Abstract
Objectives:
The purpose of the present study was to evaluate the effects of superimposed electromyostimulation (E) during cycling on myokines and markers of muscle damage, as E might be a useful tool to induce a high local stimulus to skeletal muscle during endurance training without performing high external workloads.
Methods:
13 subjects participated in three experimental trials each lasting 60 min in a randomized order. 1) Cycling (C), 2) Cycling with superimposed E (C+E) and 3) E. Interleukin-6 (IL-6), brain-derived neurotrophic factor (BDNF), creatine kinase (CK) and myoglobin were determined before (pre) and 0’, 30’, 60’, 240’ and 24h after each intervention.
Results:
Only C+E caused significant increases in levels of CK and myoglobin. BDNF and IL-6 significantly increased after C and C+E, however increases for IL-6 were significantly higher after C+E compared to C.
Conclusion:
The present study showed that superimposed E during cycling might be a useful tool to induce a high local stimulus to skeletal muscle even when performing low to moderate external workloads. This effect might be due the activation of additional muscle fibers and mild eccentric work due to the concomitant activation of agonist and antagonist. However the higher load to skeletal muscle has to be taken into account.
Keywords: IL-6, BDNF, Creatine Kinase, Myoglobin, Perceived Physical Pain
Introduction
The endocrine system (the exercise-induced hormonal response) is important for mediating signaling pathways for both short-term homeostatic control and long-term cellular adaptations to exercise training[1]. Previous studies have shown that especially high-intensity training (HIT) induces high acute hormonal responses, which are known to be critical for adaptation processes[2] and that HIT is a time-saving strategy to improve endurance performance. However, HIT might not be practical for all kinds of subjects such as patients with certain (cardiovascular) diseases or athletes in certain situations (i.e., injuries), as HIT is very demanding for different kind of tissues and organs[3]. Therefore, it seems to be promising to look for methods that allow an intensification of endurance training without performing high external loads.
Electromyostimulation (E) is an alternative training method mainly performed in strength training and is used for its intensification. Only a few studies used isolated E in matters of improvements of endurance performance[4]. However, two main limitations of E are the strong discomfort associated with the peripheral stimulation[5] and the limited spatial recruitment of muscle fibers, which is quite superficial[6] and largely incomplete. Despite this limited spatial recruitment, we were able to show significantly greater metabolic changes (lactate, respiratory exchange ratio, base excess (BE), HCO3-, pCO2) during cycling with superimposed E compared with normal cycling[7]. Also Paillard[8] described that isolated E is highly demanding on muscle metabolism and can enhance energy consumption and carbohydrate oxidation more than voluntary contraction (VC) can. Hamada et al. showed that E strongly activates anaerobic glycolysis for energy production with lactate formation and acidifies more cytoplasm than VC leading to early fatigue[9]. Furthermore, even at lower exercise intensities, additional E may allow one to induce a high local stimulus on skeletal muscle. The non-selective recruitment (activation of a mix of both fast and slow twitch fibers located within the “range” of the electrodes[10]) may provide (clinical) advantages in that all fibers, regardless of type, have the potential to be activated at relatively low exercise intensities, which might lead to greater skeletal muscle adaptations and improvements of endurance performance, especially in fast twitch fibers[11].
In order to gauge the additional load of E during cycling on skeletal muscle, the present study aimed to investigate the acute response of myokines and muscle damage markers. Skeletal muscle has been identified as an endocrine organ, which releases myokines like IL-6 and BDNF[12]. The increase in the plasma concentration of IL-6 during exercise has been a consistent finding, shown to be dependent on the intensity and duration of exercise[13]. Thereby the expression of IL-6 in skeletal muscle is regulated via calcium (Ca2+) and the intracellular glycogen content and it has been demonstrated to be released by muscle tissue in response to inflammatory action[13].
BDNF is a neurotrophic growth factor which is expressed in the brain and in skeletal muscle, mainly regulating neuronal development, but also regulating metabolism in skeletal muscle. Physical activity was shown to increase circulating BDNF levels 2-3-fold, whereupon the brain contributes 70-80% of the circulating BDNF during exercise[12,14].
The serum level of creatine-kinase (CK) is routinely measured as an index of muscle damage. An increase in CK may be an index of cellular necrosis and tissue damage following acute or chronic muscle injuries[15]. Thereby post-exercise levels of CK are related to the intensity of exercise and muscular strain. Also myoglobin, the oxygen binding protein in muscle, is released into the bloodstream in increasing amounts upon muscle damage[15].
