Abstract
Objectives:
To evaluate the association between radiographically-assessed knee osteoarthritis and femoral neck bone characteristics in women with mild knee radiographic osteoarthritis and those without radiographic osteoarthritis.
Methods:
Ninety postmenopausal women (mean age [SD], 58 [4] years; height, 163 [6] cm; weight, 71 [11] kg) participated in this cross-sectional study. The severity of radiographic knee osteoarthritis was defined using Kellgren-Lawrence grades 0=normal (n=12), 1=doubtful (n=25) or 2=minimal (n=53). Femoral neck bone mineral content (BMC), section modulus (Z), and cross-sectional area (CSA) were measured with DXA. The biochemical composition of ipsilateral knee cartilage was estimated using quantitative MRI measures, T2 mapping and dGEMRIC. The associations between radiographic knee osteoarthritis grades and bone and cartilage characteristics were analyzed using generalized linear models.
Results:
Age-, height-, and weight-adjusted femoral neck BMC (p for linearity=0.019), Z (p for linearity=0.033), and CSA (p for linearity=0.019) increased significantly with higher knee osteoarthritis grades. There was no linear relationship between osteoarthritis grades and knee cartilage indices.
Conclusions:
Increased DXA assessed hip bone strength is related to knee osteoarthritis severity. These results are hypothesis driven that there is an inverse relationship between osteoarthritis and osteoporosis. However, MRI assessed measures of cartilage do not discriminate mild radiographic osteoarthritis severity.
Keywords: Osteoarthritis, Bone Strength, Kellgren and Lawrence Grade, Quantitative MRI, Postmenopausal Women
Introduction
Osteoarthritis (OA) and osteoporosis (OP) may cause serious morbidity and impose a substantial burden on the health care system, especially among elderly women. Although OA affects all of tissues within a joint, a characteristic aspect of an osteoarthritic joint is a progressive degeneration of articular cartilage. In OP, the related bone loss leads to an increased risk of fracture. The relationship between these two diseases is not well understood and is a topic of controversy[1].
Even though clinical experience and some studies indicate that OA and OP are not mutually exclusive[2], previous reports, including large epidemiological studies and subsequent cross-sectional studies, suggested that OA is associated with higher bone mineral mass or density[3-6]. Moreover, subjects with OA seem to not only have higher bone mineral mass, but also bigger bone size compared with healthy controls[7]. Also, women with OA are observed to have larger muscle mass and force compared with age- and body size-matched osteoporotic women[8]. From the perspective of bone and cartilage interaction, there is some evidence showing that bone mineral mass is associated with knee cartilage volume in healthy adults[9,10]. In a longitudinal analysis, bone loss was associated with loss of cartilage volume in subjects with knee OA[11].
In addition to cartilage volumetric assessment, recent advances in quantitative magnetic resonance imaging (qMRI) techniques have enabled the use of biomarkers for structure and biochemical composition of cartilage. T2 relaxation time mapping is a qMRI technique sensitive to the integrity of the collagen network, collagen orientation, and hydration[12], whereas the delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) is sensitive to cartilage glycosaminoglycan (GAG) content[13-15]. Analogously, in order to be able to analyze bone structure and strength, software for dual energy x-ray absorption meter (DXA) and advanced hip structure analysis (AHA) were developed to derive hip geometry from bone mineral data for an estimate of hip strength[16]. Thus, concurrent use of qMRI and AHA provides potential tools for determining the biochemical composition of cartilage, and assessing bone structure and strength prior to gross morphologic changes of cartilage or bone fracture have occurred. However, to our knowledge there are no studies in subjects with mild OA and who may be at risk of OP focused on bone mass and strength and cartilage biochemical composition. Therefore, the aim of the present study was to investigate the association between radiographic knee OA with femoral neck bone characteristics using AHA in a sample of postmenopausal women with mild knee radiographic OA and those without radiographic OA.
Materials and methods
Study participants
Baseline data from the previously reported randomized controlled trial (RCT)[17] with two trial arms: 1) a high-impact exercise and 2) a nonintervention were used in the current cross-sectional study. Seventy-eight postmenopausal women with mild knee OA were recruited via newspaper advertisement from the Central-Finland area. During the screening process of study participants, knee OA was radiographically confirmed at the symptomatic or most symptomatic knee. Subjects with Kellgren-Lawrence (K/L) grade 1 or 2 radiographic OA changes[18] in the tibiofemoral joint were included, while subjects with moderate or severe knee OA (K/L grades 3 or 4) were excluded after screening process. In addition of having knee K/L grade 1 or 2, other eligibility criteria for the RCT and the current cross-sectional study were postmenopausal status, age 50-65 years, knee pain on most days within the preceding year, no more than twice weekly regular intensive exercise, and no illness that contraindicated or limited participation in the exercise intervention. A subject was excluded if her T-score for femoral neck bone mineral density (BMD, g/cm2) was lower than -2.5; body mass index (BMI, kg/m2) was higher than 35 kg/m2; and if she had any previous knee instability or severe trauma, inflammatory joint disease, or knee intra-articular steroid injections in the preceding 12 months. Moreover, a subject was excluded if she had any known contraindications to MRI, any known allergies to contrast agents, or renal insufficiency. Additionally, an age-, weight-, and height-matched sample of 12 women with no considerable knee symptoms were recruited to the study as a reference group from the same source population as those who were recruited into the parent RCT. The inclusion and exclusion criteria were same as for the women with mild knee OA, except that the women without clinically significant symptoms should not have had any frequent pain, aching or stiffness in or around the knee joint in either knee in the preceding year. The suitability of the women without significant symptoms was confirmed by the radiographs, and only subjects who had no radiographic OA changes (i.e., K/L grade 0) in both tibiofemoral joints were entered into the study. The exercise study protocol with the amendment of the study protocol of women without significant symptoms and radiographic OA (hereafter called K/L 0 group) were in agreement with Helsinki declaration with the approval of the Ethics Committee of the Central Finland Health Care District. Written informed consent was obtained from all participants prior to enrollment.
