Abstract
The AP-1 factor, basic leucine zipper transcription factor, ATF-like (BATF) is important for CD4+ T-helper (Th) 17, Th9 and follicular T helper (Tfh) cell development. However, its precise role in Th2 differentiation and function remains unclear, and the necessity of BATF in non-allergic settings of type-2 immunity has not been explored. Here, we show that in response to parasitic helminths, Batf−/− mice are unable to generate both Tfh and Th2 cells. As a consequence, Batf−/− mice fail to establish productive type-2 immunity during primary and secondary infection. Batf−/− CD4+ T cells do not achieve type-2 cytokine competency, which implies that BATF plays a key role in the regulation of interleukin(IL)-4 and IL-13. In contrast to Th17 and Th9 cell subsets where BATF binds directly to promoter and enhancer regions to regulate cytokine expression, our results show that BATF is significantly enriched at Rad50 hypersensitivity sites (RHS) 6 and 7 of the locus control region (LCR) relative to AP-1 sites surrounding type-2 cytokine loci in Th2 cells. Indeed, Batf−/− CD4+ T cells do not obtain permissive epigenetic modifications within the Th2 locus, which have been linked to RHS6 and RHS7 function. In sum, these findings reveal BATF as a central modulator of peripheral and humoral hallmarks of type-2 immunity, and begin to elucidate a novel mechanism by which BATF regulates type-2 cytokine production through its modification of the Th2 LCR.
Keywords: BATF, helminth, allergic immunity, Tfh, Th2 cells, IL-4, AP-1, Th2 locus control region
Introduction
Over three billion people worldwide are afflicted with type-2 or allergic inflammation (1). In the context of parasitic helminth infections, type-2 inflammatory responses serve a protective role by reducing worm burden in exposed individuals. In contrast, allergic asthma and allergies often result from the improper activation of type-2 inflammation. Recent studies posit that type-2 immune responses are divided into two distinct arms (2–4). One arm is modulated by canonical T-helper (Th) 2 cells, which promote innate cell recruitment, mucus production, smooth muscle contractility, and tissue remodeling at mucosal barriers. The second arm is regulated by IL-4-producing follicular T-helper cells (Tfh) to promote humoral aspects of type-2 responses such as IgE and high-affinity IgG1. Type-2 cytokine production by both Th2 and Tfh cells underlies the unique hallmarks associated with type-2 immunity. However, type-2 cytokine regulation in Th2 cells is distinct from mechanisms used by Tfh cells. Th2 cells utilize canonical factors STAT6 and GATA3 for optimal IL-4 production (2, 5–10), while Tfh cells produce IL-4 independent of these classical Th2 lineage-determining factors (2, 5, 11, 12).
One candidate factor that may regulate IL-4 expression in both Th2 and Tfh cells is the AP-1 factor basic leucine zipper transcription factor, ATF-like (BATF). BATF is essential for Tfh cell generation (13, 14), and modulates Tfh-mediated IL-4 expression by binding to the 3’ CNS2 enhancer of the Il4 locus (15–17). The role of BATF in Th2 cell generation and IL-4 production is less clear. Initial in vitro studies demonstrated that BATF was required for Th17 cell differentiation but not Th1 or Th2 cell development (13, 18, 19). Other studies also concluded that BATF had a minor role in Th2 differentiation in vitro (15), however, data from a different Batf−/− mouse line suggested a greater role for BATF in Th2 generation (14, 20). Although the reasons for such differences remain unclear, the genetic background of Batf-deficient strains has been proposed to contribute to the varied results (21).
An in vivo role for BATF in allergic immunity is clear. Models of ovalbumin (OVA)-induced allergic airway inflammation show reduced type-2 cytokine expression and allergic hallmarks in Batf−/− lungs (15, 20, 22) regardless of the genetic background (BALB/c and C57BL/6) or different targeting strategies used for the generation of Batf−/− mice (14, 18). However, despite a clear decrease in allergic inflammation, a consensus as to the mechanisms involved has not been reached. Currently, increased Th1 cell accumulation and defects in Th9 development, Th17 cell generation, and Tfh cell function have all been used to explain the diminished type-2 inflammation found in Batf−/− mice (15, 20, 22). Although defects in Th2 cell function and generation have been implied as a result of data obtained from bulk cultures or total tissue extracts from Batf−/− lungs (20, 22), a recent study concluded that Th2 differentiation and IL-4 production remain largely intact after ovalbumin sensitization and challenge of Batf−/− mice (15). As such, further studies are needed to understand the full impact of BATF-mediated regulation in type-2 immunity and Th2 cell biology.