The aim of the present study was to investigate the myokine responses (IL-6, BDNF) and to quantify the muscular strain (CK, myoglobin), to cycling (C), to cycling with superimposed E (C+E) and to E (E). It is hypothesized that C+E leads to higher increases in myokines and muscle damage markers than C or E alone.
Materials and methods
Subjects
Thirteen healthy, nonsmoking sport students (mean±SD, age: 24.8±3.7 years, weight: 79.4±7.5 kg, height: 186.3±4.1 cm, relative VO2max: 51.6±5.4 ml·min-1·kg-1) volunteered and gave written informed consent to participate in this study. The study protocol was approved by the University’s ethics review board and is in accordance with the declaration of Helsinki. All subjects were inexperienced with E.
Exercise study protocol
Before the participation, subjects performed an incremental cycling test to determine VO2max (Zan 600, Zan Messgeräte, Oberthulba, Germany) and maximal performance in order to determine the proper intensities for each subject for the main experiments. The incremental cycling test consisted of cycling at a cadence ≥80 rpm with an initial workload of 100 W for 5 minutes and incremental 40-W increases every 5 minutes until volitional exhaustion was reached. Afterwards, subjects were familiarized with E during C for 15 min.
Subjects participated in three experimental trials each lasting 60 min and each separated by one week in a randomized order. 1) Cycling (C), 2) Cycling with superimposed E (C+E) and 3) E (E). C and C+E were carried out on a bicycle ergometer (Schoberer Rad Meßtechnik SRM GmbH, Jülich, Germany) both times adjusted to 70% peak power output (PPO) and a cadence between 80-85 rpm. E was carried out in a sitting position on a chair. For the 2 interventions with E, two circular electrodes were placed all around the thigh (44 x 4 cm; half of the distance between spina iliaca anterior superior and the proximal patellar pole) and two circular electrodes were placed all around the calf (27 x 4 cm; 1/3 of the distance between fossa poplitea and the calcaneus) stimulating the major muscles of the thigh and the calf. Two laminar electrodes (13 x 10 cm) were centrally placed at the buttocks. A bipolar rectangular pulse waveform with an impulse width of 400 μs was continuously applied with 60 Hz. The settings of the EMS device (miha bodytec, Emersacker, Germany) were kept constant, including the intensity, for C+E and E. The intensity (current) was set individually for each subject and each muscle group at the maximum tolerated intensity[16,19] (thigh: 18.5±2.3 mA; calf: 14.9±1.4 mA; buttocks: 14.9±1.8 mA) according to each athlete’s discomfort threshold and at an intensity at which proper pedaling was still possible. However, due to the resistance of different tissue structures it is not possible to precisely determine the impulse intensity (mA)[18] that ultimately reaches the muscle. In order to maximize the spatial recruitment, subjects were allowed to increase the intensity of E every 10 min. Electrical stimulation was continuous and independent from cycling.
A biphasic impulse type (in comparison to monophasic current) was chosen, as it was applied in several other studies[16,17], and because it offers advantages for applying high stimulation intensities. In regard to stimulation frequency, authors recommend a wide range between 2-200 Hz. Comprehensive recommendations for high stimulation intensities range between 50-100 Hz[20]. Furthermore, in a previous study we found no differences in the metabolic response between 30 and 85 Hz[7]. For the level of impulse width, a compromise should be found to activate deeper motor units without being unpleasant for the athlete. Impulse durations between 300-400 microseconds were recommended, as long impulse durations result in deeper and more intensive muscle stimulation and thus more motor units will be recruited[20].
Before each experimental trial, subjects warmed up (WU) for 5 min at an intensity of 50% PPO on a cycle ergometer.
Before each experiment subjects had a standardized resting-phase of 1 hour in which 0.5 mL of water was ingested in order to assure a well-hydrated status and to provide a good conductance of the skin where the electrodes are placed. During each session environmental conditions (temperature and humidity) were kept constant and all three tests were carried out at the same time of day in order to prevent diurnal variations in performance and the hormonal status.
The food intake before the tests was standardized to the extent that subjects recorded their food intake on the day before the first test, and then were advised to reproduce their diet before each test day. In addition carbohydrate-rich foods were recommended to the subjects. A last snack was allowed 2 hours before the test. The subjects were not allowed to perform strenuous exercise 24 hours before testing. 30 minutes after each of the three tests, subjects received 500 ml of a low fat chocolate milk and additional energy-bars. Food intake was adjusted so that energy intake matched the calculated energy expenditure of each trial. After the ingestion, subjects were only allowed to drink water until the last blood sample was withdrawn.
