Abstract
Objective:
We investigated acute bone turnover marker (BTM) responses to high-intensity resistance exercise with and without whole-body vibration (WBV) in young men (n=10).
Methods:
In this randomized crossover study, subjects performed 2 protocols separated by 2-week wash out periods: 1) resistance exercise only (RE) (3 sets 10 repetitions 80% 1RM for 9 exercises); and 2) WBV + RE (side-alternating vibration platform 5 intermittent, 1-minute bouts 20 Hz, 3.38 mm peak-to-peak displacement followed by RE). Fasting morning blood draws were taken before RE or WBV (PRE), immediately post RE (IP), and 30 minutes post RE (30P). WBV + RE also had a blood draw after the WBV exposure (POST WBV). Blood samples were analyzed for lactate, hematocrit, bone-specific alkaline phosphatase (Bone ALP, U/L), C-terminal telopeptide of type I collagen (CTX-I, ng/mL) and tartrate-resistant acid phosphatase 5b (TRAP5b, U/L).
Results:
Lactate, hematocrit, and Bone ALP significantly increased (p<0.05) IP for both protocols. Bone resorption markers did not change during RE only. CTX-I significantly decreased POST WBV. TRAP5b increased POST WBV, then significantly decreased at 30P.
Conclusions:
Generally, BTM changes to RE only were not significant when adjusted for hemoconcentration. The WBV stimulus altered bone resorption marker but not bone formation marker responses.
Keywords: Vibration Exercise, Bone Resorption, Bone Formation, Resistance Exercise, Men
Introduction
Whole-body vibration (WBV) induces mechanical oscillations that affect the skeleton both directly by strains sensed by bone cells, and indirectly, by augmenting the force of muscle contraction resulting in greater mechanical loads on bone[1]. There is growing in vitro evidence that vibration signals stimulate bone formation[2] and inhibit osteoclastic activity[2-4]. In vivo animal models have documented decreased bone resorption markers[5,6], increased bone mineral density (BMD)[5,7], and increased bone strength[5] with WBV treatment. Although the human results are less consistent (refer to[8,9] for reviews), recent longitudinal WBV studies have demonstrated beneficial effects on bone health by increasing BMD[10], ameliorating bone loss with unloading[11], and altering bone turnover markers[12].
Bone turnover markers (BTM) are enzymes or degradation products released by bone cells into the circulation, reflecting the bone remodeling processes[13]. BTM have clinical utility as they are reported to be associated with bone microarchitecture in older men[14] and to predict fracture risk[13]. Thus, BTM can be useful indicators of bone metabolism when measured with sufficient control for factors that contribute to the large variability in serum concentrations, such as circadian rhythm, food intake, and exercise[13]. BTM have been measured in response to traditional resistance exercise training[15] and to WBV training[16,17]. In addition, significant changes in BTM concentrations have been documented in response to acute aerobic[18] and resistance exercise[19-22] protocols.
Since the inception of WBV training, much of the literature has focused on the use of WBV in conjunction with resistance exercise, to determine whether WBV confers additional benefit for muscular strength, power, and performance (refer to[23,24] for reviews). Generally, the literature supports that performing WBV with a traditional exercise program has additive effects on muscle performance[24]. Since increasing muscle force production is one of the mechanisms through which WBV can stimulate bone metabolism, adding WBV to a traditional resistance exercise program may potentially exert an additive effect on bone responses[1]. Few studies, however, have investigated the combined effects of WBV and resistance exercise on either acute or chronic bone BTM responses. In a previous training study we conducted in postmenopausal women, resting BTM concentrations were not affected by combined WBV and resistance exercise[25]. More recently, we compared acute BTM responses to the same two protocols implemented in this study, RE only and RE preceded by 5 intermittent bouts of WBV, in young women taking oral contraceptives. We found that the bone resorption marker, C-terminal telopeptide of type I collagen (CTX-I) had a significantly greater reduction in response to the combined WBV and RE protocol versus the RE only condition[22]. However, these findings may not be applicable to young men since there are sex differences in BTM concentrations[26] and oral contraceptives can lower serum BTM concentrations[27].
The purpose of this study was to compare acute BTM responses to resistance exercise (RE) and to resistance exercise combined with WBV (WBV + RE) in young men. We hypothesized that both protocols would stimulate increases in the bone formation marker, bone-specific alkaline phosphatase (Bone ALP). Based on animal models, we expected that adding the WBV stimulus to RE would result in a greater decrease bone resorption markers than the RE alone.
Methods
Subjects
Ten healthy, recreationally active men, aged 20-30 years, not resistance or endurance trained within the previous 12 months, volunteered for this study. Physical activity status was determined by the Bone-Specific Physical Activity Questionnaire (BPAQ)[28] and subjects were included in the study if they had participated in resistance and/or aerobic exercise less than 3 times per week for the previous 12 months. Dual energy x-ray absorptiometry (DXA) was used to assess body composition and bone status (Z-scores) of the subjects. Exclusion criteria for this study were: (1) current smokers; (2) body weight greater than the weight limit of the DXA table (>136 kg); (3) a spine or hip Z-score <-2.0 in conjunction with a secondary cause of osteoporosis29; (4) taking medications that affect bone or muscle metabolism; and (5) contraindications to WBV exposure (e.g., epilepsy, fresh bone fracture, knee or hip implants, bone cancers, open wounds, recent surgery, acute thrombosis). This study was approved from the University of Oklahoma Institutional Review Board and written informed consent was obtained from all participants.