To date, studies have focused on the role BATF plays in allergic airway models of type-2 inflammation. To extend our understanding of BATF in non-allergic settings of type-2 immunity, we utlilized the helminth Nippostrongylus brasiliensis, a mouse model of human hookworm infection. This model drives a robust type-2 immune response in both the lung and intestine of helminth colonized animals. Using cytokine reporter mice to assess Th2 and Tfh differentiation at the single cell level, we demonstrate that BATF is essential for both Tfh- and Th2-driven hallmarks of type-2 inflammation during helminth infection. BATF deficiency prevented Tfh cell formation, type-2 cytokine production from Th2 cells, and the recruitment of innate type-2 immune cells to mucosal sites of infection, all of which contribute to the defects observed in helminth clearance during primary and secondary infection.
This study also reveals that BATF binds to the locus control region (LCR) within the Th2 cytokine locus and modulates early aspects of LCR activity during Th2 but not Th1 differentiation. Given that optimal type-2 cytokine expression is dependent on the Th2 LCR, this work has identified a novel mechanism of BATF-mediated regulation of type-2 cytokine expression in Th2 cells. Importantly, this mechanism is distinct from that described for BATF-mediated IL-4 production in Tfh cells. In sum, these findings demonstrate that BATF is a central regulator of both Tfh- and Th2-driven arms of type-2 immunity in response to helminth exposure.
Materials and Methods
Mice
C57BL/6 Batf−/− mice used in this study were generated by Kenneth Murphy at Washington University (St. Louis, MO) and purchased from Jackson Laboratories (18). Batf−/− mice were breed at least 10 generations onto the C57BL/6 background and 8–10 generations onto the BALB/c background. IL-4 reporter mice: Il4KN2 and Il44get and IFN-gamma reporter mice: IfngGreat, were graciously provided by Richard Locksley (UCSF). All mice were maintained in pathogen-free animal facilities in accordance to guidelines established by the Division of Laboratory Animal Resources, Institutional Animal Care and Use Committee, and Duke University Medical Center.
Infection and worm clearance
N. brasiliensis was prepared as described (23). Mice were injected in the rear flank with 500 L3 larvae in saline solution.
T cell isolation and culture
Lymph nodes from naïve mice were obtained, and CD4+ T cells were negatively selected (Stemcell Technologies; 19860). CD4+ T cells of >90% purity, were cultured at a density of 3x106 cells/ml on plate-bound anti-CD3 and anti-CD-28 (5ug/ml; 145-2C11; 2ug/ml; 37.51 respectively). For Th1 cultures, cells were polarized with 10ug/ml anti-IL-4 (XMG1.2), 10ug/ml IL-12 (Biolegend: 577002), and 10ug/ml IL-2 (Biolegend: 575402); For Th2 cultures, cells were polarized with 10ug/ml anti-IFN-gamma (XMG1.2), 10ug/ml IL-4 (Biolegend: 574302), and 10ug/ml IL-2. All cultures used RPMI (Gibco) supplemented with 2% fetal calf serum, 55uM 2-mercaptoethanol, 100U Penicillin, and 100ug/mL Streptomycin. Cells were cultured for 3 (Th1) and 2 or 4 (Th2) days unless otherwise stated.