Knee radiography
The radiographs were acquired during screening examinations from both knees with a postero-anterior view of the tibiofemoral joint in a semi-flexed weight-bearing position. An experienced musculoskeletal radiologist blinded to subjects graded the X-rays according the radiological grading scale of Kellgren and Lawrence (K/L). This system uses the following global grades (for tibiofemoral OA): 0 = normal, 1=doubtful (possible osteophytes), or 2=minimal (definite osteophytes, possible joint space narrowing)[18]. In subjects with OA, the final grade was the highest one for the most severely-affected knee.
Bone assessment
Proximal femurs were scanned with dual-energy X-ray absorptiometry (DXA, GE Medical System, Lunar Prodigy, Madison WI, USA) at the narrowest femoral neck section from the side of the higher K/L grade knee in subjects with knee OA. Femoral neck bone mineral content (BMC, g) was used in the analysis, whereas femoral neck areal bone mineral density (BMD, g/cm2) was used during screening of the study participants. Femoral neck cross-sectional area (CSA, [mm2], the surface area of bone in the cross-section after excluding all trabecular and soft tissue space), and the section modulus (Z, [mm3], an index of bending strength) were calculated with advanced hip structure analysis (AHA). In K/L 0 group, the average bone trait value of both femoral neck sites was calculated for the analysis.
The root mean square coefficient of variation (CVRMS) for femoral neck BMC measurements with this population was 0.6%. Reproducibility of the DXA measurements was tested from the duplicate measurements of 8 participants within an average 6 (SD 2) days between imaging sessions. The scanner was calibrated daily with bone phantoms (GE Medical System, Lunar Prodigy, Madison WI, USA) for quality assurance.
Knee cartilage assessment
Transverse relaxation time (T2) and dGEMRIC index, i.e., T1 relaxation time in the presence of Gd-DTPA2- were determined using a Siemens Magnetom Symphony Quantum 1.5 T scanner (Siemens AG, Medical Solutions, Erlangen, Germany) with a standard transmit/receive knee array coil. Prior to the measurements, experienced radiologists and technicians were specifically trained to run the MRI research protocol. The participants were imaged lying supine. In the subjects with radiographically-confirmed OA, scans were performed on the side with the higher K/L grade knee, and in the right knee of K/L 0 group. The flexion angle and rotation of the knee was controlled by stabilizing the leg in a fixed position with a leg holder and a custom-made inflatable cushion. T2 mapping, which was performed prior to the dGEMRIC experiment, was carried out using a sagittal multislice, multiecho, fast-spin echo sequence (field of view [FOV] 140 mm, acquisition matrix 256×256, repetition time [TR] 2090 ms, eight echo times [TE] between 13 and 104 ms, echo train length [ETL] 8, 3-mm slice thickness, imaging time=8 min 55 s). The slices were positioned perpendicular to a line tangential to the posterior femoral condyles in the axial scout view. One slice covering the central region of the medial and lateral condyles, were chosen for the analyses. Monoexponential fitting was used to compute relaxation time maps.
For the dGEMRIC imaging, a double dose i.e., 0.4 ml/kg (0.2 mM/kg) intravenous administration of Gd-DTPA2- (Magnevist, Schering, Berlin) was followed by a 90-minute delay with active flexion-extension exercises of the knee for 5 min while sitting, then while walking for 5 min and stair climbing for 5 min in order to enhance the penetration of contrast agent into the knee cartilage. T1 mapping was performed in the sagittal plane using a single slice inversion recovery fast-spin echo sequence (FOV=14 cm, matrix 256×256, TR=1800 ms, TE=13 ms, inversion time TI=50, 100, 200, 400, 800, 1600 ms, echo train length [ETL] 5, 3-mm slice thickness, imaging time per image=6 min 50 s). The slices were positioned perpendicular to a line tangential to the posterior femoral condyles in the axial scout view. The remaining slice was then positioned at the center of the medial and lateral condyles as viewed on the axial scout image.