Research design
We utilized a randomized, repeated measures crossover design for this study, where each participant completed 2 acute resistance exercise (RE) protocols in random order: (1) WBV performed immediately before RE (WBV + RE); and (2) RE only. During the first visit to the laboratory, participants completed the informed consent form, a health screening questionnaire, a physical activity readiness questionnaire (PAR-Q), a calcium intake questionnaire[30], and the BPAQ. Body composition and BMD were measured by DXA during the first visit. Finally, participants were familiarized with resistance training equipment and the vibration platform. In the second visit, participants underwent the muscular strength assessment by one repetition maximum (1RM) testing to determine 80% 1RM intensity for the acute exercise protocols that were performed in random order during visits 3 and 4.
Bone mineral density
DXA (GE Lunar Prodigy, enCORE software, version 13.31.016, GE Healthcare, Madison, WI) was used to assess BMD of the total body, AP lumbar spine (L1-L4), and dual proximal femur (femoral neck, trochanter, and total hip). Body composition variables (% body fat, fat mass, bone free lean body mass) were obtained from the total body scan analysis. Urine specific gravity was measured prior to the scans using a refractometer (VEE GEE®, Model CLX-1) to ensure normal hydration status. Participants removed all attenuating materials before the DXA scans. Scan speeds were determined by software based on the thickness of the subject at the navel (thick= >25, standard= 13-25 and thin= <13 cm). In this laboratory, in vivo precision (% coefficient of variation [%CV]) is 0.6% for the total body BMD, 0.9% for the spine, and 0.4-0.8% for the dual proximal femur BMD sites. Scan acquisition and analyses were performed by the same technician.
Muscular strength testing
Strength was assessed by standardized 1RM procedures for leg press, hip extension (right and left), hip abduction (right and left), hip adduction (right and left), shoulder press and seated row resistance exercises using Cybex isotonic weight training equipment (Cybex International, Inc., Medway, MA). Following a 5 minute cycling warm-up, participants performed a set of 8-10 repetitions at 50% of their predicted 1RM. After a 2 minute rest, subjects completed a single repetition at 80% of their predicted 1RM; thereafter, the weight was progressively increased after each successful lift until a failed attempt occurred. Two minutes rest was given between the lifts, and the 1RM for each exercise was obtained within 5 attempts. This muscular strength testing protocol has been found to be reliable in our laboratory, with intraclass correlation coefficients >0.91[31].
Exercise protocols
Participants completed the 2 exercise protocols, in random order, and separated by a 2 week washout period to eliminate potential 48-72 hour last bout effects. Also, the exercise protocols were performed in the morning (~8:00-10:00 h) after an overnight fast. Figure 1 depicts the timing of the blood draws for each protocol. Participants were instructed to be well hydrated and to eat a good meal with about 400-600 grams of carbohydrate about 18:00 h and a snack about 20:00 h on the evening before each test session. The RE only protocol included 3 sets of 10 repetitions of each exercise at 80% 1RM. The whole-body vibration plus resistance exercise (WBV + RE) included the RE protocol described above preceded by 5 intermittent WBV bouts (1 minute exposures separated by 1 minute rest) at 20 Hz with 3.38 mm peak-to-peak displacement using a Vibraflex Vibration Platform (Orthometrix, Inc., Naples, FL). Total workload for each condition was calculated by summing the workloads (weight lifted × total number of repetitions) for the resistance exercises. The peak acceleration for each vibration bout, calculated using the formula G-Force= (A(2πf)2)/9.81, was approximately 2.7 g[32]. Subjects stood barefoot on the platform with knees flexed at 30o to minimize the transmission of vibrations to the head for safety purposes[33]. They placed their second toe in line with the dot midway between foot positions 1 and 2.
Figure 1.
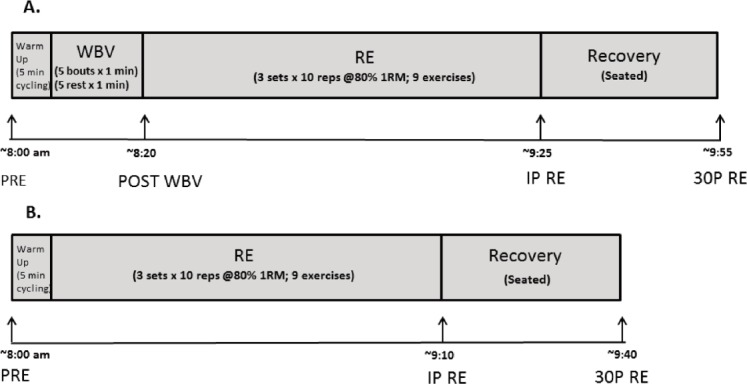
Timeline for blood draws for the WBV + RE (A.) and RE (B.) protocols.
Blood sampling and biochemical assays
Acute bone marker responses were assessed in the morning after an overnight fast (minimum 8 hours) for both protocols at the same time of the day, beginning at about 8:00 h and ending at about 10:00. Four venipuncture blood samples were obtained from an antecubital vein using butterfly needles and 7 mL serum separator tubes during WBV + RE; at rest before WBV (PRE); immediately post WBV (POST WBV), immediately post resistance exercise (IP), and 30 minutes post RE (30P). Three blood samples were obtained for the RE only protocol; at rest before the exercise (PRE), immediately post RE (IP), and 30 minutes post RE (30P). Blood samples were allowed to clot, then centrifuged to obtain the serum, which was transferred into microtubes, and stored in a -84°C freezer until the assays were performed. All samples were thawed only once before performing the assays.