Flow cytometry
Mice were perfused with 15 mL of PBS. Mediastinal lymph nodes and lungs (left lobe only) were collected for analysis. Single-cell suspensions were prepared: lung was digested with: 250ug/ml Collagenase (Sigma: C7657), 50ug/ml Liberase (Roche: 145495), 1mg/ml Hyaluronidase (Sigma: h3506), 200ug/mL DnaseI (Sigma: DN25) in RPMI. Surface staining was performed with the following antibodies: Alexa Fluor 647 conjugated to anti-mouse/human GL7 (GL7); APC/Cy7 conjugated to anti-mouse CD90.2 (30-H12); PECy7 conjugated to anti-mouse PD-1 (RMP1-30); PE conjugated to Streptavidin, anti-mouse CD49b (DX5), anti-human/mouse/rat ICOS (C398.4A), to IL-4Rα (I015F8) and PerCP/Cy5.5 conjugated to anti-mouse CD8 (53-6.7), B220 (RA3-6B2) were from Biolegend; PE conjugated to anti-mouse SiglecF (E50-2440), anti-mouse CD131 (J0R050), anti-mouse CD95 (Jo2) were from BD Pharmingen. Brilliant violet 605 conjugated to anti-mouse CD4 (RM4-5); APC-eFluor 780 conjugated to anti-human/mouse CD45R/B220 (RA3-6B2) and biotinylated anti-mouse CD185/CXCR5 (SPRCL5) were from eBioscience; APC or PE conjugated to anti-human CD2 (S5.5) were from Invitrogen. Live lymphocytes were gated by DAPI exclusion, size and granularity based on forward and side scatter, and singlet gates. A lineage negative gate was used to identify ILC2 cells (Linneg: Ly6G−, B220−, CD8−, CD4−, SiglecF−, CD131−). Data was collected using a FACSCanto (BD Biosciences) and analyzed with FlowJo software (TreeStar).
Intracellular staining
Cells were stained for surface markers followed by Live/Dead exclusion (Invitrogen). For transcription factor staining, cells were fixed and permeablized using the FOXP3 staining kit (eBioscience) as recommended by the manufacturer. For cytokine staining, cells were incubated with PMA and ionomycin for 5 hours with monensin added during the final 2 hours of culture. Cells were harvested and fixed in 2% formaldehyde, and then permeabilized (0.5% Saponin, 2% FCS, 0.1% Sodium Azide in PBS) as previously described (24). Antibodies used to detect cytokines were FITC-conjugated anti-mouse-IFNγ (XMG1.2;) or PE-conjugated anti-mouse-IL-13 (eBio13A).
Enzyme-linked immunosorbent assay
96-well plates were coated with Rat-anti-mouse-IgE (R35-72). Total IgE was detected by biotinylated anti-mouse-IgE (R35-118), followed by streptavidin-horseradish peroxidase and o-phenylenediamine. Concentrations of total IgE were determined by Emax-precision microplate reader (Molecular Devices) and compared to standard curves generated with purified IgE (MEB-38).
Immunohistochemistry
Mice were exposed to N. brasiliensis and sacrificed 10 days later. The draining mediastinal lymph nodes were isolated, fixed in 4% paraformaldehyde, placed in 30% sucrose, embedded and frozen in Optimum Cutting Temperature (O.C.T.) embedding compound (Sakura Finetek USA). Sections were cut at 5 microns and quenched of endogenous peroxidase activity with 1% H2O2 and 0.5% Sodium Azide in PBS. Slides were subsequently blocked for non-specific Fc Receptor binding (TruStain Fc block-clone 93, Biolegend) and endogenous biotin (Avidn/biotin block; Vector Labs). eGFP was detected by anti-mouse-GFP (Novus Biologicals: NB600-308), followed by biotinylated F(ab’)2 donkey anti-rabbit (1:500; Jackson Immuno Research Laboratories). Tyramide amplification was applied to maximize detection of biotinylated-anti-eGFP. Briefly, following addition of biotinylated F(ab’)2 antibodies, tissue sections were incubated with streptavidin-HRP followed by fluorescein isothiocyanate–tyramide from the TSA Fluorescein System according to the manufacturer's instructions (PerkinElmer: NEL 701001KT). Prior to CD4 detection, a second round of peroxidase quenching and blocking of biotin (Avidn/Biotin Block; Vector labs) was performed. CD4 was detected using biotinylated-anti-CD4 antibody (RM4-5; Biolegend), and signal was amplified with tyramide–Alexa Fluor 555 (Invitrogen). After a third round of blocking and quenching of biotin and peroxidase, B220 was detected by biotinylated-anti-B220 (RA3-6B2; Biolegend), followed by Dylight649 conjugated to anti-Streptavidin (Biolegend). Nuclei were counterstained with DAPI (4′, 6-diamidine-2′-phenylindole dihydrochloride; Roche) in PBS before mounting (Vectashield: Vector Laboratories) on coverslips. Digital images were collected with Zeiss Axio Imager. Images were converted to red-green-blue, colored and overlaid with Photoshop CS5 software (Adobe Systems). Concentrations of antibodies used are available on request.
