Abstract
Objectives:
We tested whether intermittent short-radius centrifugation was effective for mitigating alteration in balance and gait following bed rest.
Methods:
Ten male subjects were exposed to 5 days of 6° head-down tilt bed rest with: (a) no countermeasure; (b) daily 1-g centrifugation for a continuous 30-min period; and (c) daily 1-g centrifugation for six periods of 5 min. During and after the bed rest, subjects were asked to scale the severity of neurovestibular symptoms that followed centrifugation or 80º head-up tilt. Following the bed rest, equilibrium scores were derived from anterior-posterior sway while standing on a foam pad with the eyes open or closed while making pitch head movements, and gait was evaluated by grading subjects’ performance during various locomotion tasks.
Results:
At the beginning of bed rest, one single 30-min period of centrifugation induced more severe neurovestibular symptoms than six periods of 5-min centrifugation. After bed rest, although equilibrium scores and gait performance were not significantly altered, subjects felt less neurovestibular dysfunction with orthostatic stress when centrifugation was used.
Conclusion:
Centrifugation was effective at reducing the severity of neurovestibular symptoms after bed rest, but this decrease was not different between one or multiple daily sessions.
Keywords: Posturography, Balance, Bed Rest, Centrifugation, Artificial Gravity
Introduction
Artificial gravity generated by short-radius centrifugation has been proposed as a countermeasure against neurovestibular, cardiovascular, and musculoskeletal deconditioning following prolonged exposure to weightlessness[1-2]. Because a human-rated centrifuge is currently not available on board the International Space Station, the effectiveness of short-radius centrifugation can only be assessed in analogs of space flight conditions, such as bed rest or dry immersion. In these investigations subjects are periodically exposed to short-radius centrifugation generating centripetal acceleration along their longitudinal body axis[3]. Results have shown that daily 1-h exposure to 1-2 g at the heart significantly reduced cardiovascular and muscular deconditioning following bed rest[4-11].
In 2005 the European Space Agency (ESA) commissioned a multi-disciplinary Topical Team to advise the agency on artificial gravity research. The outcome of this Topical Team was a detailed set of recommendations for undertaking a program of ground-based research into the feasibility of using intermittent gravity loading as a multi-system countermeasure. The first step of this program was very short-duration (5 days) bed rest studies to establish the effective ranges of intermittent gravity loading on sensory-motor, cardiovascular, and musculoskeletal systems, using either real gravity or short-radius centrifugation. These studies used a crossover design that required all subjects to experience each test condition. Thus, multiple campaigns were required, with each subject spending one week in the facility (under a different experimental condition) during each campaign and recovering for at least four weeks between campaigns. The full set of ESA standard bed rest dependent measures were collected on each subject before and after each campaign. As the 5-day bed rests are very short relative to the deconditioning time constants of some of the physiological systems (e.g., bone demineralization, muscle atrophy), a few longer duration campaigns (14-21 days) will be scheduled later in the program to validate the effects predicted in these systems and to develop optimal intermittent artificial gravity prescriptions.
The present study was part of a series of 5-day bed rest studies organized by ESA. The results presented here focus on neurovestibular function, referring to the sensory illusions, motor deficits and motion sickness associated with intermittent short-radius centrifugation. Several studies with widely differing objectives were performed in parallel, concerning for example cardiovascular[11-12]; endocrine[13-14], and musculoskeletal[15] functions.
In a previous paper, we reported the effects of intermittent standing or a combination of locomotion-like exercises on sensory-motor performance[16]. Our results showed that when subjects stood upright by their bed or executed a combination of locomotion-like exercises for a continuous 25-min exposure each day, they performed better than when in bed 24 hours per day. Indeed, the postural instability and the number of falls decreased after the 5-day bed rest compared to the control condition. In the present study, subjects were actually exposed to short-radius centrifugation during the bed rest. The objectives were to compare the neurovestibular effects: (a) between intermittent short-radius centrifugation and intermittent standing or locomotion-like exercises, and (b) between one single daily centrifugation session and multiple daily centrifugation sessions. It was hypothesized that several shorter centrifugation periods with rest in-between would not only be better tolerated by the subjects, but also prove more efficient as a countermeasure. The rationale for this theory stems from studies on hind-limb suspension in rats[17] and in-orbit exercises in astronauts[18], which showed that repetitive short-duration, high-load exercise training was the most effective and efficient means of reducing physiological deconditioning. Vernikos et al.[19] also suggested that the frequency of gravity exposure rather than the duration was a critical factor for mitigating physiological deconditioning due to unloading.
Material and methods
General design
A total of three 5-day bed rest campaigns were scheduled. Each campaign consisted of 5 days of baseline data collection (BDC-5 through BDC-1), 5 days of bed rest in 6° head-down tilt (HDT1 through HDT5), and 5 days of recovery (R+0 through R+4). Each subject randomly performed bed rest only (CON), bed rest with daily centrifugation during one period of 30 min (AG1), or bed rest with daily centrifugation during six periods of 5 min each (AG2). During the AG1 and AG2 interventions, the subjects were lying horizontally on the gurney during transportation from their bed to the centrifuge and back for 15 min/day, and on the centrifuge for 45 min/day. During the control condition (CON) subjects were also placed horizontally for 1 h/day. The rest of the time, subjects remained in HDT and refrained from any type of physical exercise and/or upright posture.