Lactate was measured at PRE, POST WBV, and IP time points using a Lactate Plus Portable Lactate Analyzer (Nova Biomedical, Waltham, MA). Hematocrit (Hct) was measured in duplicate using a microhematocrit centrifuge (StatSpin, Norwood, MA) to estimate plasma volume changes (%ΔPV) using the following equation: %ΔPV= (100/(100-Hct Pre) × 100 ((Hct Pre-Hct Post) / Hct Post)[34]. Since plasma volume tends to decrease during acute exercise, changes in BTM concentrations could be the result of hemoconcentration rather than a direct response of bone cells[35]. In order to assist with interpretation, we used the following formula to adjust BTM concentrations for hemoconcentration: Corrected BTM Concentration = Uncorrected BTM concentration × ((100 + %PV∆)/100). We were unable to obtain a Hct for the RE Pre sample for 1 subject and a POST WBV sample for 1 subject, therefore, n=9 for calculated %PV∆.
Serum Bone ALP concentrations were determined in duplicate by MicroVue™ BAP EIA kits (Quidel Corporation, San Diego CA). Intra-assay CVs were 0.7-10.9% and inter-assay CVs ranged from 10-15.5%. TRAP5b was measured in duplicate using MicroVue™ TRAP5b EIA kits (Quidel Corporation, San Diego, CA). The intra-assay CVs ranged from 0.5-4.4% and inter-assay CVs were 6.5-7.7% for TRAP5b. Serum CTX-I (Immunodiagnostic Systems, Ltd., Scottsdale, AZ) was measured in duplicate using Serum CrossLaps® ELISA kits. The intra-assay CVs ranged from 0.2-3.5% and inter-assay CVs ranged from 0.7-4.4% for serum CTX-I. The CTX-I/TRAP5b ratio was used as a bone resorption index since CTX-I is an indicator of osteoclast activity and TRAP5b is an indicator of osteoclast number[36].
Data analyses
Data are reported as means ± standard error (SE). IBM SPSS Statistics 19 (SPSS Inc., Chicago, IL) was used for the statistical analyses. All the descriptive statistics were calculated for the dependent variables for each condition and time point. All data were determined to be normally distributed by the Kolmogorov-Smirnov test. Two (condition) × 2 (time) repeated measures ANOVA was computed to assess lactate responses and percent changes in BTM concentrations. Two (condition) × 3 (time) repeated measures ANOVA was used to determine significant BTM and hematocrit responses. When a significant condition × time interaction occurred, the model was decomposed using one way (time) repeated measures ANOVA with a Bonferroni post hoc test separately for each condition. The effect of the vibration bouts on dependent variables was determined by comparing PRE and POST WBV time points with paired t-tests. The level of significance was set at p≤0.05.
Results
Subject characteristics
[Table 1] shows the physical characteristics and bone density data. One subject had trochanter Z-scores below the expected range for age (-2.1 left, -2.3 right)[29]. [Table 2] shows the 1RM muscular strength descriptive data for each resistance exercise and the total workload (sum of weight lifted × total number of repetitions for the resistance exercises) for each session. There was no significant difference between WBV + RE and RE for the total workload.
Table 1.
Subject characteristics (n=10).
| Variables | Mean ± SE |
| Age (yr) | 23.08±0.58 |
| Weight (kg) | 76.81±5.03 |
| Height (cm) | 179.00±3.02 |
| % Body fat | 20.09±2.04 |
| Fat mass (kg) | 16.18±2.48 |
| Bone free LBM (kg) | 58.26±3.09 |
| Calcium intake (mg/d) | 1115±326 |
| BPAQ scores | |
| Current | 9.18±1.82 |
| Past | 41.82±9.06 |
| BMD (g/cm2) | |
| Total body | 1.249±0.037 |
| Lumbar spine (L1-L4) | 1.213±0.044 |
| Left total hip | 1.164±0.041 |
| Left femoral neck | 1.165±0.050 |
| Left trochanter | 0.945±0.042 |
LBM, lean body mass; BPAQ, Bone-Specific Physical Activity Questionnaire; BMD, bone mineral density.
Table 2.
1RM muscular strength and total workload for each condition (n=10).
| Resistance exercise (kg) | Mean ± SE |
|---|---|
| Leg press | 202.7±21.4 |
| Hip extension (left) | 108.6±7.9 |
| Hip extension (right) | 110.8±7.6 |
| Hip abduction (left) | 59.5±2.5 |
| Hip abduction (right) | 61.5±2.8 |
| Hip adduction (left) | 70.8±6.6 |
| Hip adduction (right) | 70.8±6.3 |
| Seated row | 59.8±2.9 |
| Shoulder press | 65.4±7.3 |
| Total Workload (RE) | 18,903.3±971.3 |
| Total Workload (WBV+ RE) | 19,028.6±979.3 |
Total Workload = sum of workloads (weight lifted × repetitions x sets) for all exercises.
Hematocrit and plasma volume changes
There were no significant differences between the two conditions for hematocrit or lactate responses (Table 3). Both protocols showed significant increases in hematocrit and blood lactate at IP compare to Pre (p<0.05), as well as at POST WBV compared to PRE for WBV + RE (Table 4). Plasma volume decreased from PRE to IP for both RE (-10.74% ± 2.21) and WBV + RE (-12.05% ± 4.71); and it also decreased immediately post vibration (-14.61% ± 5.73) for the WBV + RE protocol.
Table 3.