Measurements
Venous blood samples were collected for the determination of interleukin-6 (IL-6), brain-derived neurotrophic factor (BDNF), creatine kinase (CK) and myoglobin. One venous blood sample was taken before exercise (pre), and five post-exercise samples were taken at 0 min (0´), 30 min (30´), 60 min (60´), 240 min (240´) and 24 h (24h) after cessation of each of the three tests. 8.5 ml of blood was collected by the Vacutainer blood withdrawal system (Becton Dickinson). After storage at 7°C for ~30 min for deactivation of coagulation factors, the blood samples were centrifuged for 10 min at 1861 g and 4°C (Rotixa 50, Hettich Zentrifugen, Mühlheim, Germany). The serum was stored at -80°C till analysis. Serum levels of Myoglobin (ng·ml-1), BDNF, (pg·ml-1) and IL-6 (pg·ml-1) were determined using commercial ELISA kits (Myoglobin ELISA EIA-3955, DRG Instruments GmbH Germany; human BDNF Immunoassay, Quantikine ELISA-DBD00; human IL-6 Immunoassay, Quantikine HS ELISA-HS600B, R&D Systems USA). CK was analyzed with an autoanalyser (ADVIA, Siemens healthcare, USA) by using an enzymatic-photometric method. All results were adjusted for changes in plasma volume (PV): PV changes in percentage of pre values= [(Hbpre/Hbpost)·(100-Hctpost)/[(100-Hctpre)-1]]·100.
The rating of muscle soreness was assessed by sitting down on a chair from an upright posture and standing up again from this position without using the arms. The subjects were then asked to rate their perceived physical pain using a 0-10 visual analog scale (VAS) pre, directly after (0´), 240 min after (240´) and 24 h (24 h) after each intervention.
Statistics
Statistical analyses of the data were performed by using a statistics software package (Statistica for Windows, 7.0, Statsoft, Tulsa, OK). Descriptive statistics of the data are presented as means±SD. To assess the effect of the three different interventions on circulating IL-6, BDNF, CK and myoglobin, a 2-factor [intervention (C, C+E, E); time (pre, 0´, 30´, 60´, 240´, 24h)] repeated-measures ANOVA with Fisher post-hoc test was used. For each growth factor we reported the p-value corresponding to the main intervention effect and time effect. Statistical differences were considered to be significant for p<0.05. Furthermore, the effect size “partial η2” was calculated according to the main effect over time for each intervention and parameter. It has been suggested that for ANOVA, an effect size of 0.1 represents a small effect size; 0.25, a medium effect; and 0.4, a large effect. Power (1-β) was calculated post-hoc for ANOVA repeated measures using α, sample size and effect size.
Results
Heart rate showed no significant differences between C (151±11 bpm) and C+E (153±11 bpm), but both interventions showed significantly higher levels compared to E (56±12 bpm).
Power output during C and C+E was 212±30 W (70% PPO).
Creatine Kinase (CK)
Over-all ANOVA showed a significant time effect (p=0.008; partial η2=0.28; power=0.4), no intervention effect (p=0.07; partial η2=0.25; power=0.51) and a significant interaction effect (intervention * time) (p<0.001; partial η2=0.38; power=0.57). Post-hoc analysis revealed that C+E significantly increased CK levels 24h after exercise compared to pre, whereas C and E did not induce any significant changes (Figure 1b).
Figure 1.