Centrifugation
Subjects were lying supine on the arm of a 2.8-m radius centrifuge, with their head closer to the axis of rotation and their feet pointing radially outwards[20]. For each subject, the center of mass was estimated using a ratio of center of mass to height of 0.5621. The subjects were positioned so that their calculated center of mass was located between 118 cm and 122 cm from the center of rotation. This small variation was inevitable due to variation in subjects’ height and due to mechanical constraints. Accordingly, the rotation rates ranged from 27.8 to 27.2 rpm, respectively, so that all subjects were exposed to 1 g along the body longitudinal axis at their estimated center of mass. Because a short-arm centrifuge is associated with a large centripetal force gradient along the body, a normal 1 g level at the center of mass leads to approximately 2 g at the feet, thus giving rise to blood congestion and pain in some subjects. Nevertheless, previous studies have shown that short sessions (<1 h) were well tolerated and that subjects generally felt to be standing upright during centrifugation[20,22].
The centrifuge accelerated at 5 deg/s2 for 32-33 sec until the target rotation rate was achieved. Rotation at constant velocity then lasted either 30 min for the AG1 protocol or 5 min for the AG2 protocol. The centrifuge then decelerated at 5 deg/s2 until a complete stop. For AG2, a 3-min rest period was used between two successive runs. The direction of centrifuge rotation (clockwise or counterclockwise) varied each day.
During centrifugation, subjects were in complete darkness with their head placed on a headrest. They were instructed to not move the head or contract their leg muscles. They could however crimp their toes every 10-20 s to prevent calf pain. Medical monitoring included continuous heart rate, blood pressure, and respiration rate. Subjects wore headsets to communicate with the medical operator. The termination criteria were: syncope, severe signs of orthostatic intolerance (sweating, dizziness, fainting), fast and persistent decrease in systolic blood pressure, abrupt changes in heat rate, tachycardia, irregular cardiac rhythm, and the subject’s request to stop.
Subjects
The study design was approved by the local Ethics Committee (Comité de Protection des Personnes Sud-Ouest et Outre-Mer I). This ESA-sponsored investigation was organized by MEDES in the Hospital Rangueil of Toulouse, France. All volunteers signed a consent form and were aware of their right to withdraw from the experiment without prejudice at any time. Twelve healthy male were originally included in the study after receiving a complete description of the experimental methods, and after passing a comprehensive medical assessment and a centrifuge tolerance test.
Before the investigation all the participants filled in a Motion Sickness Susceptiblity Questionnaire. This questionnaire relates to the subject’s past experiences with and perceptions of potentially provocative situations[23]. Subjects ranked on a scale from 0 to 3 if they felt sick (0=never; 1=rarely; 2=sometimes; 3=frequently) for each of the ten following types of transport or entertainment: car, bus/coach, train, aircraft, small boat, ship, channel ferry, swing in playground, roundabout in playground, or big dippers/funfair ride, A composite MSSQ score was tabulated as the sum of individual items. The MSSQ scores for the subjects participating in this study ranged from 1 to 19, which suggested moderate motion sickness susceptibility.
One subject developed appendicitis during his second bed rest campaign, and he was subsequently withdrawn from the study. Another subject had a severe vasovagal syncope on his second centrifuge run during the first day of his third bed rest campaign. After recovery, this subject continued the bed rest, but without centrifugation and without performing the 80º head-up tilt test at the end. The data from these two subjects were therefore discarded from the present analysis. Consequently, this study includes complete data sets from ten subjects (mean±SEM age: 34.2±2.1 years, height: 178.0±2.3 cm; weight: 76.2±2.0 kg).
Neurovestibular symptoms
Neurovestibular symptoms were recorded within minutes after the end of each centrifugation session during the bed rest, and within minutes after the 80º head-up tilt test at the end of the bed rest. Subjects were presented with the same questionnaire as that used by NASA to review the list and severity of neurovestibular symptoms experienced by crewmembers returning from space[24]. The questionnaire included a list of 15 potential symptoms that the subjects were asked to rate on a scale from 0 to 3 according to their severity (0=none; 1=mild, symptom awareness; 2=moderate, symptom present, no performance impact; 3=severe, symptom present and interferes with performance). Symptoms were off balance, feel abnormally heavy, problems when making rapid head movements, clumsiness, dizziness, difficulty turning corners, motion illusions, sweating, nausea, stomach awareness, malaise/sluggishness, disorientation, vomiting, dry heaves, and fatigue. A composite Readaptation Symptom Severity (RSS) score was tabulated as the sum of the scores for individual symptoms.
Posturography
This test utilized dynamic posturography to quantitatively assess both sensory and motor components of postural control[25]. The subjects with their hands across the waist stood with the feet together, while looking straight ahead for 30 s on a foam pad that rested on a force platform. The foam pad was made of 12-cm thick medium-density foam (Sunmate, Dynamic Systems Inc., Leicester NC, USA). Standing on foam alters somatosensory inputs, and is a useful option for testing balance control when more expensive dynamic posturography testing equipment (e.g. EquiTest) is not available[26].
There is evidence that the diagnostic assessment of postural instability is more pronounced during unstable support conditions requiring active head movements. For this reason, in some trials, subjects were oscillating their head in pitch (frequency 0.33 Hz; amplitude ±20º) in phase with a sinusoidally varying auditory tone. These dynamic head tilts stimulate the vestibular system, which renders the maintenance of upright posture more challenging. This method improves the diagnostic sensitivity of posturography, as shown in healthy subjects and astronauts returning from space flight[27-28].