Hematocrit, lactate, and bone turnover marker responses before (PRE), immediately after RE (IP), and 30 min after RE (30P) for conditions (n=10; Mean ± SE).
| Variables | WBV+RE | RE | Significant Effects | p |
|---|---|---|---|---|
| Hematocrit (%)a | ||||
| PRE | 46.6 ± 1.4 | 48.2 ± 0.9 | Time | 0.004 |
| IP | 49.9 ± 1.3 | 51.1 ± 1.2 | IP>PRE | 0.025 |
| 30P | 47.4 ± 1.2 | 46.9 ± 0.7 | IP>30P | 0.005 |
| Lactate (mmol/L) | ||||
| PRE | 1.11 ± 0.16 | 1.53 ± 0.41 | Time | <0.001 |
| IP | 9.40 ± 0.91 | 9.08 ± 0.58 | IP>PRE | |
| Bone ALP (U/L) | ||||
| PRE | 42.08 ± 5.33 | 42.41 ± 4.45 | Time | 0.003 |
| Uncorr IP | 45.47 ± 5.41 | 47.54 ± 6.00 | IP >PRE | 0.013 |
| Uncorr 30P | 41.79 ± 5.60 | 43.03 ± 5.81 | IP>30P | 0.043 |
| PRE a | 42.57 ± 5.93 | 43.16 ± 4.90 | N.S. | |
| Corr IP a | 42.15 ± 7.34 | 42.92 ± 5.45 | ||
| Corr 30P a | 42.23 ± 7.14 | 45.81 ± 6.43 | ||
| TRAP5b (U/L) | ||||
| PRE | 3.21 ± 0.39 | 2.59 ± 0.22 | Condition, Time | 0.047, 0.036 |
| Uncorr IP | 3.27 ± 0.39 | 2.74 ± 0.24 | Condition × Time | 0.031 |
| Uncorr 30P | 2.98 ± 0.34 | 2.66 ± 0.21 | WBV+RE PRE, IP>30P | 0.016,0.014 |
| PRE a | 3.24 ± 0.44 | 2.57 ± 0.24 | Time | 0.024 |
| Corr IP a | 2.92 ± 0.45 | 2.39 ± 0.23 | N.S. post hoc | |
| Corr 30P a | 2.98 ± 0.45 | 2.71 ± 0.22 | ||
| CTX-I (ng/mL) | ||||
| PRE | 0.868 ± 0.127 | 0.743 ± 0.089 | N.S. | |
| Uncorr IP | 0.853 ± 0.110 | 0.731 ± 0.065 | ||
| Uncorr 30P | 0.785 ± 0.125 | 0.716 ± 0.076 | ||
| PRE a | 0.890 ± 0.140 | 0.756 ± 0.099 | N.S. | |
| Corr IP a | 0.779 ± 0.109 | 0.663 ± 0.065 | ||
| Corr 30P a | 0.805 ± 0.151 | 0.781 ± 0.092 | ||
| CTX-I/TRAP5b ratio | ||||
| PRE | 0.274 ± 0.022 | 0.287 ± 0.022 | N.S. | |
| Uncorr IP | 0.263 ± 0.016 | 0.269 ± 0.013 | ||
| Uncorr 30P | 0.254 ± 0.021 | 0.274 ± 0.026 | ||
| PRE a | 0.279 ± 0.024 | 0.293 ± 0.023 | N.S. | |
| Corr IP a | 0.272 ± 0.016 | 0.278 ± 0.011 | ||
| Corr 30P a | 0.261 ± 0.023 | 0.288 ± 0.025 | ||
RE, resistance exercise; WBV, whole-body vibration; Bone ALP, bone-specific alkaline phosphatase; CTX-I, C-terminal telopeptides of type I collagen; TRAP5b, tartrate-resistant acid phosphatase 5b; Uncorr - uncorrected concentrations; Corr - corrected for plasma volume changes;
n=9, N.S. - not significant.
Table 4.
Hematocrit, lactate and bone turnover marker concentrations before (PRE) and immediately after whole-body vibration (POST WBV) (n=10; Mean ± SE).
| Variables | PRE | POST WBV | p | % Change |
|---|---|---|---|---|
| Hematocrit (%)a | 46.7±1.4 | 50.2±1.3 | 0.032 | 8.2±3.4 |
| Lactate (mmol/L) | 1.11±0.15 | 1.38±0.13 | 0.044 | 37.8±13.9 |
| Bone ALP (U/L) | ||||
| Uncorr | 42.08±5.32 | 43.75±6.03 | 0.093 | 3.0±1.5 |
| Corr a | 42.03±5.95 | 37.53±5.88 | 0.062 | -11.4±5.7 |
| TRAP5b (U/L) | ||||
| Uncorr | 3.21±0.39 | 3.45±0.44 | 0.013 | 6.7±2.9 |
| Corr a | 3.08±0.42 | 2.75±0.38 | 0.033 | -9.9±4.5 |
| CTX-I (ng/ml) | ||||
| Uncorr | 0.868±0.127 | 0.805±0.012 | 0.046 | -6.8±3.1 |
| Corr a | 0.767±0.087 | 0.600±0.071 | 0.002 | -20.8±4.6 |
Bone ALP, bone-specific alkaline phosphatase; CTX-I, C-terminal telopeptides of type I collagen; TRAP5b, tartrate-resistant acid phosphatase 5b; Uncorr - uncorrected concentrations; Corr - corrected for plasma volume changes;
n=9.
Bone turnover markers
There was a significant time effect for Bone ALP (p<0.01), which increased from PRE to IP for both conditions (Table 3). As shown in Figure 2A, percent changes in Bone ALP from PRE to IP for RE and WBV + RE were similar in magnitude to the plasma volume changes. The Bone ALP response was no longer significant after correcting the concentrations for plasma volume changes. Also, Bone ALP concentrations (both uncorrected and corrected for PV changes) at the POST WBV time point were not significantly different from PRE (Table 4).
Figure 2.
