Abstract
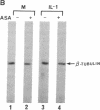
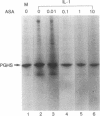
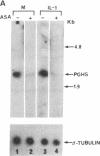
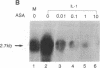
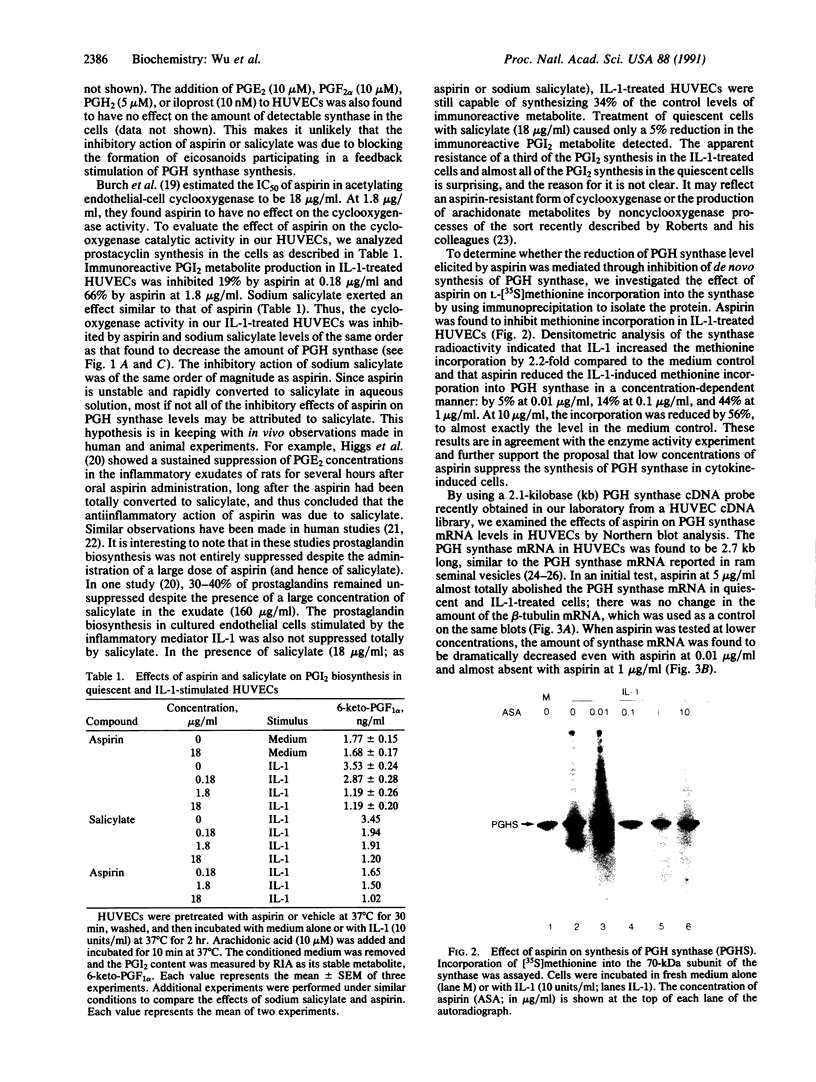
Prostaglandin H (PGH) synthase (EC 1.14.99.1) is a key enzyme in the biosynthesis of prostaglandins, thromboxane, and prostacyclin. In cultured human umbilical vein endothelial cells, interleukin 1 (IL-1) is known to induce the synthesis of this enzyme, thereby raising the level of PGH synthase protein severalfold over the basal level. Pretreatment with aspirin at low concentrations (0.1-1 micrograms/ml) inhibited more than 60% of the enzyme mass and also the cyclooxygenase activity in IL-1-induced cells with only minimal effects on the basal level of the synthase enzyme in cells without IL-1. Sodium salicylate exhibited a similar inhibitory action whereas indomethacin had no apparent effect. Similarly low levels of aspirin inhibited the increased L-[35S]methionine incorporation into PGH synthase that was induced by IL-1 and also suppressed expression of the 2.7-kilobase PGH synthase mRNA. These results suggest that in cultured endothelial cells a potent inhibition of eicosanoid biosynthetic capacity can be effected by aspirin or salicylate at the level of PGH synthase gene expression. The aspirin effect may well be due to degradation of salicylate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrightson C. R., Baenziger N. L., Needleman P. Exaggerated human vascular cell prostaglandin biosynthesis mediated by monocytes: role of monokines and interleukin 1. J Immunol. 1985 Sep;135(3):1872–1877. [PubMed] [Google Scholar]
- Burch J. W., Baenziger N. L., Stanford N., Majerus P. W. Sensitivity of fatty acid cyclooxygenase from human aorta to acetylation by aspirin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5181–5184. doi: 10.1073/pnas.75.10.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DeWitt D. L., Smith W. L. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Fagan J. M., Goldberg A. L. Inhibitors of protein and RNA synthesis cause a rapid block in prostaglandin production at the prostaglandin synthase step. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2771–2775. doi: 10.1073/pnas.83.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G. A., Oates J. A., Hawiger J., Maas R. L., Roberts L. J., 2nd, Lawson J. A., Brash A. R. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983 Mar;71(3):676–688. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Frasier-Scott K., Hatzakis H., Seong D., Jones C. M., Wu K. K. Influence of natural and recombinant interleukin 2 on endothelial cell arachidonate metabolism. Induction of de novo synthesis of prostaglandin H synthase. J Clin Invest. 1988 Dec;82(6):1877–1883. doi: 10.1172/JCI113805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heavey D. J., Barrow S. E., Hickling N. E., Ritter J. M. Aspirin causes short-lived inhibition of bradykinin-stimulated prostacyclin production in man. Nature. 