Full-thickness cartilage regions-of-interest (ROIs) were manually segmented from the most load-bearing areas from single sagittal slices at the center of the medial and lateral femoral and tibial condyles using an in-house MATLAB application (Mathworks, Inc. Natick, MA, USA). The femoral ROI was defined anteriorly at a point opposite the middle of the tibial plateau regarding antero-posterior position, and posteriorly at a point of posterior end of meniscus towards the femoral bone. The tibial ROI was defined anteriorly at a point of meniscus’ anterior tip of horn towards the tibial bone, and posteriorly at a point of meniscus’ posterior tip of horn towards the tibial bone. Figure 1 shows ROIs from the center of the lateral tibiofemoral compartment. dGEMRIC index is an average T1 spin lattice relaxation time in the presence of Gd-DTPA2- in a given ROI. The dGEMRIC indices were corrected by body mass indices (BMIs) as suggested by Tiderius et al.[19]. On average, the precision (CVRMS) of dGEMRIC in asymptomatic subjects has been shown to be 7% for full-thickness ROIs and 5% for bulk cartilage[20]. The inter-observer error in our laboratory between two independent cartilage investigators (J.M. and E.L., with 6 and 12 years of experience in cartilage analyzing, respectively) was on average 2% for T2 full-thickness ROIs and 3% for dGEMRIC.
Figure 1.
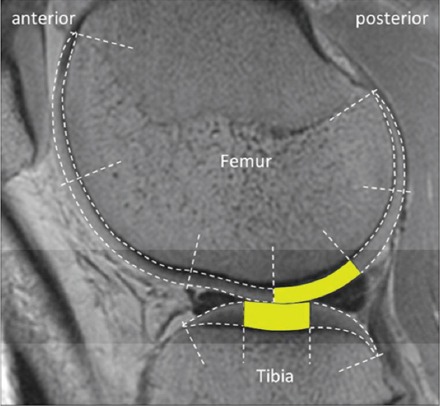
Sagittal T2 image of the segmented and analyzed cartilage regions-of-interest (ROIs) in lateral tibiofemoral compartment. The analyzed ROIs are shown as yellow-colored areas. In the study, the corresponding ROIs were also analyzed from a single sagittal slice in medial tibiofemoral compartment.
Physical function
Agility or dynamic balance was assessed with a standardized figure-of-eight running test of two laps around two poles placed 10 m apart[21]. Maximal isometric knee extension and flexion force was measured in a sitting position with a knee angle of 60° using a dynamometer chair (Good Strength, Metitur Oy, Jyväskylä, Finland)[22]. Leg power was determined by a maximal vertical counter movement jump on a force platform (University of Jyväskylä, Finland)[23]. Cardiorespiratory fitness (estimated oxygen consumption at maximum exertion i.e., VO2max, mL/kg/min) was assessed with a standardized 2-km Walk Test (UKK Institute, Finland)[24].
Questionnaires
Knee pain, stiffness, and self-rated physical functioning were assessed with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)[25]. General health and leisure time physical activity were assessed by a questionnaire. A health questionnaire addressed medical conditions, current medications and leisure time physical activity, which was converted into metabolic equivalent (MET) hours per week[26].
Statistical analysis
The results are presented using means, standard deviations (SD) and frequency distributions. Statistical significance for the hypothesis of linearity between radiographic knee OA grades (0 normal, 1 doubtful and 2 minimal) and bone and cartilage characteristics were evaluated by using generalized linear models with appropriate distribution and link function. In addition, the associations of bone and cartilage traits with K/L grading were tested with an effect size, which was calculated using partial Eta-squared (η2). By convention, values of 0.01, 0.06, and 0.14 are called small, medium, and large effect sizes, respectively[27]. The bone traits and cartilage T2 relaxation times were adjusted by age, body mass, and height. The cartilage dGEMRIC indices were adjusted for age. The normality of the variables was tested by using the Shapiro-Wilk W test. All reported p values are two sided, and statistical significance was set at <0.05. Statistical analyses were conducted using Stata v.12.1 (StataCorp, College Station, TX, USA).
Results
[Table 1] shows the demographic and clinical characteristics of the subjects according to radiographic K/L grades. There was a linear relationship between perceived knee pain, stiffness, physical functioning, and OA grade, showing that the higher the OA grade was, the more the subjects experienced knee pain (p=0.008 for linearity), stiffness (p=0.036), and physical disability (p=0.033). There were no linear relationships between the OA grade and use of medication, leisure time physical activity, or physical function measures (Table 1).
Table 1.
Demographic and clinical characteristics of the study population according to radiographic Kellgren-Lawrence (K/L) grades.