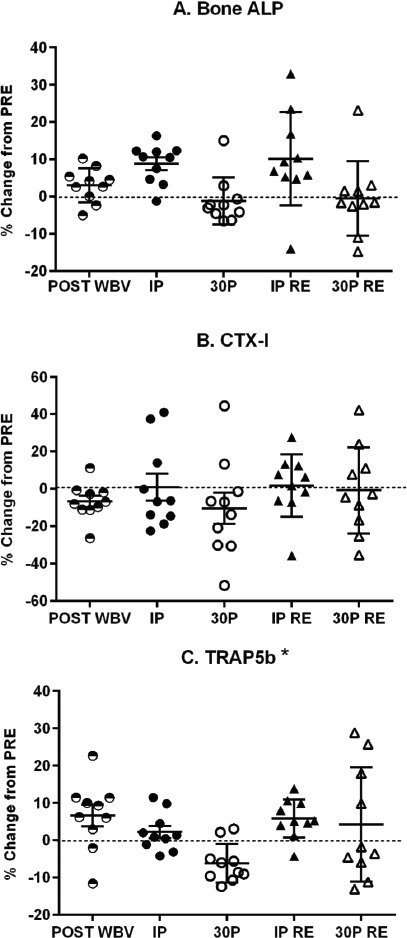
Individual percent changes (%) in uncorrected bone turnover marker concentrations from pre-exercise (PRE) for WBV + RE (circles) and RE (triangles) protocols. Bars represent mean ± SE. Panel A - Bone ALP - Bone-specific alkaline phosphatase; Panel B - CTX-I - C-terminal telopeptide of type I collagen; Panel C - TRAP5b - Tartrate-resistant acid phosphatase 5b; WBV - Whole-body Vibration; RE - Resistance Exercise. * p<0.05 significant condition effect.
CTX-I concentrations (both uncorrected and corrected for PV changes) significantly decreased immediately after the WBV stimulus (POST WBV) (Table 4); otherwise, there were no significant CTX-I responses to resistance exercise for either condition (Tables 3). There were no significant differences between conditions for percent changes in CTX-I (Figure 2B).
TRAP5b showed significant (p<0.05) condition, time and condition × time interaction effects (Table 3). Marginal means for the conditions showed that TRAP5b was significantly higher in the WBV + RE condition. Post hoc analyses for the WBV + RE condition determined that the 30P time point was significantly (p<0.05) lower than PRE and IP, whereas TRAP5b did not change in the RE condition. Correcting TRAP5b concentrations for hemoconcentration retained the significant time effect but eliminated the significant condition and condition × time interaction effects. Percent change in TRAP5b (uncorrected) showed a significant condition effect (p<0.05) as it decreased from PRE to 30P for WBV + RE (Figure 2C). TRAP5b at the POST WBV time point significantly (p<0.05) increased from PRE, but was significantly (p<0.05) lower than PRE when corrected for plasma volume change (Table 4). The CTX-I/TRAP5b ratio did not have any significant condition, time, or condition × time interaction effects (Table 3).
Discussion
To date, there are limited data in the literature on acute BTM responses to WBV alone or in combination with resistance exercise. Recently, we reported that an acute bout of combined WBV and RE significantly altered bone resorption marker concentrations in young women taking oral contraceptives[22]. The current preliminary study adds to the literature by reporting BTM responses to the same WBV + RE protocol in young men, whose responses may differ due to sex differences in BTM concentrations[26]. We found an increase in the bone formation marker, Bone ALP, immediately post RE for both protocols; however, these responses were no longer significant when adjusted for hemoconcentration. Generally, the WBV + RE protocol had greater effects on the bone resorption markers. CTX-I showed a significant decrease immediately post WBV, even when corrected for plasma volume shifts. TRAP5b had a small but significant increase immediately post WBV, followed by a significant decrease at 30P for the WBV + RE protocol. Adjusting for hemoconcentration eliminated the significant differences between conditions, and resulted in a significant decrease in TRAP5b at the POST WBV time point. As previously mentioned, decreased plasma volume and the resulting hemoconcentration during exercise would artificially raise circulating BTM concentrations without any direct response from the bone cells[35]. Therefore, adjusting the concentrations for plasma volume changes accounts for this source of variability and would provide greater insight into the bone responses. Although this analysis resulted in some interesting patterns in corrected BTM responses, we interpret these data cautiously since the sample size was reduced to 9 for those analyses.
Other potential mechanisms for acute alterations in BTM concentrations in response to mechanical loading include lactate acidosis, and changes in serum concentrations of hormones that affect bone metabolism. Metabolic acidosis can affect calcium metabolism as shown by Ashizawa et al.[37] who found that a single bout of strenuous resistance exercise that increased blood lactate and decreased urine pH also decreased serum ionized calcium and increased urinary calcium excretion in young men. However, the bone resorption marker, urinary deoxypyridinoline, showed a concomitant decrease suggesting the increased urinary calcium excretion was independent of osteoclast-mediated bone resorption. Interestingly, Cardinale et al.[38] found that 5 days of WBV exposures decreased urinary calcium excretion and urine CTX levels in participants with metabolic acidosis induced by high dietary protein intakes. Parathyroid hormone (PTH) has been shown to increase in response to aerobic exercise[39,40] but it was reported to decrease after resistance exercise[21,37]. The different PTH response patterns for exercise mode may be confounded by circadian rhythm as the resistance exercise studies measured PTH in the late afternoon[37] or did not test all participants at the same time of day[21].
Diurnal variation is an important source of biological variation for serum CTX-I concentrations, which exhibit large decreases from early morning (8:00 h) to late morning (11:00 h)[41]. Fasting reduced the magnitude of the decrease from ± 40.5% (non-fasting) to ± 16.5% of the mean 24 hour value[41]. It is possible that the 6.7% decrease in CTX-I immediately post WBV reflects the diurnal pattern; however, this was followed by an increase so that IP was similar to the pre-exercise concentrations. Since we did not have a resting control condition, we are not able to determine the magnitude of diurnal change that may have occurred in the 15-20 minutes separating the PRE and POST WBV blood draws.