Quantification of IL-4 competent cells in the B cell follicles
Individual images in the Fitc (anti-GFP), Tyramide-555 (anti-CD4), and Cy5 (B220) channels were captured using a 5x objective and processed in Fiji (ImageJ). First, B220 and CD4+ staining were overlaid and used to demarcate the B cell follicles. A region of interest (ROI) corresponding to the B cell follicle was outlined and saved. Total pixels were measured within this region of interest to obtain the total area of the follicular region. Next, thresholding was used to select GFP+ events that were above background within the GFP image. The saved ROI depicting the follicular region was applied to the adjusted GFP image, and total pixels within the threshold set for the GFP+ events was obtained. Total GFP+ pixels divided by the total pixels in the follicles gave the relative area of the follicles that was occupied by GFP+ (IL-4+) cells.
H&E and PAS staining
Lungs and small intestines were fixed in 4% formaldehyde overnight. Tissues were paraffin embedded, sectioned, and stained by the Duke University histology core. Digital images were acquired with Zeiss Axio Imager.
DNA Methylation analysis and bisulfite sequencing
Cells were lysed with proteinase K in lysis buffer (50mM Tris pH8, 100mM EDTA, 0.5% SDS), and genomic DNA was prepared by phenol-chloroform extraction and ethanol precipitation. Bisulfite treatment and purification was performed with the EpiTect (Qiagen) kit according to the manufacturer’s protocol. Primers used to amplify bisulfite-treated genomic DNA of RHS7 were previously described (25). Amplicons were cloned into the TOPO-TA pCR2.1 vector (Invitrogen) and individual clones were sequenced.
Chromatin Immunoprecipitation (ChIP)
5x106 to 15x106 live cells were cross-linked with 1% paraformaldehyde for 10 minutes. Nuclei were generated with Farnham Lysis buffer. Nuclear lysates were isolated with RIPA buffers and fragmented with a Vibra-Cell VCX130PB (Sonics & Materials). For immunoprecipitation, anti-mouse IgG1 Dynabeads or Protein G were incubated overnight at 4°C with 5ug anti-BATF (WW8), anti-JunB (sc-46x), or anti-IRF4 (sc-6059), washed and incubated overnight with sonicated chromatin at 4°C. After immunoprecipitation, protein-DNA crosslinking was reversed by heating at 65°C, followed by proteinase K and RNase A treatment. DNA was purified using phenol-chloroform and ethanol precipitation and amplified by qPCR (LightCycler 480II, Roche) with indicated primers (see Supplementary Table 1) for conserved AP1 binding sites at the Th2 locus and a conserved non-AP-1 site within the locus (negative control). Conserved binding sites were identified with the Evolutionary Conserved Region (ECR) browser (http://ecrbrowser.dcode.org/), and promoter regions were defined to include approximately 500 basepairs upstream of the transcription start sites of Il4 and Il13.
Native ChIP
Chromatin was prepared from 5x106 cultured Th1 or Th2 cells and immunoprecipitated using antibodies specific for acetylated histone H3 (H3ac; EMD Millipore, 06-599), trimethylated histone H3 lysine 4 (H3K4me3; EMD Millipore; 07-473) following a protocol described previously (26) with modifications: MNase was purchased from NEB, and sonication step was omitted. Immunoprecipitated and input samples were quantified by SYBR green (Bio-Rad) qPCR (LightCycler 480II, Roche) with previously described primers against the Il4 promoter (27). Values were normalized to that obtained for β2-microglobulin (26) and enrichment was expressed as percent input.