Consequently, three conditions were used to objectively evaluate the subject’s ability to make effective use of (or suppress inappropriate) visual, vestibular, and proprioceptive information for balance control: (a) eyes open with the head erect (EO); (b) eyes closed with the head erect (EC); and (c) eyes closed with dynamic head tilts (ECDHT). Each condition was performed three times. The order of conditions was randomized. Between trials, subjects could rest and sit on a chair. Duration of the test was 15 min.
Center-of-mass sway angles were estimated from instantaneous anterior-posterior (AP) and medial-lateral center-of-force positions, which were computed from force transducers mounted within the force platform (Leonardo Mechanograph, Novotec Medical GmbH, Pforzheim, Germany). The AP peak-to-peak sway angle, Θ (in degrees), was used to compute the equilibrium score (EQ): EQ=100 x (1-(Θ/12.5)) where 12.5º is the maximum theoretical peak-to-peak sway in the sagittal plane. For Θ = 12.5º, which is scored as a fall, the EQ score is zero[29].
Dynamic gait index
The Dynamic Gait Index (DGI) was developed as a clinical tool to assess gait, balance and fall risk[30]. It evaluates not only usual steady state walking, but also walking during more challenging tasks. Eight functional walking tests were performed by the subject: (1) walk a distance of 6 m on a level surface at normal pace; (2) walk the same distance while changing gait speed at instructor’s command; (3) walk 6 m at normal pace while looking to the right or to the left upon instructor’s command; (4) walk 6 m at normal pace while looking up or down; (5) walk at normal pace, then turn as quickly as possible to face the opposite direction and stop; (6) walk at normal pace and step over an obstacle (shoebox); (7) walk at normal pace and step around the right side of a cone placed at 3 m, and around the left side of a cone placed at 6 m; and (8) walk up stairs, using the railing if necessary, and when arrived at the top, turn around and walk down. Duration of the test was 30 min.
Each of these eight tasks was performed once, in random order. Performance of each task was rated by a trained observer on a scale of 0 to 3 (where 3 indicated the best score), based on the following criteria:
– 3 (normal): successful execution of task with no assistance, no evidence for imbalance, normal and smooth gait pattern.
– 2 (mild impairment): slow execution speed, may require verbal cueing, mild gait deviations, no signs of imbalance.
– 1 (moderate impairment): very slow execution speed, abnormal gait pattern, evidence for imbalance, must use rail, staggers but recovers before continuing the task.
– 0 (severe impairment): cannot safely complete the task, severe gait deviations or imbalance, unable to clear obstacles, subject stops and reaches for wall or requires physical assistance.
The Dynamic Gait Index (DGI) was obtained by summing scores for all eight tasks. Twenty-four is the maximum individual DGI possible. Based on previous studies in older adults, DGI ranging from 22-24 are indicators of safe ambulators, whereas scores of 19 or less have been related to increased incidence of falls[30].
Data analysis
Subjects were familiarized with the test procedures three days before the first bed rest. Both posturography and DGI tests were then performed by the subjects one day before each bed rest (BDC-1), and again on the last day of each bed rest (R+0), i.e., approximately 20 min after the subjects first stood upright during a 5-30 min orthostatic tolerance (80º head-up tilt) test, and then finally three days later (R+3). EQ and DGI scores were compared across days (BDC-1, R+0, R+3) and bed rest countermeasures conditions (CON, AG1, AG2).
All statistical analyses were performed using SPSS (V20, IBM Corp, Armonk NY, USA). RSS and DGI scores were analyzed using repeated-measures ANOVA and paired t-tests. Since EQ scores cannot be treated as continuous, normally distributed data when falls are present[31], posture data were analyzed using the non-parametric repeated measures Friedman test. When Friedman tests were significant, follow-up (post hoc) analyses between paired data were achieved using the Wilcoxon Signed Ranked test. Statistical significance was accepted at P<0.05 before appropriate Bonferroni correction was applied.
Results
Neurovestibular function
Nine out of 10 subjects performed nominally the 30 centrifugation sessions of the intermittent AG2 protocol. The remaining subject asked to interrupt the second centrifugation session on the second day of the second bed rest campaign because of motion sickness symptoms (malaise, nausea, sweating, dizziness). It is interesting to note that all 5 centrifugation sessions of the continuous AG1 protocol were also prematurely stopped during the first bed rest campaign with this subject because of increased heart rate, drop in blood pressure, and nausea. These interruptions occurred later and later (5 min, 7 min, 18 min, 27 min) during rotation. Three other subjects asked to interrupt one or two centrifugation sessions of the AG1 protocol because of malaise, dizziness, and nausea. In two occasions, the centrifuge was stopped because subjects reported visual problems associated with a drop in blood pressure. These interruptions occurred during the first session of the first bed rest campaign after 12 min, 13 min, 19 min, and 25 min of rotation.
The difference in tolerance between AG1 and AG2 during the first centrifugation session and the habituation of the effects throughout the centrifugation sessions are reflected in the subjective ratings of symptom severity shown in Figure 1A. During the AG1 protocol, nine out of ten subjects reported neurovestibular symptoms after the first centrifugation session. These symptoms were mostly related to motion sickness (nausea, stomach awareness, sweating). The number of subjects reporting symptoms and the severity of the symptoms progressively decreased afterwards. A repeated-measures ANOVA indicated that the changes in RSS scores for AG1 from HDT1 to HDT5 were significant [F(4,49)=2.95; P=0.03]. Only three subjects reported symptoms following AG2 on HDT1. The difference in RSS scores between AG1 and AG2 was significant on HDT1 (P=0.04), but not on subsequent days.