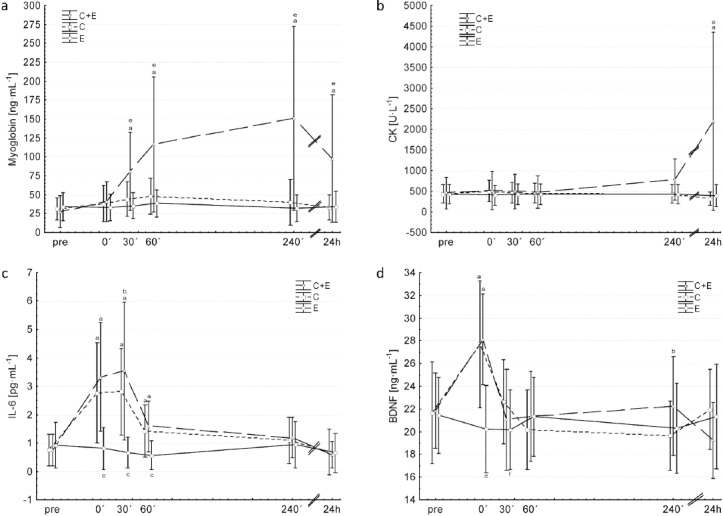
Changes in circulating myoglobin (a), creatine kinase (b), IL-6 (c) and BDNF (d) before (pre) and after (0’, 30’, 60’, 240’, 24h) each intervention: C+E (triangles, broken line), C (Squares, dotted line) and E (circles, solid line). aSignificant different to pre of the same condition; bsignificant difference between C and C+E at respective time points; csignificant difference between E and C+E/C at respective time points; esignificant difference between C+E and C/E at respective time points; fsignificant difference between C and E at respective time points. Values are presented as means±SD.
Myoglobin
Over-all ANOVA showed a significant time effect (partial η2=0.46; power=0.97), intervention effect (partial η2=0.48; power=0.9) and a significant interaction effect (intervention * time) (partial η2=0.4; power=0.9) (all p<0.001). Post-hoc analysis revealed that C+E significantly increased myoglobin levels 30´, 60´, 240´and 24h after exercise compared to pre, whereas E and C induced no significant changes. C+E showed significantly higher values 30´, 60´, 240´and 24h compared to C and E (Figure 1a).
IL-6
Over-all ANOVA showed a significant time effect (partial η2=0.74; power=1.0), intervention effect (partial η2=0.56; power=0.98) and a significant interaction effect (intervention * time) (partial η2=0.55; power=0.99) (all p<0.001). Post-hoc analysis revealed that C+E and C significantly increased IL-6 levels 0´, 30´, and 60´after exercise. 30´ after C+E IL-6 values were significantly higher compared to C. IL-6 levels were significantly lower 0´, 30´ and 60´ after E compared to C+E and C (Figure 1c).
BDNF
Over-all ANOVA showed a significant time effect (p<0.001; partial η2=0.52; power=1.0), intervention effect (p=0.03; (partial η2=0.3; power=0.68) and interaction effect (intervention * time) (p<0.001; partial η2=0.33; power=1.0)). Post-hoc analysis revealed that C+E and C significantly increased BDNF levels 0´ after exercise compared to pre, whereas E induced no significant changes. BDNF levels were significantly lower 0´and 30´ after E compared to C+E and C. 240´after exercise C+E showed significantly higher values compare to C (Figure 1d).
Perceived physical pain
Muscle soreness was highest after C+E compared to both other interventions at all time points post intervention. C and E did not differ between each other (Figure 2).
Figure 2.
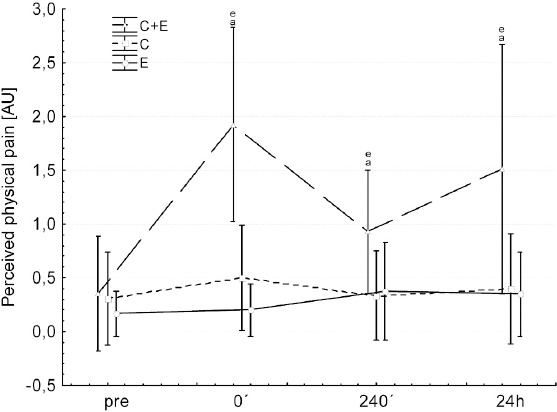
Changes in perceived physical pain before (pre) and after (0’, 240’, 24h) each intervention: C+E (triangles, broken line), C (Squares, dotted line) and E (circles, solid line). asignificant different to pre of the same condition; esignificant difference between C+E and C/E at respective time points. Values are presented as means±SD.
Discussion
The present study investigated the effects of C, E and C+E on markers of muscle damage (myoglobin & CK) and on two myokines (IL-6 & BDNF). The major findings of the present study are that C+E caused the highest increases in myoglobin and creatine kinase accompanied with the highest ratings of muscle soreness, whereas C and E had no major effects. Furthermore, IL-6 showed the highest increases after C+E followed by C and E. Serum BDNF levels were significantly increased by C and C+E, whereas E induced no significant changes.