Results
BATF is required for type-2 inflammation and immunity to Nippostrongylus brasiliensis
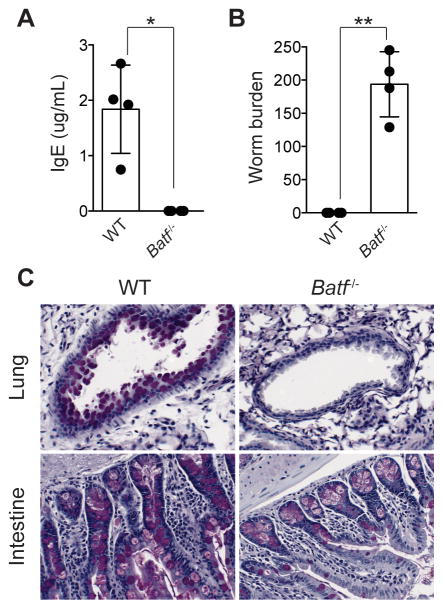
Wild-type and Batf−/− mice were analyzed for hallmarks of type-2 inflammation 10 days after exposure to the helminth Nippostrongylus brasiliensis. Consistent with previous findings that showed BATF is required for antibody isotype class-switch recombination, we found that serum IgE was undetectable in Batf−/− mice (Fig. 1A) (13, 14). Furthermore, worms were cleared from the intestine in wild-type animals, but remained abundant in Batf−/− mice (Fig. 1B). Batf−/− mice also failed to induce mucus production in the lung and intestine during helminth infection (Fig. 1C). Together, these results indicated that Batf−/− mice exhibit significant impairments in both humoral and peripheral arms of type-2 inflammation.
Fig. 1. BATF deficiency impairs type-2 immunity against N. brasiliensis.
Wild-type and Batf−/− mice were exposed to N. brasiliensis. (A) Serum IgE and (B) worm burden ten days post exposure. (C) Periodic Acid-Schiff staining was performed on the lung and intestine of wild-type and Batf−/− mice eight days after helminth infection. Images are representative of multiple fields of view in each tissue and animal. *P = 0.004 (two-tailed t-test), **P = 0.0002 (two-tailed t-test). Data are representative of (A,B) 3 or (C) 2 independent experiments. (A,B) n= 4 mice per group, (C) n=2–3 mice per group. Data are represented as mean +/− SD.
Lasting immunity to N. brasiliensis is dependent on CD4+ T cells and type-2 cytokine production (28–30). To determine the impact of BATF deficiency on anti-helminth immunity, we evaluated the generation and function of Tfh and Th2 memory cells with IL-4 reporter mice. Il44get mice report Il4 transcription by GFP expression and Il4KN2 mice report recent IL-4 protein production by human CD2 (huCD2) expression (5, 31). Dual IL-4 reporter mice (Il44get/KN2) were re-exposed to N. brasiliensis 21 days following primary infection (Fig. 2A). Wild-type mice elicited a rapid memory response indicated by increased CXCR5+, PD-1+ Tfh cell numbers (Fig. 2B, C) in the mediastinal lymph node and IL-4+ (CD4+, GFP+) Th2 cell numbers (Fig. 2D, E) in the lung five days after secondary exposure (2°). These responses were significantly above what is observed 5 days into the primary infection (1°). In contrast, Batf−/− mice showed no significant enhancement of Tfh cells in the draining lymph node or Th2 cell numbers in the lung upon primary or secondary exposure (Fig. 2C, E).
Fig. 2. BATF deficiency prevents the establishment of immunological memory.