| Variable | K/L 0 (n=12) | K/L 1 (n=25) | K/L 2 (n=53) | P for linearity |
|---|---|---|---|---|
| Mean age (SD), years | 58 (3) | 58 (4) | 59 (4) | 0.52 |
| Mean height (SD), cm | 161 (6) | 163 (5) | 163 (6) | 0.64 |
| Mean body mass (SD), kg | 67.5 (10.8) | 69.9 (9.6) | 72.1 (11.3) | 0.15 |
| Mean body mass index (SD), kg/m2 | 25.8 (3.7) | 26.3 (3.1) | 27.2 (3.9) | 0.16 |
| Mean time from menopause (SD), years | 8.3 (5.5) | 9.1 (5.9) | 9.0 (5.4) | 0.81 |
| Current HRTa users, n (%) | 3 (25) | 13 (52) | 18 (34) | 0.88 |
| Pain killers, n (%) | 7 (58) | 13 (52) | 28 (53) | 0.80 |
| Occasional glucosamine use, n (%) | 0 (0) | 7 (28) | 14 (26) | 0.15 |
| WOMAC, range 0–100 | ||||
| Pain | 2 (5) | 6 (5) | 8 (8) | 0.008 |
| Stiffness | 5 (10) | 8 (9) | 11 (12) | 0.036 |
| Physical functionb | 2 (4) | 4 (4) | 5 (5) | 0.033 |
| Leisure time physical activity, METh/week | 18.1 (12.6) | 15.6 (7.0) | 19.9 (17.8) | 0.41 |
| Muscle force, N | ||||
| knee extension | 381 (49) | 384 (79) | 417 (82) | 0.11e |
| knee flexion | 172 (44) | 174 (53) | 184 (54) | 0.55e |
| Power, W | 1794 (286) | 1888 (375) | 1871 (363) | 0.97e |
| Dynamic balancec, s | 16.5 (1.7) | 17.2 (1.3) | 17.2 (2.4) | 0.87e |
| VO2maxd, mL/kg/min | 29.4 (2.9) | 29.4 (2.9) | 28.8 (4.5) | 0.61e |
Hormone replacement therapy.
Negative number indicates better physical functioning.
Negative number indicates better balance.
VO2max, estimated maximal oxygen uptake.
Adjusted for age and body mass.
In the femoral neck, there was a statistically significant linear trend showing that BMC, Z, and CSA increased with higher OA grades (Table 2). The effect sizes indicated that in the bone traits the linear association was at moderate level, i.e. in BMC the η2 was 0.08, in Z 0.04 and in CSA it was 0.05. The age, body mass, and height-adjusted mean ratio of BMC was 1.10 (95% CI: 1.01 to 1.19), Z was 1.12 (95% CI: 1.02 to 1.21), and CSA was 1.08 (95% CI: 1.01 to 1.16) in the K/L 2 group compared with the K/L 0. The ratios did not differ between the K/L 1 and K/L 0 grades.
Table 2.
Bone and cartilage trait values (mean, SD) from different anatomical regions according to radiographic Kellgren-Lawrence (K/L) grades.
| Variable | K/L 0 (n=12) | K/L 1 (n=25) | K/L 2 (n=53) | P for linearity |
|---|---|---|---|---|
| Bone trait | ||||
| Bone mineral content (BMC), g | 4.183 (0.836) | 4.463 (0.874) | 4.726 (0.575) | 0.019a |
| Section modulus (Z), mm3 | 543 (98) | 611 (170) | 628 (101) | 0.033a |
| Cross sectional area (CSA), mm2 | 135 (22) | 142 (28) | 150 (19) | 0.019a |
| T2, ms | ||||
| MEDIAL CONDYLE | ||||
| Posterior part of central femur | 50.4 (2.8) | 49.6 (6.3) | 50.2 (5.4) | 0.85a |
| Central tibia | 44.2 (5.2) | 44.9 (4.8) | 45.1 (4.2) | 0.60a |
| LATERAL CONDYLE | ||||
| Posterior part of central femur | 50.8 (5.4) | 50.5 (6.0) | 50.4 (5.6) | 0.83a |
| Central tibia | 38.4 (2.4) | 43.5 (6.5) | 42.0 (7.3) | 0.20a |
| dGEMRIC index, ms | ||||
| MEDIAL CONDYLE | ||||
| Posterior part of central femur | 446 (60) | 461 (55) | 456 (78) | 0.79b |
| Central tibia | 362 (67) | 412 (72) | 402 (58) | 0.17b |
| LATERAL CONDYLE | ||||
| Posterior part of central femur | 474 (67) | 512 (75) | 469 (59) | 0.28b |
| Central tibia | 426 (107) | 433 (82) | 437 (77) | 0.63b |
Adjusted for age, body mass, and height.
Adjusted for age.
In knee cartilage regions, none of the T2 or dGEMRIC indices in the center of the medial or lateral femoral or tibial condyles were linearly related with radiographic grades (Table 2). The effect sizes indicated that in the cartilage traits, the linear associations were considered as not meaningful. There were no relationships between BMC and T2 or dGEMRIC index at any anatomical site (Figures 2 and 3, respectively).
Figure 2.
