the controls, the utility of MPV in predicting preeclampsia has been shown in several studies.12,14–16,18–20 It is worth to mention that Yavuzcan et al21 observed no significant difference in the MPV between women with severe preeclampsia, healthy pregnant women, and healthy non-pregnant women. It is noteworthy that some researchers failed to confirm PC and MPV as predictors of preeclampsia,25,26 probably because of the differences in the methods and/or equipment used to obtain hemogram.
Contact of platelets with the injured endothelium activates the coagulation system, which can increase both consumption and bone marrow production of platelets. Enhanced thrombopoiesis produces younger platelets, which are larger (increased MPV) than older platelets, and they are metabolically and enzymatically more active.8
Aside from platelet parameters, there are several biomarkers of preeclampsia including soluble endoglin (sEng) or soluble fms-like tyrosine kinase-1 (sFlt-1).27 One limitation of this study is the lack of a comparative analysis between platelet parameters and sEng/sFlt-1. However, platelet parameters are simple laboratory markers and easy to check during antenatal care.
Conclusion
The current study suggests that PC and the PC/MPV ratio are useful in risk evaluation of preeclampsia. Large scale prospective studies are needed from early pregnancy to reach a definitive conclusion.
Acknowledgments
The study was supported by the Deanship of Scientific Research, Qassim University, KSA, grant number # 3195.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Adam GK, Bakheit KH, Adam I. Maternal and perinatal outcomes of eclampsia in Gadarif Hospital, Sudan. J Obs Gynaecol. 2009;29(7):619–620. doi: 10.1080/01443610903150802. [DOI] [PubMed] [Google Scholar]
- 2.Redman Christopher W, Sargent Ian L. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 3.Walker JJ. Pre-eclampsia. Lancet (London, England) 2000;356(9237):1260–1265. doi: 10.1016/S0140-6736(00)02800-2. [DOI] [PubMed] [Google Scholar]
- 4.Anderson UD, Olsson MG, Kristensen KH, Åkerström B, Hansson SR. Review: biochemical markers to predict preeclampsia. Placenta. 2012;33(Suppl):S42–S47. doi: 10.1016/j.placenta.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Burton GJ, Woods AW, Jauniaux E, Kingdom JCP. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton GJ, Charnock-Jones DS, Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction. 2009;138(6):895–902. doi: 10.1530/REP-09-0092. [DOI] [PubMed] [Google Scholar]
- 7.Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011;31(1):33–46. doi: 10.1016/j.semnephrol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juan P, Stefano G, Antonella S, Albana C. Platelets in pregnancy. J Prenat Med. 2011;5(4):90–92. [PMC free article] [PubMed] [Google Scholar]
- 9.Tzur T, Sheiner E. Is there an association between platelet count during the first trimester and preeclampsia or other obstetric complications later in pregnancy? Hypertens Pregnancy. 2013;32(1):74–82. doi: 10.3109/10641955.2012.704109. [DOI] [PubMed] [Google Scholar]
- 10.Altınbas S, Toğrul C, Orhan A, Yücel M, Danısman N. Increased MPV is not a significant predictor for preeclampsia during pregnancy. J Clin Lab Anal. 2012;26(5):403–406. doi: 10.1002/jcla.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dundar O, Yoruk P, Tutuncu L, et al. Longitudinal study of platelet size changes in gestation and predictive power of elevated MPV in development of pre-eclampsia. Prenat Diagn. 2008;28(11):1052–1056. doi: 10.1002/pd.2126. [DOI] [PubMed] [Google Scholar]
- 12.Freitas LG, Alpoim PN, Komatsuzaki F, Carvalho Md, Dusse LM. Preeclampsia: are platelet count and indices useful for its prognostic? Hematology. 2013;18(6):360–364. doi: 10.1179/1607845413Y.0000000098. [DOI] [PubMed] [Google Scholar]