(A) Model of experimental procedure: mice were exposed to N. brasiliensis either once (1°) or were rechallenged 21 days after initial infection (2°). Mice were then analyzed five or eight days after the last exposure. (B) Representative contour plots of total CD4+ T cells isolated from the lung-draining mediastinal lymph nodes five days post-secondary exposure. Gates indicate Tfh cells (CXCR5+, PD1+). (C) Total number of Tfh cells from the mediastinal lymph node five days after primary (1°) and secondary (2°) exposure. (D) Representative contour plots of total CD4+ T cells isolated from the lung five days post-secondary infection. Gates represent Th2 cells (CD4+, GFP+). (E) Total number of Th2 cells in the lung 5 days after primary (1°) and secondary (2°) infection. (F) Serum IgE post-secondary infection. (G) Worm burden post-secondary infection. *P<0.05; **P<0.005, ***P<0.0001 (two-tailed t-test). n= 3–6 per group. Data are represented as mean +/− SD.
Although B cells and antibodies do not appear to play a significant role in protection during the primary response, they are important for reducing worm burden upon re-infection (32, 33). In agreement, we found that previously exposed wild-type mice exhibited enhanced IgE production and expedited worm clearance during the secondary exposure to N. brasiliensis (Fig. 2F, G). In contrast, Batf−/− mice did not exhibit an increase in either humoral or cell-mediated immunity during the secondary response to helminths as serum IgE levels remained low and intestinal worm burden remained similar to that achieved during the primary infection (Fig.1B; 2F, G). These data demonstrate that BATF is required for the establishment of protective immunity against intestinal helminth infection.
BATF is required for Tfh cell generation, follicular migration, and IL-4 production after infection with N. brasiliensis.
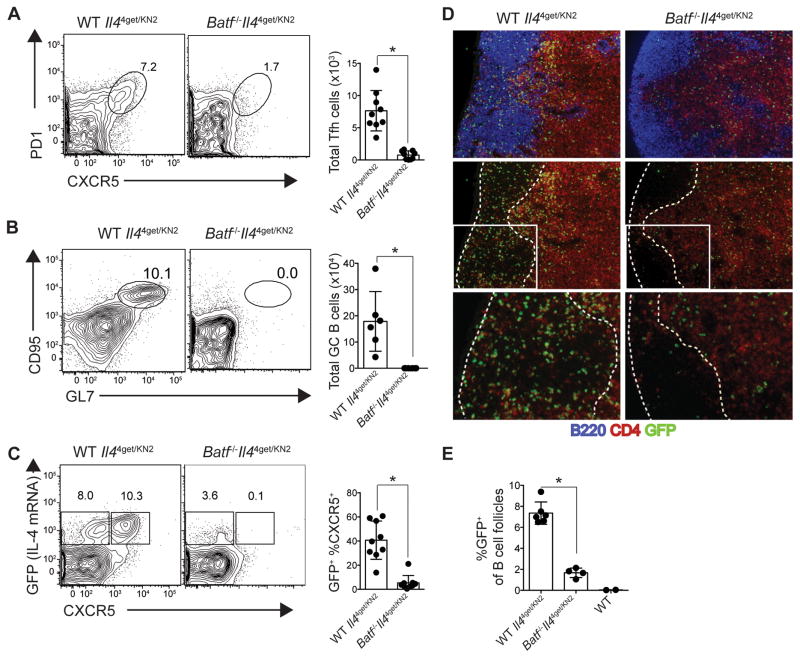
To assess the humoral response in more detail, Tfh cell generation and germinal center formation were evaluated in mice exposed to N. brasiliensis. As expected, mediastinal lymph nodes from wild-type mice revealed robust Tfh cell (CXCR5+, PD1+) and germinal center B cell (CD95+, GL7+) generation eight days after helminth exposure (Fig. 3A, B). In contrast, Batf−/− mice were devoid of both Tfh cells and germinal center B cells. Consistent with previous studies (3, 34), GFP (IL-4) expression was enriched among CD4+CXCR5+ Tfh cells, while BATF deficiency resulted in the absence of CD4+, CXCR5+ T cells expressing GFP (Fig. 3C). As a whole, GFP+ CD4+ T cells in the lymph nodes were significantly reduced in Batf−/− mice.
Fig. 3. BATF is required for Tfh cell generation, follicular migration, and germinal center formation.