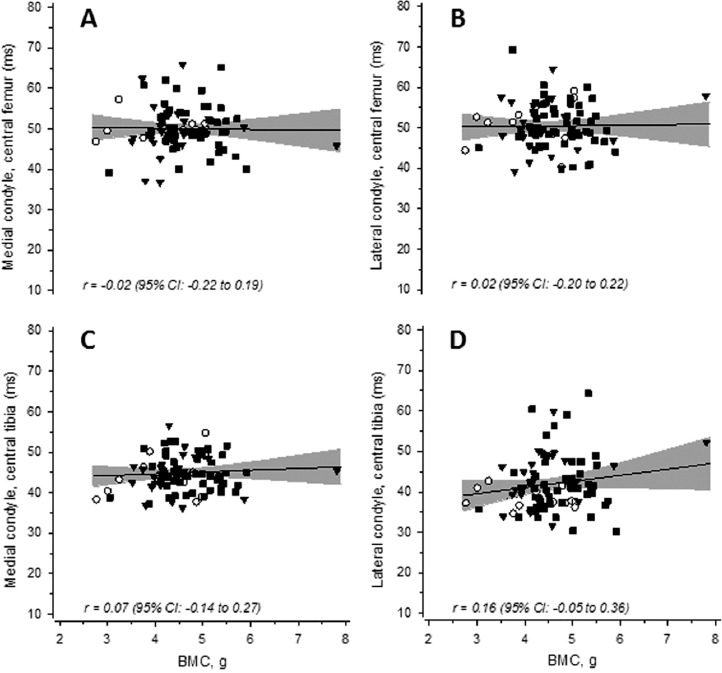
Scatter plots showing the association between the femoral neck bone mineral content (BMC) and knee cartilage T2 relaxation times. Panels in the upper row show the association between BMC and T2 in the posterior part of the central femoral cartilage in both the medial (A) and lateral (B) condyles. Panels in the lower row show the corresponding associations in the central part of the medial (C) and lateral (D) tibial condyles. ○= K/L 0; ▼= K/L 1; ■= K/L 2. The gray band shows the 95% confidence intervals.
Figure 3.
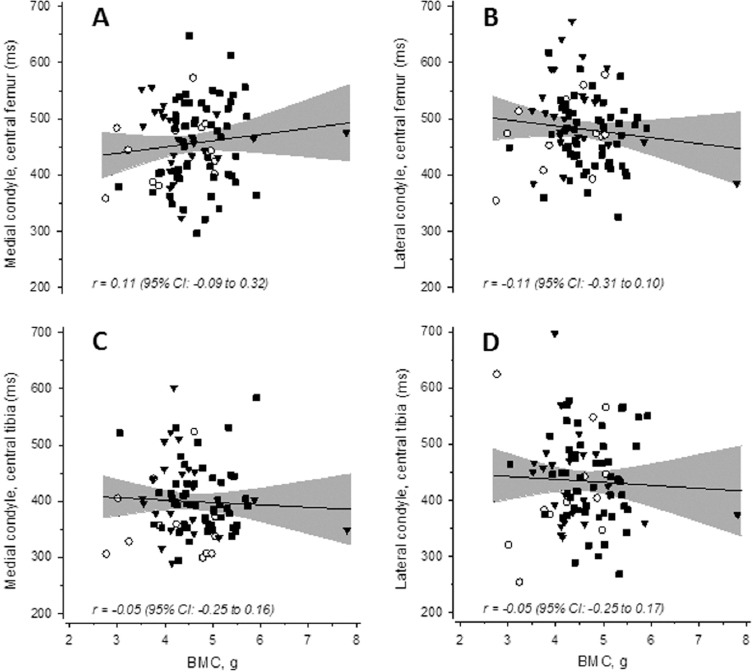
Scatter plots showing the associations between femoral neck bone mineral content (BMC) and knee cartilage dGEMRIC indices. Panels in the upper row show the association between the BMC and dGEMRIC index in the posterior part of the central femoral cartilage in both the medial (A) and lateral (B) condyles. Panels in the lower row show the corresponding associations in the central part of the medial (C) and lateral (D) tibial condyles. ○= K/L 0; ▼= K/L 1; ■= K/L 2. The gray band indicates the 95% confidence intervals.
Discussion
The primary finding of this study was that there was a linear association between femoral neck mineral mass and bending strength, and radiographic grades from K/L 0 to K/L 2. In other words, as the knee OA became radiographically worse the femoral neck bone mineral mass and strength increased. We also found that T2 or dGEMRIC index which reflect the biochemical composition of knee cartilage, was not related to radiographic OA grades. It is also noteworthy that knee pain, stiffness, and physical function disability increased significantly with OA grades, although the symptom values were relatively low even in the highest OA grade (K/L 2).
The bone results of this study are consistent with those of previous large epidemiological surveys showing that radiographic knee OA is associated with high femoral neck bone mineral mass[28-31]. However, so far there are no studies that have simultaneously investigated the relationship between hip bone structure and strength, and estimated biochemical composition of cartilage in mild knee OA. The mechanism whereby OA subjects have more advanced bone structure and strength than non-OA subjects remains unknown. However, Burr & Gallant[32] have suggested that because of the rapid turnover of bone in early OA, the subchondral bone plate and calcified cartilage thickens leading to a subchondral sclerosis while the subchondral trabecular bone may even remain osteopenic. Whether these subchondral bone changes occur at the same time as cartilage deteriorates, and whether these changes occur in more local or general level, is still debatable. Nevertheless, most of the studies in hip and knee OA subjects have demonstrated increased bone mineral mass or density values of different skeletal sites compared with age-matched healthy controls[6,31,33]. In contrast, there are also indications that knee OA is associated with significantly lower BMD values in the affected side compared with the contralateral hip[34], thus, it is important to investigate associations between cartilage and bone at the different sites in same limb.