The major source of serum IL-6 levels during exercise is the skeletal muscle[12,13], and previous studies showed that the intensity and duration of exercise influence the increase in serum IL-6 levels[21]. This sensitivity to exercise intensity indirectly represents the muscle mass involved in the activity[13]. IL-6 is important for the maintenance of glucose homeostasis and fat oxidation. Low intracellular glycogen concentrations stimulate IL-6 production and release via an AMPK-dependent pathway[22] in order to mobilize glucose and FFA from liver and fat[13]. The higher increases of IL-6 after C+E compared to C of the present study might be due to the previously described additional recruitment of muscle fibers and MUs during (superimposed) E[11], increasing the muscle mass involved, the intensity of exercise and therefore the energy demand of the muscle. Besides the energetic regulation of IL-6, the expression in skeletal muscle is also regulated via changes in calcium (Ca2+) homeostasis[23]. The additional recruitment of muscle fibers during C+E might alter the Ca2+ homeostasis in skeletal muscle more than C and might therefore be a trigger for an increased expression/release of IL-6 as well. Furthermore, IL-6 has been demonstrated to be released by muscle tissue in response to inflammatory action and muscle damage[13]. As we were able to show significantly higher muscle damage after C+E, a higher inflammatory response might also explain the higher IL-6 release. However, this mechanism might only play a minor role, as inflammatory induced IL-6 release might occur later than directly after exercise[24].
BDNF plays a key role in regulating survival, growth and maintenance of neurons[25], however, BDNF has also been identified as a key component of central metabolic pathways and as a regulator of metabolism in skeletal muscle[12,26]. The results of BDNF of the present study are in line with previous studies, showing significant increases after exercise[27]. Although, previous studies suggested a link between BDNF elevations and exercise intensity[27,28], the intensification of endurance training with superimposed E in the present study did not lead to higher increases in BDNF levels. However, previous studies mainly compared more distinct graded exercise test to exhaustion, with low intensity, short duration exercise. This might explain the previously observed link to exercise intensity and the link to metabolic challenges imposed by strenuous exercise on the brain and BDNF production. However, up to now, the underlying molecular mechanisms driving the elevation of BDNF remain mainly unknown. Wrann et al.[29] recently showed that endurance exercise stimulates hippocampal Fndc5 gene expression (irisin) through a PGC-1α/Errα transcriptional complex. This elevated Fndc5 gene expression in turn stimulates BDNF gene expression. These findings link endurance exercise and important metabolic mediators (PGC-1a and FNDC5) with BDNF expression in the brain[29] and might explain the influence of exercise intensity. Furthermore, studies showed that BDNF is released from the cerebral vascular endothelium following hypoxic stress[30]. It can be speculated that exercise could result in cerebral hypoxic stress, since cerebral oxygen tension decreases during strenuous exercise[31], although, this might not have been the case for the chosen intensity of the present study. The fate, however, of the BDNF in the periphery remains unclear. BDNF enhances lipid oxidation in the muscles[26] and it could thus be speculated that the muscles take up BDNF.
Previous studies showed that exercise is associated with remarkable changes in (inflammatory) cytokines and damage markers and that the exercise-related response of these markers can be used to gauge exercise load[32]. Creatine kinase (CK) and myoglobin are well known for their role in muscle disruption, where CK peaks around 24-72 hours, and myoglobin with a much shorter (hours) response[15], which is supported by the present results. Myoglobin is about half the size of CK, which makes it easier to permeate the membrane and Mb is released from damaged muscle directly into the bloodstream whereas CK is released first into the lymph[15,33]. The significant increase of both markers after C+E only, clearly showed the high muscular strain of superimposed E during cycling. These higher increases might be due to the additional recruitment of (type II) muscle fibers, but more likely due to eccentric contractions (which normally do not occur during cycling), as the agonist and antagonist were stimulated simultaneously due to the use of circular electrodes. However, Chen et al.[34] also reported that CK significantly increased by about 6-times in rats following a fatiguing swimming protocol, an activity known to lack eccentric contractions. Significant CK increases were also found in humans immediately and 24 h after an exhaustive 90 min swimming protocol[35], or 90 min cycling exercise[36], also known to lack mechanical impact and eccentric contractions. Therefore, it was suggested that the energy status (severe fatigue) of the muscle cells probably contributes to the amount of CK efflux independent from mechanically induced membrane damage[37,38], which might also have been the case in the present study.
Several previous studies reported a repeated bout effect of muscle damage (makers) and soreness induced by E[39,40]. We cannot exclude a repeated bout effect in the present study; however, we therefore randomized all three trials, so that possible repeated bout effects might have canceled each other out.