Wild-type- and Batf−/−- Il44get/KN2 mice were exposed to N. brasiliensis and draining mediastinal lymph nodes were analyzed 8 days after infection. (A) Representative contour plots and quantification (bar graph) of CXCR5+PD1+ Tfh cells (gated through CD4+ T cells). (B) Representative contour plots and quantification (bar graph) of GC B cells identified as CD95+GL7+ (gated through B220+ cells). (C) Contour plots gated through CD4+ T cells depicting IL-4 competency (GFP-expression) in non-Tfh (CXCR5−; left gate) and Tfh (CXCR5+; right gate) cells. Graph shows the frequency of CXCR5 expression among CD4+GFP+ T cells. (D) Immunohistochemistry of mediastinal lymph nodes 10 days after helminth exposure. Sections were stained for IL-4 competency (anti-GFP; green) of CD4+ T cells (anti-CD4; red) and B cell follicles (anti-B220; blue). Top panel shows all three stains, and middle and bottom panels show only CD4 and GFP staining with dashed lines outlining the boarders of the B cell follicle. Bottom panels represent an enlargement of the boxed region in the corresponding middle panel. (E) Quantification of the area in the follicles in (D) comprised of GFP staining. Data points represent individual images of separate follicle regions from indicated mice. *P<0.0001 (two-tailed t test). Data are cumulative of 3 (A, B, C) or representative of 2 (D, E) independent experiments. (A, B, D) n=3 per group, (C) n=4–5 per group, (E) n=2 per group with 1 wild-type (GFP-negative) mouse as a control. Data are represented as mean +/− SD.
Immunohistochemical analysis of the mediastinal lymph nodes 10 days after helminth exposure confirmed the absence of CD4+ T cells in the follicles of Batf−/− mice (Fig. 3D). This is highlighted by the selective reduction of CD4+ staining (red) in the follicular regions of Batf−/− mice compared to wild-type animals (Fig. 3D, 3D inset). Importantly, GFP+, CD4+ T cells also were absent from the follicles of infected Batf−/− mice as shown by the significant reduction of GFP+ cells in B220+ regions of the lymph node (Fig. 3D, E). Failure of Tfh cells to migrate into the follicles would explain the defects we observed in IgE production and may help to explain the global reduction in class-switching observed with other isotypes in prior studies (13). In summary, our data demonstrate that impaired IL-4 production, Tfh cell generation, follicular migration and germinal center development collectively contribute to the humoral defects observed in Batf−/− mice after helminth exposure.
BATF is required for type-2 inflammation
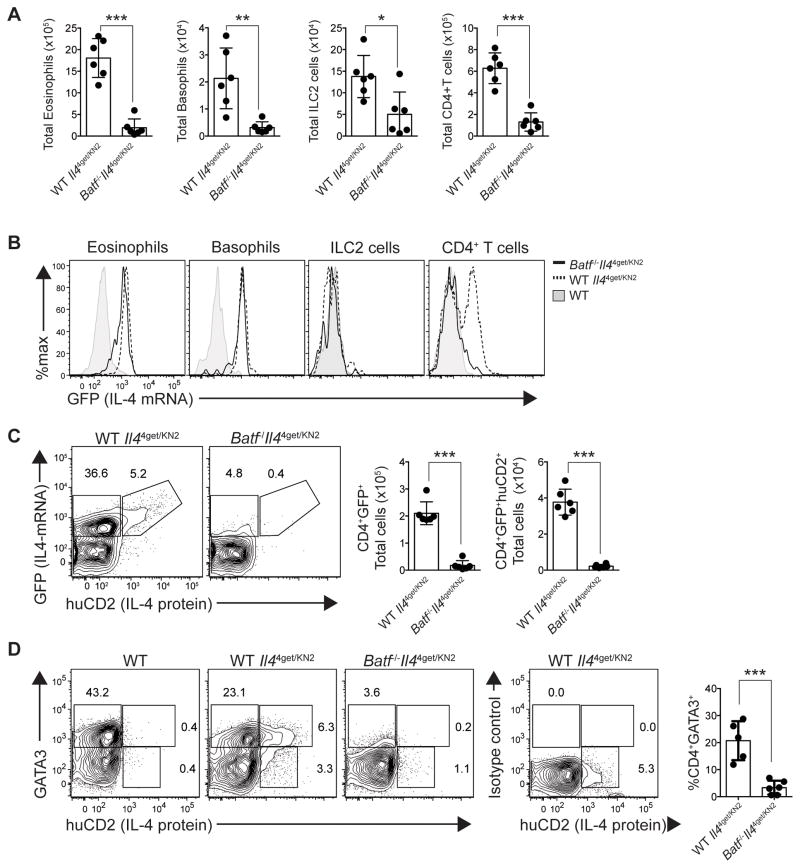