Figure 1.
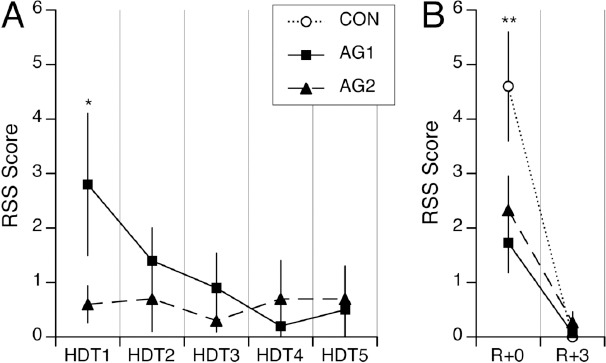
A. Mean±SEM of Readaptation Symptoms Severity (RSS) scores of ten subjects reported on each day of bed rest with head-down tilt (HDT1 to HDT5) immediately after centrifugation for one period of 30 min (AG1), or after centrifugation for six periods of 5 min each separated by 3 min of rest (AG2). B. Mean±SEM of RSS scores obtained immediately after the 80° head-up tilt test following bed rest (R+0) and three days later (R+3) for each of the three interventions (CON, AG1, AG2). *P<0.05 relative to AG2; **P<0.05 relative to AG1 and AG2.
Immediately after the 80º head-up tilt test on R+0, all ten subjects reported neurovestibular symptoms when no countermeasure was used, compared to seven and six subjects when AG1 and AG2 were used, respectively. In addition, the RSS scores following the centrifugation protocols were smaller (62% and 49% for AG1 and AG2, respectively) than in absence of countermeasure (P<0.01). However, the difference between the AG1 and AG2 protocols was not found to be significant (Figure 1B). No neurovestibular effects were reported on R+3 for any of the interventions.
After the 80º head-up tilt test on R+0, the neurovestibular disturbances that were mostly reported by the subjects were a general malaise, the sensation that their balance was off, and symptoms of motion sickness (nausea, dizziness, sweating, fatigue, stomach awareness, dry heaves) that were exacerbated when making head movements (Figure 2). In addition subjects had vertigo and felt disoriented. Malaise and off-balance were less severe when centrifugation was used during the bed rest. Symptoms of motion sickness were also greatly reduced: sweating and stomach awareness were seldom reported, and nausea, fatigue, and dry heaves were not reported at all following bed rest with centrifugation.
Figure 2.
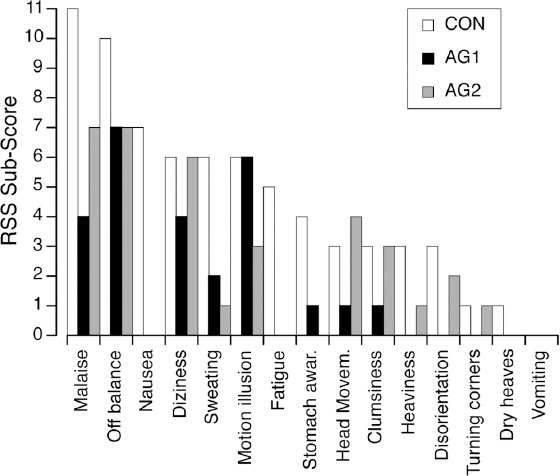
Sum of RSS scores of 10 subjects for each neurovestibular symptom obtained immediately after the 80° head-up tilt test following bed rest (R+0) for each of the three interventions (CON, AG1, AG2).
Equilibrium scores
To determine whether there were any effects of adaption/learning/familiarization to the test paradigm, we evaluated the temporal stability of the data for all BDC and R+3 data across the three bed rest campaigns. Our null hypotheses were that: (a) no differences would be observed in BDC scores from campaign to campaign; and (b) recovery would always be complete by R+3, so that no differences would be observed between BDC and R+3 scores in any campaign.
All 30 EQ Scores (3 trials x 10 subjects) for each of the three posture conditions (EO, EC, and ECDHT) conducted at the BDC and R+3 test sessions for each of the three campaigns were analyzed. Median values for each of the six test sessions are presented in [Table 1]. These data are presented in the temporal order they were collected, so, owing to the randomization process, interventions (CON, AG1, AG2) varied approximately equally among subjects between each of the three BDC and R+3 test sessions.
Table 1.