Our result showing that T2 values were not linearly associated with radiographic OA grades is in accordance with the findings by Koff et al.[35] indicating no association between T2 values and patellar cartilage OA grades. However, our finding is contrary to that of Dunn et al.[36] and Li et al.[37], who showed that regional T2 relaxation times became elongated (high value correspond to compromised cartilage structure degeneration) with higher OA grades. The results of Dunn et al.[36] and Li et al.[37] should be considered with caution, however, due to small sample sizes, and a limited number of echo images used to determine T2 values in the study by Dunn et al.[36]. Similarly, dGEMRIC index showed no association with OA grades. This is in line with the study by Williams et al.[38] who found no relationship between dGEMRIC index and knee OA (K/L grades 1-4) in erosive, narrowed, tibiofemoral joint space compartments. However, when they also analyzed compartments without joint space narrowing, there was a trend toward lower dGEMRIC indices with increasing K/L grades, yet there was significant overlap in dGEMRIC indices between different OA grades. The study was limited by the small sample sizes in different K/L grades.
All in all, the quantitative MRI methodology applied was unable to categorize subjects with mild knee OA according to K/L radiographic criteria. This result could be due to the techniques used (X-ray and qMRI) that focus on different tissue types. In OA, both osteophyte formation and increased bone mineral mass are features of bone, which were measured using the same methodology derived from increased attenuation of X-rays in bone mineral mass. Thus, these bone hypertrophy forms can be expected to be associated with each other. However, the qMRI methods are surrogates for cartilage matrix constituents, indicating cartilage biology beyond radiographic detection. Moreover, the cartilage compositional changes most likely precede changes in bone and cartilage quantity in early OA. Furthermore, there are conflicting results about the cartilage GAG content changes in early OA[39]. GAG content may increase[40,41] or diminish[42,43] in early OA. For instance, in a recent study Stubendorff et al.[44] suggested that dGEMRIC-derived sulphated glycosaminoglycan (sGAG) content may remain at rather constant levels in early hip OA due to compensative sGAG synthesis. This might explain our findings why there were no linear associations between dGEMRIC indices and radiographic knee OA grades, or between dGEMRIC indices and femoral neck BMC. An anomalous behavior of cartilage GAG content may reflect a more complex pattern of early OA progression than anticipated. Alternatively, dGEMRIC may not be a specific method for detecting early cartilage degeneration. On the other hand, previous cross-sectional studies reported differences in dGEMRIC indices between asymptomatic versus OA knees[45], and arthroscopically normal femoral compartments versus diseased compartments[46]. Nonetheless, our qMRI results challenge the role of radiographic evaluation of OA, since articular cartilage has an important role in OA development and is not adequately addressed using K/L scoring.
The strength of the present study is that, to our knowledge, it is the first to simultaneously investigate the relationship between bone mineral mass and OA by detecting bone structural characteristics and articular cartilage measures. In previous knee OA and OP relationship studies, which were published largely in the 1990s[28-31], the biochemical composition of knee cartilage was not taken into account mainly because appropriate in vivo cartilage imaging techniques were not available. Additionally, recent cross-sectional studies using standard MRI techniques have focused on delineating cartilage morphology[9,10] rather than the biochemical composition of cartilage. Standard MRI sequences, however, cannot detect the initial stages of OA, including glycosaminoglycan loss, increased water content, and disorganization of the collagen network[47].
The present study has some limitations. Only one radiologist graded knee OA according the K/L scoring system; however, it is unlikely that two radiologists would have improved the grading reliability as we had a musculoskeletal radiologist with 20 years of experience. The study was also limited because bone traits in the hips and cartilage traits in the knees according to radiographic knee OA were not obtained of the same body part. This cannot though be considered as a crucial failure due to systemic disease component of both OP and OA. The participants’ history of development of knee OA was not available. In addition, we had a rather select group of study participants with K/L grades 1 and 2 because of the related exercise intervention study[17], and a relatively small sample size in the K/L grade 0 group. To investigate this aspect, further investigations including subjects across a radiographic spectrum of OA are required with prospective longitudinal follow-ups.
In conclusion, this study demonstrated that femoral neck structure, strength and mineral mass were inversely related to mild knee OA in postmenopausal women, i.e. the femoral neck rigidity improvement was associated with severity of radiographic knee OA. These results have implications for the hypothesis that there is an inverse relationship between OA and OP. However, with this population there was no association between radiographic OA grades and knee cartilage biochemical composition assessed using quantitative MRI measures; T2 relaxation time and dGEMRIC. The reason for this inconsistent periarticular cartilage-bone interaction remains unclear, but the radiographic K/L scoring system may not be sensitive to cartilage changes in early to mid-stage knee OA.
Acknowledgements
The authors gratefully acknowledge Dr. Risto Ojala, MD in the Department of Diagnostic Radiology at Oulu University Hospital, Finland, for reading the radiographs and Dr. Timo Rantalainen in the Department of Health Sciences at University of Jyväskylä, Finland, and in the Center for Physical Activity and Nutrition Research, School of Exercise and Nutrition Sciences, Deakin University Melbourne, Australia, for helping assemble the dataset used in this analysis. This research was supported by the Academy of Finland (grants 123140, 128603 and 260321), the Finnish Ministry of Education and Culture, the Yrjö Jahnsson Foundation, the Finnish Cultural Foundation, the Finnish Rheumatism Foundation, the Juho Vainio Foundation, the Emil Aaltonen Foundation, the Central Finland Health Care District, and the Finnish Doctoral Programme of Musculoskeletal Disorders and Biomaterials (TBDP).