We are aware that a different approach would be to trigger stimulation to coincide with muscle EMG activity during the exercise, so that E contributes to the cycling (to volitional contractions), and does not interfere with normal contraction/relaxation cycles. However, the superimposed E during cycling of the present study might be a helpful and easy method to induce additional ‘mild eccentric exercise’[41]. Previous studies indicate that muscle tissue reacts specifically and differently to the combination of mechanical and metabolic stress induced by concentric and eccentric endurance-type training[42]. Repetitive submaximal concentric exercise (i.e., shortening contractions during normal cycling) mainly leads to adaptations of muscle oxidative metabolism and endurance, while eccentric exercise (i.e., lengthening contractions like during superimposed E during cycling) results in muscle growth and gain of muscle strength[41]. As preventing the loss of muscle mass with age, of patients or of athletes during and after injuries is an important aim for maintaining health and performance, the approach of the present study might be helpful to prevent this loss due to the eccentric component. Superimposed E may provide better results than volitional training for subjects, when they are unable to sustain an adequate volitional training intensity and duration to gain benefit from the intervention[10]. Orthopedic patients who cannot perform high-intensity voluntary contractions because of injury, recent surgery or impaired activation[43,44], and also athletes requiring high levels of muscle strength and power[45,46], might benefit from the use of superimposed E exercise - even at low intensity - to (re)train at least some of the fast fibers that otherwise can only be activated using high-force voluntary efforts.
A recent study showed that ‘mild eccentric exercise’ can increase Hsp72 content in skeletal muscle compared to concentric exercise, which is known to provide protection to skeletal muscle[47]. However, if this is the case for superimposed E needs to be shown in future studies and it has to be considered that ‘mild eccentric exercise’ has a molecular signature distinctly different from intensive concentric exercise as well as from maximal eccentric exercise[41].
In summary, the higher increases of CK, myoglobin and IL-6 after C+E indirectly show that a larger muscle mass is activated and that superimposed E induces a higher strain to skeletal muscles than normal C.
The present study showed that superimposed E during cycling might be a useful method to induce a high local stimulus to skeletal muscle even when performing low to moderate external workloads and therefore to intensify endurance training. This effect might be due the activation of additional muscle fibers and due to ‘mild eccentric work’ induced by the concomitant activation of agonist and antagonist. However, the higher load of superimposed E and the induced damage to skeletal muscle compared to C has to be taken into account.
Acknowledgements
This work was supported by the grant funding of the German Sport University Cologne.
Footnotes
Edited by: J. Rittweger
Refrences
- 1.Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35:339–61. doi: 10.2165/00007256-200535040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Wahl P, Mathes S, Kohler K, et al. Acute Metabolic, Hormonal, and Psychological Responses to Different Endurance Training Protocols. Horm Metab Res. 2013;45(11):827–33. doi: 10.1055/s-0033-1347242. [DOI] [PubMed] [Google Scholar]
- 3.Wahl P, Hagele M, Zinner C, et al. High intensity training (HIT) for the improvement of endurance capacity of recreationally active people and in prevention &rehabilitation. Wien Med Wochenschr. 2010;160:627–36. doi: 10.1007/s10354-010-0857-3. [DOI] [PubMed] [Google Scholar]
- 4.Kim CK, Takala TE, Seger J, et al. Training effects of electrically induced dynamic contractions in human quadriceps muscle. Aviat Space Environ Med. 1995;66:251–5. [PubMed] [Google Scholar]
- 5.Delitto A, Strube MJ, Shulman AD, et al. A study of discomfort with electrical stimulation. Phys Ther. 1992;72:410–21. doi: 10.1093/ptj/72.6.410. [DOI] [PubMed] [Google Scholar]
- 6.Vanderthommen M, Duteil S, Wary C, et al. A comparison of voluntary and electrically induced contractions by interleaved 1H- and 31P-NMRS in humans. J Appl Physiol. 2003;94:1012–24. doi: 10.1152/japplphysiol.00887.2001. [DOI] [PubMed] [Google Scholar]