Whipple et al.[19] conducted one of the few acute randomized crossover studies comparing BTM responses to moderate intensity resistance exercise and a control condition in young men. They reported no significant change in Bone ALP concentrations, but the bone resorption marker, serum N-terminal telopeptide of type I collagen, significantly decreased 1 hour post resistance exercise. This response may be explained by diurnal variation since the same pattern also was observed in the control condition. Our current findings are consistent with the previous data in young women[22] for the acute effect of WBV exposures, as CTX-I decreased and TRAP5b (uncorrected) increased at POST WBV compared to PRE in both studies. However, we observed different response patterns for the post RE time points with women exhibiting a decrease in CTX-I (from PRE) for the WBV + RE protocol and men having no change in CTX-I concentrations post resistance exercise. In the Sherk et al.[22] study, women decreased TRAP5b at 30P for both protocols, whereas in this study, men decreased TRAP5b only for WBV + RE condition. In both studies, plasma volume shifts contributed to the TRAP5b responses. Another important consideration is that oral contraceptive use by the women may have affected their BTM responses since oral contraceptive use can affect resting BTM levels[27].
In vitro animal models provide evidence supporting a suppressive effect of mechanical vibration on bone resorption by decreasing osteoclast formation[3,4]. WBV also decreased bone resorption markers in in vivo rodent models[5,6]. The human studies are less consistent as many training studies on the effects of WBV on bone have produced conflicting results (refer to[8,9] for reviews). Recently, Elmantaser et al.[17] conducted an 8 week training study in young men comparing the effects of low intensity (0.3 g) vertical vs. high intensity (2.6 to 3.8 g) side-alternating vibration platforms on resting BTM concentrations. They found a significant decrease in CTX-I in response to the high-intensity side-alternating WBV treatment only. Our WBV protocol settings were similar to Elmantaser’s side-alternating vibration protocol as we used a frequency of 20 Hz (vs. 18 and 22 Hz) and a peak-to-peak displacement of 3.38 mm (vs. 4 mm) to induce a 2.7 g load. However, we also included a resistance exercise component to test the hypothesis that adding WBV to regular resistance exercise would yield greater responses. Presently, it is unclear whether there is any advantage to adding WBV to RE for bone adaptations. Li et al.[42] used a hindlimb unloading rodent model to compare combined WBV + RE to each treatment separately on bone characteristics and BTM. Their findings support an interaction effect for the two treatments as bone density and bone strength were better maintained in the WBV + RE hindlimb unloaded rats than in the other treatment groups. Also, the bone formation marker, Bone ALP, was significantly higher in the combined WBV + RE treatment group compared to the other groups (control, hindlimb unloaded + WBV or RE or standing). However, these positive findings may not translate to humans. Stolzenberg et al.[43] investigated the effectiveness of resistance training combined with either WBV or balance training for 9 months in osteopenic postmenopausal women. The WBV protocol involved 3 intermittent bouts (4 minutes total) on a side-alternating vibration platform that progressed in intensity from 3.9 to 10.9 g during the study. Both groups showed significant improvements in tibia trabecular BMD, therefore, the WBV stimulus did not confer additional benefits to resistance training alone.
There are several limitations to our study. We did not include a control condition in the randomized crossover experimental design because of the assay costs. We did control for circadian rhythm, pre-test food intake, last exercise bout effect, and physical activity status to reduce these sources of variation on the BTM concentrations[13]. TRAP5b does not have a diurnal variation[26], but CTX-I concentrations have a large diurnal variation, with increases during the night followed by decreases during the day[41]. Food intake also affects CTX-I as shown by a greater daytime decrease in fed individuals and an attenuated decrease in fasted individuals[41]. Previous studies reported that the pre-exercise dietary status (fed vs. fasted) had only minor effects on the acute BTM responses to exercise[18,21]. However, energy restricted diets have been reported to affect BTM markers at rest[44], therefore, it may be helpful in future studies to assess energy balance in the participants.
In conclusion, the findings of this preliminary study suggest that acute WBV treatment alter bone resorption marker concentrations in young men, but it does not seem to have an additive effect on lactate, hematocrit, or the bone formation marker responses to resistance exercise. Most of the BTM responses were attributed to plasma volume shifts that occurred during the protocols, with the exception of the decrease in CTX-I immediately post the WBV bouts. More research is needed to compare the effects of different types of WBV devices, different frequencies, amplitudes, and accelerations on bone metabolism.
Acknowledgements
This study was funded in part by a College of Arts & Sciences Faculty Enrichment Grant.