Footnotes
Edited by: S. Warden
Refrences
- 1.Im GI, Kim MK. The relationship between osteoarthritis and osteoporosis. J Bone Miner Metab. 2014;32:101–9. doi: 10.1007/s00774-013-0531-0. [DOI] [PubMed] [Google Scholar]
- 2.McDonald Blumer MH. Bone mineral content versus bone density in a population with osteoarthritis: A new twist to the controversy? J Rheumatol. 2005;32:1868–9. [PubMed] [Google Scholar]
- 3.Dequeker J, Aerssens J, Luyten FP. Osteoarthritis and osteoporosis: Clinical and research evidence of inverse relationship. Aging Clin Exp Res. 2003;15:426–39. doi: 10.1007/BF03327364. [DOI] [PubMed] [Google Scholar]
- 4.Lo GH, Tassinari AM, Driban JB, et al. Cross-sectional DXA and MR measures of tibial periarticular bone associate with radiographic knee osteoarthritis severity. Osteoarthritis Cartilage. 2012;20:686–93. doi: 10.1016/j.joca.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Kim TN, Kim SH. Knee osteoarthritis is associated with increased prevalence of vertebral fractures despite high systemic bone mineral density: A cross-sectional study in an Asian population. Mod Rheumatol. 2014;24:172–81. doi: 10.3109/14397595.2013.854060. [DOI] [PubMed] [Google Scholar]
- 6.Okano K, Ito M, Aoyagi K, Osaki M, Enomoto H, Yamaguchi K. Discrepancy in bone mineral densities at different skeletal sites in hip osteoarthritis patients. Mod Rheumatol. 2014;24:340–2. doi: 10.3109/14397595.2013.854078. [DOI] [PubMed] [Google Scholar]
- 7.Arokoski JP, Arokoski MH, Jurvelin JS, Helminen HJ, Niemitukia LH, Kröger H. Increased bone mineral content and bone size in the femoral neck of men with hip osteoarthritis. Ann Rheum Dis. 2002;61:145–50. doi: 10.1136/ard.61.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dequeker J, Goris P, Uytterhoeven R. Osteoporosis and osteoarthritis (osteoarthrosis). Anthropometric distinctions. JAMA. 1983;249:1448–51. [PubMed] [Google Scholar]
- 9.Cicuttini F, Wluka A, Davis S, Strauss BJ, Yeung S, Ebeling PR. Association between knee cartilage volume and bone mineral density in older adults without osteoarthritis. Rheumatology (Oxford) 2004;43:765–9. doi: 10.1093/rheumatology/keh171. [DOI] [PubMed] [Google Scholar]
- 10.Brennan SL, Pasco JA, Cicuttini FM, et al. Bone mineral density is cross sectionally associated with cartilage volume in healthy, asymptomatic adult females: Geelong osteoporosis study. Bone. 2011;49:839–44. doi: 10.1016/j.bone.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Harvey WF, Price LL, Paulus JK, Dawson-Hughes B, McAlindon TE. Relationship of bone mineral density to progression of knee osteoarthritis. Arthritis Rheum. 2013;65:1541–6. doi: 10.1002/art.37926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9:393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- 13.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–73. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 14.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857–65. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Nieminen MT, Nissi MJ, Mattila L, Kiviranta I. Evaluation of chondral repair using quantitative MRI. J Magn Reson Imaging. 2012;36:1287–99. doi: 10.1002/jmri.23644. [DOI] [PubMed] [Google Scholar]
- 16.Beck TJ. Extending DXA beyond bone mineral density: Understanding hip structure analysis. Curr Osteoporos Rep. 2007;5:49–55. doi: 10.1007/s11914-007-0002-4. [DOI] [PubMed] [Google Scholar]
- 17.Multanen J, Nieminen MT, Häkkinen A, et al. Effects of high-impact training on bone and articular cartilage:12-month randomized controlled quantitative MRI study. J Bone Miner Res. 2014;29:192–201. doi: 10.1002/jbmr.2015. [DOI] [PubMed] [Google Scholar]
- 18.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiderius C, Hori M, Williams A, et al. dGEMRIC as a function of BMI. Osteoarthritis Cartilage. 2006;14:1091–7. doi: 10.1016/j.joca.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Multanen J, Rauvala E, Lammentausta E, et al. Reproducibility of imaging human knee cartilage by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1.5 tesla. Osteoarthritis Cartilage. 2009;17:559–64. doi: 10.1016/j.joca.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Vartiainen MV, Rinne MB, Lehto TM, Pasanen ME, Sarajuuri JM, Alaranta HT. The test-retest reliability of motor performance measures after traumatic brain injury. Advances in Physiotherapy. 2006;8:50–9. [Google Scholar]
- 22.Sipilä S, Multanen J, Kallinen M, Era P, Suominen H. Effects of strength and endurance training on isometric muscle strength and walking speed in elderly women. Acta Physiol Scand. 1996;156:457–64. doi: 10.1046/j.1365-201X.1996.461177000.x. [DOI] [PubMed] [Google Scholar]