- 7.Wahl P, Schaerk J, Achtzehn S, et al. Physiological responses and perceived exertion during cycling with superimposed electromyostimulation (EMS) J Strength Cond Res. 2011;26(9):2383–8. doi: 10.1519/JSC.0b013e31823f2749. [DOI] [PubMed] [Google Scholar]
- 8.Paillard T. Combined application of neuromuscular electrical stimulation and voluntary muscular contractions. Sports Med. 2008;38:161–77. doi: 10.2165/00007256-200838020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hamada T, Hayashi T, Kimura T, et al. Electrical stimulation of human lower extremities enhances energy consumption, carbohydrate oxidation, and whole body glucose uptake. J Appl Physiol. 2004;96:911–6. doi: 10.1152/japplphysiol.00664.2003. [DOI] [PubMed] [Google Scholar]
- 10.Maffiuletti NA. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol. 2010;110:223–34. doi: 10.1007/s00421-010-1502-y. [DOI] [PubMed] [Google Scholar]
- 11.Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther. 2005;85:358–64. [PubMed] [Google Scholar]
- 12.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214:337–46. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 13.Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 14.Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–9. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 15.Sayers SP, Clarkson PM. Short-term immobilization after eccentric exercise Part II: creatine kinase and myoglobin. Med Sci Sports Exerc. 2003;35:762–8. doi: 10.1249/01.MSS.0000064933.43824.ED. [DOI] [PubMed] [Google Scholar]
- 16.Jubeau M, Sartorio A, Marinone PG, et al. Comparison between voluntary and stimulated contractions of the quadriceps femoris for growth hormone response and muscle damage. J Appl Physiol 1985. 2008;104:75–81. doi: 10.1152/japplphysiol.00335.2007. [DOI] [PubMed] [Google Scholar]
- 17.Maffiuletti NA, Bramanti J, Jubeau M, et al. Feasibility and efficacy of progressive electrostimulation strength training for competitive tennis players. J Strength Cond Res. 2009;23:677–82. doi: 10.1519/JSC.0b013e318196b784. [DOI] [PubMed] [Google Scholar]
- 18.Lake DA. Neuromuscular electrical stimulation. An overview and its application in the treatment of sports injuries. Sports Med. 1992;13:320–36. doi: 10.2165/00007256-199213050-00003. [DOI] [PubMed] [Google Scholar]
- 19.Maffiuletti NA, Cometti G, Amiridis IG, et al. The effects of electromyostimulation training and basketball practice on muscle strength and jumping ability. Int J Sports Med. 2000;21:437–43. doi: 10.1055/s-2000-3837. [DOI] [PubMed] [Google Scholar]
- 20.Filipovic A, Kleinoder H, Dormann U, et al. Electromyostimulation - a systematic review of the influence of training regimens and stimulation parameters on effectiveness in electromyostimulation training of selected strength parameters. J Strength Cond Res. 2011;25:3218–38. doi: 10.1519/JSC.0b013e318212e3ce. [DOI] [PubMed] [Google Scholar]
- 21.Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans - effect of intensity of exercise. Eur J Appl Physiol. 2000;83:512–5. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- 22.Glund S, Treebak JT, Long YC, et al. Role of adenosine 5’-monophosphate-activated protein kinase in interleukin-6 release from isolated mouse skeletal muscle. Endocrinology. 2009;150:600–6. doi: 10.1210/en.2008-1204. [DOI] [PubMed] [Google Scholar]
- 23.Keller C, Hellsten Y, Steensberg A, et al. Differential regulation of IL-6 and TNF-alpha via calcineurin in human skeletal muscle cells. Cytokine. 2006;36:141–7. doi: 10.1016/j.cyto.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 24.MacIntyre DL, Sorichter S, Mair J, et al. Markers of inflammation and myofibrillar proteins following eccentric exercise in humans. Eur J Appl Physiol. 2001;84:180–6. doi: 10.1007/s004210170002. [DOI] [PubMed] [Google Scholar]
- 25.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–94. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Matthews VB, Astrom MB, Chan MH, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–18. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 27.Rojas VS, Struder HK, Vera WB, et al. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121:59–65. doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- 28.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39:728–34. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 29.Wrann CD, White JP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18:649–59. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Ward N, Boswell M, et al. Secretion of brain-derived neurotrophic factor from brain microvascular endothelial cells. Eur J Neurosci. 2006;23:1665–70. doi: 10.1111/j.1460-9568.2006.04682.x. [DOI] [PubMed] [Google Scholar]