Median equilibrium scores (± median absolute deviation) on the three posture conditions (EO: eyes open; EC: eyes closed; ECDHT: eyes closed during dynamic head tilt) for the BDC and R+3 test sessions of each bed rest campaign. *P<0.05 with respect to all other test sessions.
| Campaign 1 | Campaign 2 | Campaign 3 | ||||
|---|---|---|---|---|---|---|
| BDC-1 | R+3 | BDC-1 | R+3 | BDC-1 | R+3 | |
| EO | 86.8±2.6 | 85.5±2.4 | 86.4±2.0 | 85.8±2.4 | 87.4±2.2 | 87.1±1.5 |
| EC | 63.9*±4.4 | 70.2±5.3 | 68.8±6.4 | 72.7±3.3 | 68.4±6.2 | 70.4±4.0 |
| ECDHT | 45.4±7.0 | 40.8±6.8 | 43.2±7.7 | 44.4±7.9 | 47.3±9.4 | 50.9*±7.5 |
The repeated-measures Friedman test on EQ scores from the EO condition indicated a non-significant (P=0.74) overall effect, i.e., all test sessions were the same. However, for the EC and ECDHT conditions, the repeated-measures Friedman test suggested a significant (P=0.002 and P=0.001, respectively) overall effect, i.e., all test sessions were not the same. Removing the first test session from consideration and repeating the Friedman test on the five remaining test sessions resulted in a non-significant (P=0.395) overall effect for EC, suggesting that the first test session was different from others. Removing the last test session from consideration and repeating the Friedman test on the five remaining test sessions (including the first) also resulted in a non-significant overall effect (P=0.164) suggesting that subjects might have adapted to the ECDHT protocol by their very last test session.
Because of the above observations, and for comparison with our previous study[16], the effects of the interventions during bed rest were investigated using data where the results of the first condition for each subject were removed. For example, subject A received the AG1 intervention first, so for subject A the first AG1 data were removed from his data set. Similarly subject B received the AG2 intervention first, so the first AG2 data were removed, and so on.
For each posture condition (EO, EC, ECDHT), no significant differences in the EQ scores were observed between before and after bed rest (R+0 or R+3) for each of the interventions (CON, AG1, AG2) (Figure 3). The number of falls after ECDHT remained the same after the bed rest (N=3) whether centrifugation was used or not. Three days after the bed rest, EQ scores for ECDHT were significantly larger than on R+0 (Wilcoxon, P=0.006 for AG1, α=0.017 after applying Bonferroni adjustment), but not significantly different from BDC.
Figure 3.
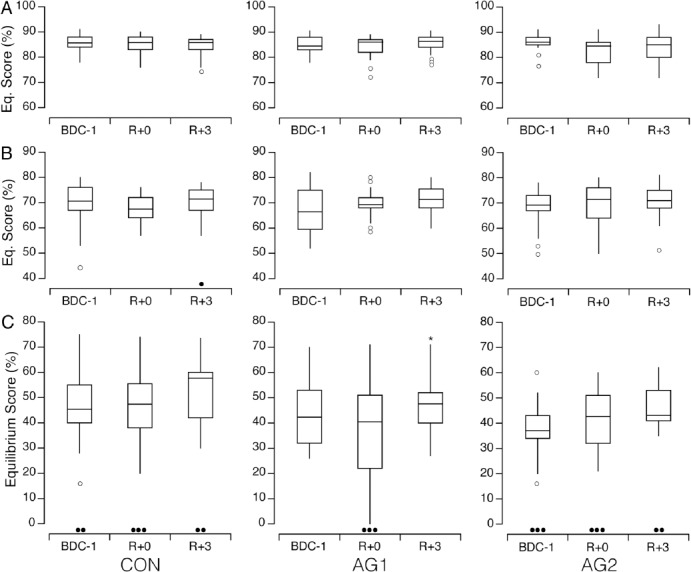
Equilibrium scores for ten subjects trying to stand upright for 30 s on a 12-cm thick foam pad with the eyes open (A), with the eyes closed (B), and with the eyes closed while making dynamic head tilts (C) before (BDC-1), and then immediately (R+0) and three days (R+3) after 5-day bed rests for the three interventions (CON, AG1, AG2). Unfilled circles show outliers. Black symbols show the number of falls in each condition. *P<0.05 relative to R+0.
Dynamic gait index
Most subjects got nearly perfect DGI scores (24/24) when tests were performed one day before and three days after bed rest. Immediately after bed rest though, DGI scores were lower by one point in half of the subjects (Figure 4). This occurred for the tasks where they were instructed to look sideways or up or down when walking on a level surface, or when they walked up and down the stairs. However, this difference was not statistically significant. In addition, there was no significant difference in the DGI scores before and after the bed rest between CON, AG1, and AG2.
Figure 4.
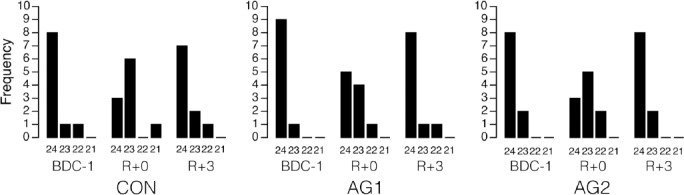
Frequency of Dynamic Gait Index scores obtained for the three interventions (CON, AG1, AG2) before and after bed rest. None of the subjects scored less than 21 on any trial.
Discussion
During the first days of bed rest, the subjective rating of neurovestibular symptom severity (RSS score) indicated that six periods of 5-min centrifugation were better tolerated than one continuous period of 30-min centrifugation. After bed rest, subject also felt some degree of neurovestibular dysfunction following the 80º head-up tilt. However, the severity of symptoms was reduced by half when centrifugation, intermittent or continuous, was used during bed rest. In contrast with these subjective judgments, objective measurements of sensory-motor performance, such as postural stability (EQ score) and gait (DGI index), did not significantly change after bed rest compared to before for any of the interventions, so the effectiveness of centrifugation on these responses could not be evaluated.