- 13.Kashanian M, Hajjaran M, Khatami E, Sheikhansari N. Evaluation of the value of the first and third trimester maternal mean platelet volume (MPV) for prediction of pre-eclampsia. Pregnancy Hypertens. 2013;3(4):222–226. doi: 10.1016/j.preghy.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Doğan K, Guraslan H, Senturk MB, Helvacioglu C, İdil S, Ekin M. Can platelet count and platelet indices predict the risk and the prognosis of preeclampsia? Hypertens Pregnancy. 2015;34(4):434–442. doi: 10.3109/10641955.2015.1060244. [DOI] [PubMed] [Google Scholar]
- 15.Han L, Liu X, Li H, et al. Blood coagulation parameters and platelet indices: changes in normal and preeclamptic pregnancies and predictive values for preeclampsia. PLoS One. 2014;9(12):e114488. doi: 10.1371/journal.pone.0114488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanat-Pektas M, Yesildager U, Tuncer N, Arioz DT, Nadirgil-Koken G, Yilmazer M. Could mean platelet volume in late first trimester of pregnancy predict intrauterine growth restriction and pre-eclampsia? J Obstet Gynaecol Res. 2014;40(7):1840–1845. doi: 10.1111/jog.12433. [DOI] [PubMed] [Google Scholar]
- 17.Atilla Karateke, Keskin Kurt Raziye, Ali Baloğlu. Relation of platelet distribution width (PDW) and platelet crit (PCT) to preeclampsia. Ginekol Pol. 2015;86(5):372–375. doi: 10.17772/gp/2425. [DOI] [PubMed] [Google Scholar]
- 18.Moraes D, Munhoz TP, Pinheiro da Costa BE, et al. Immature platelet fraction in hypertensive pregnancy. Platelets. 2016;27(4):333–337. doi: 10.3109/09537104.2015.1101060. [DOI] [PubMed] [Google Scholar]
- 19.Özdemirci Ş, Başer E, Kasapoğlu T, Karahanoğlu E, Kahyaoglu I, Yalvaç S, Tapısız Ö. Predictivity of mean platelet volume in severe preeclamptic women. Hypertens Pregnancy. 2016 Jun;17:1–9. doi: 10.1080/10641955.2016.1185113. Epub. [DOI] [PubMed] [Google Scholar]
- 20.Vilchez G, Londra L, Hoyos LR, Sokol R, Bahado-Singh R. Intrapartum mean platelet volume is not a useful predictor of new-onset delayed postpartum pre-eclampsia. Int J Gynaecol Obstet. 2015;131(1):59–62. doi: 10.1016/j.ijgo.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 21.Yavuzcan A, Cağlar M, Ustün Y, et al. Mean platelet volume, neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in severe preeclampsia. Ginekol Pol. 2014;85(3):197–203. [PubMed] [Google Scholar]
- 22.ACOG technical bulletin. Hypertension in pregnancy. Number 219 – January 1996 (replaces no. 91, February 1986). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1996;53(2):175–183. [PubMed] [Google Scholar]
- 23.ACOG Committee on Obstetric Practice ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77(1):67–75. [PubMed] [Google Scholar]
- 24.Yang SW, Cho SH, Kwon HS, Sohn IS, Hwang HS. Significance of the platelet distribution width as a severity marker for the development of preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2014;175:107–111. doi: 10.1016/j.ejogrb.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Makuyana D, Mahomed K, Shukusho FD, Majoko F. Liver and kidney function tests in normal and pre-eclamptic gestation – a comparison with non-gestational reference values. Cent Afr J Med. 2002;48(5–6):55–59. [PubMed] [Google Scholar]
- 26.Ceyhan T, Beyan C, Başer I, Kaptan K, Güngör S, Ifran A. The effect of pre-eclampsia on complete blood count, platelet count and mean platelet volume. Ann Hematol. 2006;85(5):320–322. doi: 10.1007/s00277-006-0091-7. [DOI] [PubMed] [Google Scholar]
- 27.He Y, Xu B, Song D, Yu F, Chen Q, Zhao M. Correlations between complement system’s activation factors and anti-angiogenesis factors in plasma of patients with early/late-onset severe preeclampsia. Hypertens Pregnancy. 2016 Jun;17:1–11. doi: 10.1080/10641955.2016.1190845. Epub. [DOI] [PubMed] [Google Scholar]