- 31.Nybo L, Rasmussen P. Inadequate cerebral oxygen delivery and central fatigue during strenuous exercise. Exerc Sport Sci Rev. 2007;35:110–8. doi: 10.1097/jes.0b013e3180a031ec. [DOI] [PubMed] [Google Scholar]
- 32.Eliakim A, Nemet D. Interval training and the GH-IGF-I axis - a new look into an old training regimen. J Pediatr Endocrinol Metab. 2012;25:815–21. doi: 10.1515/jpem-2012-0209. [DOI] [PubMed] [Google Scholar]
- 33.Heavens KR, Szivak TK, Hooper DR, et al. The effects of high intensity short rest resistance exercise on muscle damage markers in men and women. J Strength Cond Res. 2014;28:1041–9. doi: 10.1097/JSC.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Serfass RC, Apple FS. Alterations in the expression and activity of creatine kinase-M and mitochondrial creatine kinase subunits in skeletal muscle following prolonged intense exercise in rats. Eur J Appl Physiol. 2000;81:114–9. doi: 10.1007/PL00013783. [DOI] [PubMed] [Google Scholar]
- 35.Haralambie G, Senser L. Metabolic changes in man during long-distance swimming. Eur J Appl Physiol Occup Physiol. 1980;43:115–25. doi: 10.1007/BF00422442. [DOI] [PubMed] [Google Scholar]
- 36.Han R, Kanagawa M, Yoshida-Moriguchi T, et al. Basal lamina strengthens cell membrane integrity via the laminin G domain-binding motif of alpha-dystroglycan. Proc Natl Acad Sci U S A. 2009;106:12573–9. doi: 10.1073/pnas.0906545106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukhutdinova FI. [During fever lymphatic system transports creatine phosphokinase into systemic circulation] Biull Eksp Biol Med. 1999;128:21–3. [PubMed] [Google Scholar]
- 38.Gupta RC, Goad JT, Kadel WL. Energy related metabolic alterations in diaphragm muscle resulting from acute methomyl toxicity. Neurotoxicology. 1994;15:321–30. [PubMed] [Google Scholar]
- 39.Aldayel A, Jubeau M, McGuigan M, et al. Comparison between alternating and pulsed current electrical muscle stimulation for muscle and systemic acute responses. J Appl Physiol (1985) 2010;109:735–44. doi: 10.1152/japplphysiol.00189.2010. [DOI] [PubMed] [Google Scholar]
- 40.Black CD, McCully KK. Muscle injury after repeated bouts of voluntary and electrically stimulated exercise. Med Sci Sports Exerc. 2008;40:1605–15. doi: 10.1249/MSS.0b013e3181788dbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klossner S, Dapp C, Schmutz S, et al. Muscle transcriptome adaptations with mild eccentric ergometer exercise. Pflugers Arch. 2007;455:555–62. doi: 10.1007/s00424-007-0303-6. [DOI] [PubMed] [Google Scholar]
- 42.Zoll J, Steiner R, Meyer K, et al. Gene expression in skeletal muscle of coronary artery disease patients after concentric and eccentric endurance training. Eur J Appl Physiol. 2006;96:413–22. doi: 10.1007/s00421-005-0082-8. [DOI] [PubMed] [Google Scholar]
- 43.Petterson S, Snyder-Mackler L. The use of neuromuscular electrical stimulation to improve activation deficits in a patient with chronic quadriceps strength impairments following total knee arthroplasty. J Orthop Sports Phys Ther. 2006;36:678–85. doi: 10.2519/jospt.2006.2305. [DOI] [PubMed] [Google Scholar]
- 44.Stevens JE, Mizner RL, Snyder-Mackler L. Neuromuscular electrical stimulation for quadriceps muscle strengthening after bilateral total knee arthroplasty: a case series. J Orthop Sports Phys Ther. 2004;34:21–9. doi: 10.2519/jospt.2004.34.1.21. [DOI] [PubMed] [Google Scholar]
- 45.Babault N, Cometti G, Bernardin M, et al. Effects of electromyostimulation training on muscle strength and power of elite rugby players. J Strength Cond Res. 2007;21:431–7. doi: 10.1519/R-19365.1. [DOI] [PubMed] [Google Scholar]
- 46.Malatesta D, Cattaneo F, Dugnani S, et al. Effects of electromyostimulation training and volleyball practice on jumping ability. J Strength Cond Res. 2003;17:573–9. doi: 10.1519/1533-4287(2003)017<0573:eoetav>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Lewis EJ, Ramsook AH, Locke M, et al. Mild eccentric exercise increases Hsp72 content in skeletal muscles from adult and late middle-aged rats. Cell Stress Chaperones. 2013;18:667–73. doi: 10.1007/s12192-013-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