Astronauts typically exhibit performance decrements in postural control immediately after space flight when exposed to sensory organization tests[32-33]. However, no significant decrements in performance on a standard battery of sensory organization tests, i.e. with the head erect, were observed after 42-63 days of bed rest[34]. In a previous study[16], we observed significant alterations in EQ scores after a 5-day bed rest when subjects performed dynamic head tilts while standing with the eyes closed. Postural stability is more compromised during dynamic head tilts because of the decreased ability of the vestibular system to discern the orientation of gravity. It has been shown that the addition of dynamic head tilts to the posturography protocol allows detecting subtle deficits in balance function in both human subjects recovering from temporary balance control deficits[27] and in astronauts returning from space flight[28].
EQ scores were lower and falls were less frequent in the present study than in our previous study[16]. Research on postural control has shown that individuals use an ankle or hip strategy during the posturography tests. In use of the ankle strategy, both the upper and lower body segments move in the same direction. In contrast to the ankle strategy, when using the hip strategy, the upper body moves in a direction opposite to that of the lower body. Because the amount of torque that can be generated by the muscles surrounding the ankle joint is relatively small, the ankle strategy can only be used to control sway through a very small range of motion. When the center of gravity is displaced beyond the maximum limits of stability individuals take one or more steps in the direction of the loss of balance[35]. The individuals using an ankle strategy typically show larger EQ scores and more falls than those using a hip strategy. It is therefore possible that the subjects in our earlier study[16] favored an ankle strategy, whereas the subjects in the present study favored a hip strategy. One recommendation for further studies aimed at comparing countermeasures on postural stability is to record the EMG activity of leg muscles during centrifugation and select subjects who preferably use the ankle strategy in posturography tests.
Our results showed that continuous 30-min centrifugation provoked more severe neurovestibular symptoms than six sessions of 5-min centrifugation during the first days of bed rest. The symptoms showed a habituation and from HDT3, both protocols were equally well tolerated by the subjects. A parallel study using the same subjects indicated that daily salivary cortisol levels were significantly higher after both continuous and intermittent centrifugation during the first few days of HDT, but that a habituation of the responses took place when the intermittent centrifugation protocol was used[14].
Intermittent centrifugation was also associated with better orthostatic tolerance after bed rest than continuous centrifugation[11]. By contrast, continuous centrifugation produced superior results as a countermeasure to bone loss, as monitored by changes in bone remodeling markers[15]. However, the superior effect of intermittent centrifugation against orthostatic intolerance was not related to concomitant changes in plasma volume or aerobic power[11]. Also, intermittent centrifugation did not counteract the decrease in the heart left ventricular mass and volume, which were presumably due to decreased physiological loading and dehydration, resulting in hypovolemia[12]. One possible interpretation for the superior effect of intermittent centrifugation on the cardiovascular responses is that a centrifuge run is virtually equivalent to a head-up tilt test, during which changes in the direction of the gravity vector stimulate the otoliths and the other body graviceptors, and changes in the hydrostatic pressure gradient stimulate the baroreceptors, which all affect cardiovascular function. The intermittent centrifugation protocol generated six of these virtual head-up tilts each day of the bed rest, compared to only one per day for the continuous centrifugation protocol. The repetition of this cardiovascular stress test presumably prepared the cardiovascular system for the actual head-up tilt at the end of the bed rest after intermittent centrifugation[19]. Also, the repetitive character of intermittent centrifugation is more likely to induce a habituation of endocrine stress response.
The neural basis for adaptation and habituation is known to depend on sensory coding. Slowly adapting receptors are able to signal stimulus magnitude for several minutes. These receptors cease firing in response to constant-amplitude stimulation and are active only when the stimulus intensity increases or decreases. By contrast, rapidly adapting receptors respond only at the beginning and end of a stimulus, signaling the velocity or acceleration of the stimulation. These sensory systems detect contrasts in discrete stimuli, i.e., changes in the pattern of stimulation in time and space, and their activation conveys information about the changing sensory environment to the brain[36]. Autonomic nervous system and neuroendocrine responses are more susceptible to rapid changes in the environment, and therefore more likely to be influenced by intermittent centrifugation. Continuous centrifugation, by counteracting body unloading and stimulating the proprioceptive reflexes in lower limbs for an extended period, is presumably more effective against neurovestibular, bone, and muscle deconditioning. However, because a 5-day bed rest results only in moderate neurovestibular and musculoskeletal changes, intermittent centrifugation should be further investigated during future long-duration bed rest studies.
The decision for implementing artificial gravity during human Mars missions will be a trade-off between mission resources and crew health benefits. Design options include spinning the whole vehicle, spinning part of the vehicle, or utilizing an onboard short-radius centrifuge. The conditions of the present study (darkness, lack of head movement) are compatible with the third option: intermittent centrifugation as a complement to physical exercise. More research is required to determine the optimal range of rotation rate, gravity gradient, and duration of exposure to centrifugation for mitigating the physiological deconditioning due to weightlessness. In addition, we need to know if any change to the current physical exercise regime is required when artificial gravity is used and whether there are gender differences in how humans adapt to rotating environments.
Acknowledgements
The authors are grateful to the subjects for their participation in this experiment, and the MEDES personnel who helped conduct the tests. This study was supported by ESA, CNES, and DLR.