- 23.Rantalainen T, Nikander R, Heinonen A, et al. Neuromuscular performance and body mass as indices of bone loading in premenopausal and postmenopausal women. Bone. 2010;46:964–9. doi: 10.1016/j.bone.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Laukkanen RM, Kukkonen-Harjula TK, Oja P, Pasanen ME, Vuori IM. Prediction of change in maximal aerobic power by the 2-km walk test after walking training in middle-aged adults. Int J Sports Med. 2000;21:113–6. doi: 10.1055/s-2000-8872. [DOI] [PubMed] [Google Scholar]
- 25.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–81. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J: Lawrence Erlbaum; 1988. [Google Scholar]
- 28.Hannan MT, Anderson JJ, Zhang Y, Levy D, Felson DT. Bone mineral density and knee osteoarthritis in elderly men and women the framingham study. Arthritis Rheum. 1993;36:1671–80. doi: 10.1002/art.1780361205. [DOI] [PubMed] [Google Scholar]
- 29.Hart DJ, Mootoosamy I, Doyle DV, Spector TD. The relationship between osteoarthritis and osteoporosis in the general population: The chingford study. Ann Rheum Dis. 1994;53:158–62. doi: 10.1136/ard.53.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burger H, van Daele PL, Odding E, et al. Association of radiographically evident osteoarthritis with higher bone mineral density and increased bone loss with age. The Rotterdam study. Arthritis Rheum. 1996;39:81–6. doi: 10.1002/art.1780390111. [DOI] [PubMed] [Google Scholar]
- 31.Sowers M, Lachance L, Jamadar D, et al. The associations of bone mineral density and bone turnover markers with osteoarthritis of the hand and knee in pre- and perimenopausal women. Arthritis Rheum. 1999;42:483–9. doi: 10.1002/1529-0131(199904)42:3<483::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 32.Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8:665–73. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- 33.Nevitt MC, Lane NE, Scott JC, et al. Radiographic osteoarthritis of the hip and bone mineral density the study of osteoporotic fractures research group. Arthritis Rheum. 1995;38:907–16. doi: 10.1002/art.1780380706. [DOI] [PubMed] [Google Scholar]
- 34.Soininvaara TA, Miettinen HJ, Jurvelin JS, Alhava EM, Kröger HP. Bone mineral density in the proximal femur and contralateral knee after total knee arthroplasty. J Clin Densitom. 2004;7:424–31. doi: 10.1385/jcd:7:4:424. [DOI] [PubMed] [Google Scholar]
- 35.Koff MF, Amrami KK, Kaufman KR. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15:198–204. doi: 10.1016/j.joca.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: Comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–8. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–97. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams A, Sharma L, McKenzie CA, Prasad PV, Burstein D. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage in knee osteoarthritis: Findings at different radiographic stages of disease and relationship to malalignment. Arthritis Rheum. 2005;52:3528–35. doi: 10.1002/art.21388. [DOI] [PubMed] [Google Scholar]
- 39.Lorenz H, Wenz W, Ivancic M, Steck E, Richter W. Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading. Arthritis Res Ther. 2005;7:R156–65. doi: 10.1186/ar1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matyas JR, Ehlers PF, Huang D, Adams ME. The early molecular natural history of experimental osteoarthritis I. progressive discoordinate expression of aggrecan and type II procollagen messenger RNA in the articular cartilage of adult animals. Arthritis Rheum. 1999;42:993–1002. doi: 10.1002/1529-0131(199905)42:5<993::AID-ANR19>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 41.Lorenzo P, Bayliss MT, Heinegård D. Altered patterns and synthesis of extracellular matrix macromolecules in early osteoarthritis. Matrix Biol. 2004;23:381–91. doi: 10.1016/j.matbio.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Grushko G, Schneiderman R, Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: A comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tissue Res. 1989;19:149–76. doi: 10.3109/03008208909043895. [DOI] [PubMed] [Google Scholar]
- 43.Yagi R, McBurney D, Laverty D, Weiner S, Horton WE., Jr Intrajoint comparisons of gene expression patterns in human osteoarthritis suggest a change in chondrocyte phenotype. J Orthop Res. 2005;23:1128–38. doi: 10.1016/j.orthres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 44.Stubendorff JJ, Lammentausta E, Struglics A, Lindberg L, Heinegård D, Dahlberg LE. Is cartilage sGAG content related to early changes in cartilage disease? implications for interpretation of dGEMRIC. Osteoarthritis Cartilage. 2012;20:396–404. doi: 10.1016/j.joca.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Williams A, Gillis A, McKenzie C, et al. Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): Potential clinical applications. AJR Am J Roentgenol. 2004;182:167–72. doi: 10.2214/ajr.182.1.1820167. [DOI] [PubMed] [Google Scholar]
- 46.Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49:488–92. doi: 10.1002/mrm.10389. [DOI] [PubMed] [Google Scholar]
- 47.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]