Footnotes
Edited by: J. Rittweger
Refrences
- 1.Judex S, Rubin CT. Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J Musculoskelet Neuronal Interact. 2010;10:3–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Y, Guan X, Liu T, Wang X, Yu M, Yang G, Wang H. Whole body vibration improves osseointegration by up-regulating osteoblastic activity but down-regulating osteoblast-mediated osteoclastogenesis via ERK1/2 pathway. Bone. 2015;74:17–24. doi: 10.1016/j.bone.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effects of low-magnitude, high frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–15. doi: 10.1016/j.bone.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni RN, Voglewede PA, Liu D. Mechanical vibration inhibits osteoclast formation by reducing DC-STAMP receptor expression in osteoclast precursor cells. Bone. 2013;57:493–98. doi: 10.1016/j.bone.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenger KH, Freeman JD, Fulzele S, Immel DM, Powell BD, Molitor P, Chao YJ, Gao H-S, Elsalanty M, Hamrick MW, Isales CM, Yu JC. Effect of whole-body vibration on bone properties in aging mice. Bone. 2010;47:746–55. doi: 10.1016/j.bone.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Nowak A, Lochynski D, Pawlak M, Romanowski W, Krutki P. High-magnitude whole-body vibration effects on bone resorption in adult rats. Aviat Space Environ Med. 2014;85:518–21. doi: 10.3357/asem.3796.2014. [DOI] [PubMed] [Google Scholar]
- 7.Pasqualini M, Lavet C, Elbadaoui M, Vanden-Bossche A, Laroche N, Gnyubkin V, Vico L. Skeletal site-specific effects of whole body vibration in mature rats: from deleterious to beneficial frequency-dependent effects. Bone. 2013;55:69–77. doi: 10.1016/j.bone.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Lau RWK, Liao LR, Yu F, Teo T, Chung RCK, Pang MYC. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25:975–88. doi: 10.1177/0269215511405078. [DOI] [PubMed] [Google Scholar]
- 9.Wysocki A, Butler M, Shamliyan T, Kane RL. Whole-body vibration therapy for osteoporosis: State of the science. Ann Intern Med. 2011;155:680–86. doi: 10.7326/0003-4819-155-10-201111150-00006. [DOI] [PubMed] [Google Scholar]
- 10.Von Stengel S, Kemmler W, Bebenek M, Engelke K, Kalendar WA. Effects of whole-body vibration training on different devices on bone mineral density. Med Sci Sports Exerc. 2011;43:1071–79. doi: 10.1249/MSS.0b013e318202f3d3. [DOI] [PubMed] [Google Scholar]
- 11.Belavy DL, Beller G, Armbrecht G, Perschel FH, Fitzner R, Bock O, Borst H, Degner C, Gast U, Felsenberg D. Evidence for an additional effect of whole-body vibration above resistive exercise alone in preventing bone loss during prolonged bed rest. Osteoporos Int. 2011;22:1581–91. doi: 10.1007/s00198-010-1371-6. [DOI] [PubMed] [Google Scholar]
- 12.Corrie H, Brooke-Wavell K, Mansfield NJ, Cowley A, Morris R, Masud T. Effects of vertical and side-alternating vibration training on fall risk factors and bone turnover in older people at risk of falls. Age Ageing. 2014:1–8. doi: 10.1093/ageing/afu136. doi:10.1093/ageing/aful36. [DOI] [PubMed] [Google Scholar]
- 13.Szulc P, Bauer DC, Eastell R. Chapter 35 Biochemical markers of bone turnover in osteoporosis. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Eighth edition. Ames, Iowa: John Wiley & Sons, Inc; 2013. pp. 297–306. [Google Scholar]
- 14.Chaitou A, Boutroy S, Vilayphiou N, Munoz F, Delmas PD, Chapurlat R, Szulc P. Association between bone turnover rate and bone microarchitecture in men: The STRAMBO study. J Bone Miner Res. 2010;25:2313–23. doi: 10.1002/jbmr.124. [DOI] [PubMed] [Google Scholar]
- 15.Lester ME, Urso ML, Evans RK, Pierce JR, Spiering BA, Maresh CM, Hatfield DL, Kraemer WJ, Nindl BC. Influence of exercise mode and osteogenic index on bone biomarker responses during short-term physical training. Bone. 2009;45:768–776. doi: 10.1016/j.bone.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Turner S, Torode M, Climstein M, Naughton G, Greene D, Baker MK, Fiatarone Singh MA. A randomized controlled trial of whole-body vibration exposure on markers of bone turnover in postmenopausal women. J Osteoporos. 2011 doi: 10.4061/2011/710387. doi:10.4061/2011/710387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmantaser M, McMillan M, Smith K, Khanna S, Chantler D, Panarelli M, Ahmed SF. A comparison of the effect of two types of vibration exercise on the endocrine and musculoskeletal system. J Musculoskelet Neuronal Interact. 2012;12:144–54. [PubMed] [Google Scholar]
- 18.Scott JR, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Effect of fasting versus feeding on the bone metabolic response to running. Bone. 2012;51:990–99. doi: 10.1016/j.bone.2012.08.128. [DOI] [PubMed] [Google Scholar]
- 19.Whipple TJ, Le BH, Demers LM, Chinchilli VM, Petit MA, Sharkey N, Williams NI. Acute effects of moderate intensity resistance exercise on bone cell activity. Int J Sports Med. 2004;25:496–501. doi: 10.1055/s-2004-820942. [DOI] [PubMed] [Google Scholar]
- 20.Bemben DA, Palmer IJ, Abe T, et al. 2007 Effects of a single bout of low intensity KAATSU resistance training on markers of bone turnover in young men. Int J KAATSU Train Res. 2007;3:21–6. [Google Scholar]
- 21.Rogers RS, Dawson AW, Wang Z, Thyfault JP, Hinton PS. Acute response of serum markers of bone turnover to a single-bout of resistance training or plyometrics. J Appl Physiol. 2011;11:1353–60. doi: 10.1152/japplphysiol.00333.2011. [DOI] [PubMed] [Google Scholar]