Footnotes
Edited by: S. Warden
Refrences
- 1.Young LR. Artificial gravity considerations for a Mars exploration mission. Ann NY Acad Sci. 1999;28:367–378. doi: 10.1111/j.1749-6632.1999.tb09198.x. [DOI] [PubMed] [Google Scholar]
- 2.Clément G, Pavy-Le Traon A. Centrifugation as a countermeasure during actual and simulated microgravity: a review. Eur J Appl Physiol. 2004;92:235–248. doi: 10.1007/s00421-004-1118-1. [DOI] [PubMed] [Google Scholar]
- 3.Hargens AR, Bhattacharya R, Schneider SM. Space physiology VI: exercise, artificial gravity, and countermeasure development for prolonged space flight. Eur J Appl Physiol. 2013;113:2183–2192. doi: 10.1007/s00421-012-2523-5. [DOI] [PubMed] [Google Scholar]
- 4.Yajima K, Iwasaki K, Sasaki T, Miyamoto A, Hirayanagi K. Can daily centrifugation prevent the haematocrit increase elicited by 6-degree, head-down tilt? Pflugers Arch. 2000;441:R95–97. doi: 10.1007/s004240000351. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki KI, Sasaki T, Hirayanagi K, Yajima K. Usefulness of daily +2Gz load as a countermeasure against physiological problems during weightlessness. Acta Astronaut. 2001;49:227–235. doi: 10.1016/s0094-5765(01)00101-1. [DOI] [PubMed] [Google Scholar]
- 6.Iwase S. Effectiveness of centrifuge-induced artificial gravity with ergometric exercise as a countermeasure during simulated microgravity exposure in humans. Acta Astronaut. 2005;57:75–80. doi: 10.1016/j.actaastro.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Caiozzo VJ, Haddad F, Lee S, Baker M, Paloski WH, Baldwin KM. Artificial gravity as a countermeasure to microgravity: A pilot study examining the effects on knee extensor and plantar flexor muscle groups. J Appl Physiol. 2009;107:39–46. doi: 10.1152/japplphysiol.91130.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symons TB, Sheffield-Moore M, Chinkes DL, Ferrando AA, Paddon-Jones D. Artificial gravity maintains skeletal muscle protein synthesis during 21 days of simulated microgravity. J Appl Physiol. 2009;107:34–38. doi: 10.1152/japplphysiol.91137.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y-C, Yang C-B, Wu Y-H, Gao Y, Lu D-Y, Shi F, Wei X-M, Sun X-Q. Artificial gravity with ergometric exercise as a countermeasure against cardiovascular deconditioning during 4 days of head-down bed rest in humans. Eur J Appl Physiol. 2011;111:2315–2325. doi: 10.1007/s00421-011-1866-7. [DOI] [PubMed] [Google Scholar]
- 10.Stenger MB, Evans JM, Knapp CF, Lee SMC, Phillips TR, Perez SA, Moore AD, Paloski WH, Platts SH. Artificial gravity training reduces bed rest-induced cardiovascular deconditioning. Eur J Appl Physiol. 2012;112:605–616. doi: 10.1007/s00421-011-2005-1. [DOI] [PubMed] [Google Scholar]
- 11.Linnarsson D, Hughson RL, Fraser K, Clément G, Karlsson LL, Mulder E, Paloski WH, Rittweger J, Wuyts F, Zange J. Effects of an artificial gravity countermeasure on orthostatic tolerance, blood volumes and aerobic capacity after short-term bed rest. J Appl Physiol. 2014 doi: 10.1152/japplphysiol.00061.2014. doi:10.1152/japplphysiol.00061. [DOI] [PubMed] [Google Scholar]
- 12.Caiani EG, Massabuau P, Weinert L, Vaïda P, Lang RM. Effects of 5 days of head-down bed rest, with and without short-arm centrifugation as countermeasure, on cardiac function in males (BR-AG1 study) J Appl Physiol. 2014;117:624–632. doi: 10.1152/japplphysiol.00122.2014. [DOI] [PubMed] [Google Scholar]
- 13.Feuerecker M, Feuerecker B, Matzel S, Long M, Strewe C, Kaufmann I, Hoerl M, Schelling G, Rehm M, Choukèr A. Five days of head-down-tilt bed rest induces noninflammatory shedding of L-selectin. J Appl Physiol. 2013;115:235–242. doi: 10.1152/japplphysiol.00381.2013. [DOI] [PubMed] [Google Scholar]
- 14.Choukèr A, Feuerecker B, Matzel S, Kaufmann I, Strewe C, Hoerl M, Schelling G, Feuerecker M. Psychoneuroendocrine alterations during 5 days of head-down tilt bed rest and artificial gravity interventions. Eur J Appl Physiol. 2013;113:2057–2065. doi: 10.1007/s00421-013-2640-9. [DOI] [PubMed] [Google Scholar]
- 15.Kos O, Hughson RL, Hart DA, Clément G, Frings-Meuthen P, Linnarsson D, Paloski WH, Rittweger J, Wuyts F, Zange J, Gorczynski RM. Elevated serum soluble CD200 and CD200R as surrogate markers of bone loss under bed rest conditions. Bone. 2013;60C:33–40. doi: 10.1016/j.bone.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Mulder E, Linnarsson D, Paloski WH, Rittweger J, Wuyts F, Zange J, Clément G. Effects of five days of bed rest with and without exercise countermeasure on postural stability and gait. J Musculoskelet Neuronal Interact. 2014;14:359–366. [PubMed] [Google Scholar]
- 17.Herbert ME, Roy RR, Edgerton VR. 1988 Influence of one-week hindlimb suspension and in intermittent high load exercise on rat muscles. Exp Neurol. 1988;102:190–198. doi: 10.1016/0014-4886(88)90093-3. [DOI] [PubMed] [Google Scholar]
- 18.Ploutz-Snyder L. Current countermeasures: Benefits, consequences and uncertainties. Proceedings of the 2014 International Workshop on Research and Operational Considerations for Artificial Gravity Countermeasures, NASA Ames Research Center, February 19-20. 2014. [Accessed November 8 2014]. NASA/TM-2014-217394. NASA Johnson Technical Reports Server http://ston.jsc.nasa.gov/collections/TRS/_techrep/TM-2014-217394.pdf .