1985 Nov 14;318(6042):186–188. doi: 10.1038/318186a0. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Salmon J. A., Henderson B., Vane J. R. Pharmacokinetics of aspirin and salicylate in relation to inhibition of arachidonate cyclooxygenase and antiinflammatory activity. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1417–1420. doi: 10.1073/pnas.84.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Fagan D., Mudd J., Needleman P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J Biol Chem. 1988 Mar 15;263(8):3550–3553. [PubMed] [Google Scholar]
- Morrow J. D., Harris T. M., Roberts L. J., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990 Jan;184(1):1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Ohki S., Ogino N., Yamamoto S., Hayaishi O. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1979 Feb 10;254(3):829–836. [PubMed] [Google Scholar]
- Raz A., Wyche A., Needleman P. Temporal and pharmacological division of fibroblast cyclooxygenase expression into transcriptional and translational phases. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1657–1661. doi: 10.1073/pnas.86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Wyche A., Siegel N., Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [PubMed] [Google Scholar]
- Rossi V., Breviario F., Ghezzi P., Dejana E., Mantovani A. Prostacyclin synthesis induced in vascular cells by interleukin-1. Science. 1985 Jul 12;229(4709):174–176. doi: 10.1126/science.2409598. [DOI] [PubMed] [Google Scholar]
- Roth G. J., Stanford N., Majerus P. W. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Willis A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971 Jun 23;231(25):235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- Van Der Ouderaa F. J., Buytenhek M., Nugteren D. H., Van Dorp D. A. Acetylation of prostaglandin endoperoxide synthetase with acetylsalicylic acid. Eur J Biochem. 1980 Aug;109(1):1–8. doi: 10.1111/j.1432-1033.1980.tb04760.x. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vane J., Botting R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987 Aug;1(2):89–96. [PubMed] [Google Scholar]
- Wu K. K., Hatzakis H., Lo S. S., Seong D. C., Sanduja S. K., Tai H. H. Stimulation of de novo synthesis of prostaglandin G/H synthase in human endothelial cells by phorbol ester. J Biol Chem. 1988 Dec 15;263(35):19043–19047. [PubMed] [Google Scholar]
- Yokoyama C., Takai T., Tanabe T. Primary structure of sheep prostaglandin endoperoxide synthase deduced from cDNA sequence. FEBS Lett. 1988 Apr 25;231(2):347–351. doi: 10.1016/0014-5793(88)80847-0. [DOI] [PubMed] [Google Scholar]
- Yokoyama C., Tanabe T. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem Biophys Res Commun. 1989 Dec 15;165(2):888–894. doi: 10.1016/s0006-291x(89)80049-x. [DOI] [PubMed] [Google Scholar]