- 22.Sherk VD, Chrisman C, Smith J, Young KC, Singh H, Bemben MG, Bemben DA. Acute bone marker responses to whole-body vibration and resistance exercise in young women. J Clin Densitom. 2012;16:104–9. doi: 10.1016/j.jocd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin PJ, Rhea MR. Effects of vibration training on muscle strength: a meta-analysis. J Strength Cond Res. 2010;24:548–556. doi: 10.1519/JSC.0b013e3181c09d22. [DOI] [PubMed] [Google Scholar]
- 24.Osawa Y, Oguma Y, Ishii N. The effects of whole-body vibration on muscle strength and power: a meta-analysis. J Musculoskelet Neuronal Interact. 2013;13:380–390. [PubMed] [Google Scholar]
- 25.Bemben DA, Palmer IJ, Bemben MG, Knehans AW. Effects of combined whole-body vibration and resistance training on muscular strength and bone metabolism in postmenopausal women. Bone. 2010;47:650–656. doi: 10.1016/j.bone.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Lombardi G, Lanteri P, Colombini A, Banfi G. Blood biochemical markers of bone turnover: pre-analytical and technical aspects of sample collection and handling. Clin Chem Lab Med. 2012;50:771–89. doi: 10.1515/cclm-2011-0614. [DOI] [PubMed] [Google Scholar]
- 27.Hermann M, Seibel MJ. The effects of hormonal contraceptives on bone turnover markers and bone health. Clin Endocrinol. 2010;72:571–83. doi: 10.1111/j.1365-2265.2009.03688.x. [DOI] [PubMed] [Google Scholar]
- 28.Weeks BK, Beck BR. The BPAQ: a bone-specific physical activity assessment instrument. Osteoporos Int. 2008;19:1567–77. doi: 10.1007/s00198-008-0606-2. [DOI] [PubMed] [Google Scholar]
- 29.Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive summary of the 2013 International Society for Clinical Densitometry position development conference on bone densitometry. J Clin Densitom. 2013;16:455–66. doi: 10.1016/j.jocd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Musgrave KO, Giambalvo L, Leclerc HL, Cook RA. Validation of a quantitative food frequency questionnaire for rapid assessment of dietary calcium intake. J Am Diet Assoc. 1989;89:1484–88. [PubMed] [Google Scholar]
- 31.Seo D, Kim E, Fahs CA, Rossow L, Young K, Ferguson SL, Thiebaud R, Sherk VD, Loenneke JP, Kim D, Lee M, Choi K, Bemben DA, Bemben MG, So W. Reliability of the one-repetition maximum test based on muscle group and gender. J Sports Sci Med. 2012;11:221–25. [PMC free article] [PubMed] [Google Scholar]
- 32.Rauch F, Sievanen H, Boonen S, Cardinale M, Degens H, Felsenberg D, Roth J, Schoenau E, Verschueren S, Rittweger J. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10:193–198. [PubMed] [Google Scholar]
- 33.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108:877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 34.Van Beaumont W. Evaluation of hemoconcentration from hematocrit measures. J Appl Physiol. 1972;32:712–13. doi: 10.1152/jappl.1972.32.5.712. [DOI] [PubMed] [Google Scholar]
- 35.Brahm H, Piehl-Aulin K, Ljunghall S. Bone metabolism during exercise and recovery: the influence of plasma volume and physical fitness. Calcif Tissue Int. 1997;61:192–98. doi: 10.1007/s002239900322. [DOI] [PubMed] [Google Scholar]
- 36.Rissanen JP, Suominen MI, Peng Z, Halleen JM. Secreted tartrate-resistant acid phosphatase 5b is a marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int. 2008;82:108–15. doi: 10.1007/s00223-007-9091-4. [DOI] [PubMed] [Google Scholar]
- 37.Ashizawa N, Fujimura R, Tokuyama K, Suzuki M. A bout of resistance exercise increases urinary calcium independently of osteoclastic activation in men. J Appl Physiol. 1997;83:1159–1163. doi: 10.1152/jappl.1997.83.4.1159. [DOI] [PubMed] [Google Scholar]
- 38.Cardinale M, Leiper J, Farajian P, Heer M. Whole-body vibration can reduce calciuria induced by high protein intakes and may counteract bone resorption: A preliminary study. J Sports Sci. 2007;25:111–119. doi: 10.1080/02640410600717816. [DOI] [PubMed] [Google Scholar]
- 39.Scott JPR, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Treadmill running reduces parathyroid hormone concentrations during recovery compared with a nonexercising control group. J Clin Endocrinol Metab. 2014;99:1774–1782. doi: 10.1210/jc.2013-3027. [DOI] [PubMed] [Google Scholar]
- 40.Shea KL, Barry DW, Sherk VD, Hansen KC, Wolfe P, Kohrt WM. Calcium supplementation and parathyroid hormone response to vigorous walking in postmenopausal women. Med Sci Sports Exerc. 2014;46:2007–2013. doi: 10.1249/MSS.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31:57–61. doi: 10.1016/s8756-3282(02)00791-3. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Tan C, Wu Y, Ding Y, Wang H, Chen W, Zhu Yu, Ma H, Yang H, Liang W, Jiang S, Wang D, Wang L, Tang G, Wang J. Whole-body vibration and resistance exercise prevent long-term hindlimb unloading-induced bone loss: independent and interactive effects. Eur J Appl Physiol. 2012;112:3743–53. doi: 10.1007/s00421-012-2355-3. [DOI] [PubMed] [Google Scholar]
- 43.Stolzenberg N, Belavy DL, Beller G, Armbrecht G, Semler J, Felsenberg D. Bone strength and density via pQCT in post-menopausal osteopenic women after 9 months resistive exercise with whole body vibration or proprioceptive exercise. J Musculoskelet Neuronal Interact. 2013;13:66–76. [PubMed] [Google Scholar]
- 44.Ihle R, Loucks AB. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004;19:1231–40. doi: 10.1359/JBMR.040410. [DOI] [PubMed] [Google Scholar]