- 19.Vernikos J, Ludwig DA, Ertl AC, Wade CE, Keil L, O’Hara D. Effect of standing or walking on physiological changes induced by head down bed rest: Implications for spaceflight. Aviat Space Environ Med. 1996;67:1069–1079. [PubMed] [Google Scholar]
- 20.Clément G, Delière Q, Migeotte PF. Perception of verticality and cardiovascular responses during short-radius centrifugation. J Vestib Res. 2014;24:1–8. doi: 10.3233/VES-130504. [DOI] [PubMed] [Google Scholar]
- 21.Kaleps I, Clauser CE, Young JW, Chandler RF, Zehner GF, McConville JT. Investigation into the mass distribution properties of the human body and its segments. Ergonomics. 1984;27:1225–1237. doi: 10.1080/00140138408963604. [DOI] [PubMed] [Google Scholar]
- 22.Jarchow T, Young LR. Neurovestibular effects of bed rest and centrifugation. J Vestib Res. 2010;20:45–51. doi: 10.3233/VES-2010-0350. [DOI] [PubMed] [Google Scholar]
- 23.Golding JF. Predicting individual differences in motion sickness susceptibility by questionnaire. Personalily & Individual Differences. 1006;41:237–248. [Google Scholar]
- 24.Richards JA, Clark JB, Oman CM, Marshburn TH. Neurovestibular effects of long-duration space flight: A summary of Mir phase 1 experiences. NASA Johnson Space Center, Houston, Texas. 2002. [Accessed November 7 2014]. NASA Technical Reports Server http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20100033747.pdf .
- 25.Black FO. What can posturography tell us about vestibular function? Ann NY Acad Sci. 2001;942:446–464. doi: 10.1111/j.1749-6632.2001.tb03765.x. [DOI] [PubMed] [Google Scholar]
- 26.Black FO, Wall C, Nashner LM. Effects of visual and support surface orientation references upon postural control in vestibular deficit subjects. Acta Otolaryngol. 1983;95:199–210. doi: 10.3109/00016488309130936. [DOI] [PubMed] [Google Scholar]
- 27.Paloski WH, Wood SJ, Feiveson AH, Black FO, Hwang EY, Reschke MF. Destabilization of human balance control by static and dynamic head tilts. Gait Posture. 2006;23:315–323. doi: 10.1016/j.gaitpost.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Jain V, Wood SJ, Feiveson AH, Black FO, Paloski W. Diagnostic accuracy of dynamic posturography testing after short-duration spaceflight. Aviat Space Environ Med. 2010;81:625–631. doi: 10.3357/asem.2710.2010. [DOI] [PubMed] [Google Scholar]
- 29.Nashner LM. Computerized dynamic posturography: Clinical applications. In: Jacobson GP, Newman CW, Kartush JM, editors. Handbook of Balance Function Testing. Chicago, IL: Mosby-Year Book Inc; 1993. pp. 308–334. [Google Scholar]
- 30.Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Physical Therapy. 2007;77:812–819. doi: 10.1093/ptj/77.8.812. [DOI] [PubMed] [Google Scholar]
- 31.Feiveson AH, Metter EJ, Paloski WH. A statistical model for interpreting computerized dynamic posturography data. IEEE Transactions on Biomedical Engineering. 2002;49:300–309. doi: 10.1109/10.991157. [DOI] [PubMed] [Google Scholar]
- 32.Reschke MF, Bloomberg JJ, Harm DL, Paloski WH, Layne C, McDonald V. Posture, locomotion, spatial orientation, and motion sickness as a function of space flight. Brain Res Rev. 1998;28:102–117. doi: 10.1016/s0165-0173(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 33.Paloski WH, Reschke MF, Black FO, Doxey DD, Harm DL. Recovery of postural equilibrium control following spaceflight. Ann NY Acad Sci. 1992;656:747–754. doi: 10.1111/j.1749-6632.1992.tb25253.x. [DOI] [PubMed] [Google Scholar]
- 34.Reschke MF, Bloomberg JJ, Paloski WH, Mulavara AP, Feiveson AH, Harm DL. Postural reflexes, balance control, and functional mobility with long-duration head-down bed rest. Aviat Space Environ Med. 2009;80:A45–A54. doi: 10.3357/asem.br06.2009. [DOI] [PubMed] [Google Scholar]
- 35.Horak FB, Nashner LM. Central programming of postural movements: Adaptation to altered support-surface configurations. J Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 36.Gardner EP, Martin JH. Coding of Sensory Information. In: Kandel ER, et al., editors. Principles of Neural Science. 4th edition. New York: McGraw Hill; 2000. pp. 425–439. [Google Scholar]