The above studies clearly demonstrate that BATF is essential for the induction of type-2 humoral immunity during helminth exposure. To investigate whether BATF is also important for peripheral hallmarks of type-2 inflammation, mice were exposed to N. brasiliensis and inflammation in the lung was analyzed eight days later. Total numbers of eosinophils, basophils, group 2 innate lymphoid (ILC2) cells and CD4+ Th2 cells were reduced significantly in the lung of helminth exposed Batf−/− animals compared to wild-type mice (Fig. 4A). To determine if there was also impairment in type-2 cytokine production, IL-4 expression was assessed. As expected, eosinophils, basophils and a subset of CD4+ T cells in wild-type lungs were IL-4 competent (GFP+) (Fig. 4B). Additionally, a large fraction of wild-type CD4+ T cells expressed both IL-4 mRNA and protein (GFP+ and huCD2+ respectively) (Fig. 4C). Interestingly, although reduced in number, eosinophils and basophils from the lung of Batf−/− mice expressed GFP to a similar extent as wild-type cells (Fig. 4B). However, Batf−/− CD4+ T cells were significantly impaired in both IL-4 transcript and protein production (Fig. 4B, C). A similar defect in Th2 cell generation was observed in Batf−/− mice bred onto a BALB/c background (Supplemental Fig. 1A). Similar to C57BL/6 mice, the defect in Th2 function corresponded to an impairment in worm clearance (Supplemental Fig. 1B). A defect in Th2 cell commitment was further confirmed, as Batf−/− CD4+ T cells did not express high levels of GATA3 in the lungs or lymph nodes, a characteristic of canonical Th2 cells (Fig. 4D; Supplemental Fig. 2). In sum, BATF is required to establish IL-4 potential in Th2 cells, but this role appears specific to CD4+ T cells as type-2 innate immune cells maintain IL-4 competency in Batf−/− mice.
Fig. 4. Type-2 inflammation and Th2 cytokine production are impaired in Batf-deficient animals.
Wild-type- and Batf−/−- Il44get/KN2 mice were exposed to N. brasiliensis, and the lung (left lobe) was analyzed 8 days later. (A) Quantification of lung eosinophils, basophils, ILC2 cells and CD4+ T cells. (B) Histogram overlays show relative IL-4 competency (GFP+) of eosinophils, basophils, ILC2 cells, and CD4+ T cells from wild-type (reporter negative control, filled), wild-type Il44get/KN2 (dashed line) and Batf−/− Il44get/KN2 (solid line) mice. (C) Representative contour plots depicting IL-4 transcript (GFP+; left gate) or IL-4 transcript and protein (GFP+huCD2+; right gate) expression in CD4+ T cells. Bar graphs quantify total IL-4 transcript-expressing (GFP+; left) and total IL-4 transcript and protein-expressing (GFP+huCD2+; right) CD4+ T cells in the lung. (D) Representative contour plots of lung CD4+ T cells stained for GATA-3 and IL-4 protein (huCD2). Gates indicate: GATA3hihuCD2− (top left); GATA3hihuCD2+ (top right); and GATA3lohuCD2+ (bottom right). Bar graph depicts the proportion of CD4+ T cells that are GATA3hi. *P=0.0132; **P=0.0029; ***P<0.0004 (two-tailed t-test). Data are representative of 4 independent experiments. (A,B,C,D) n=3–4 per group. Data are represented as mean +/− SD.
BATF is required for Th2 cell differentiation
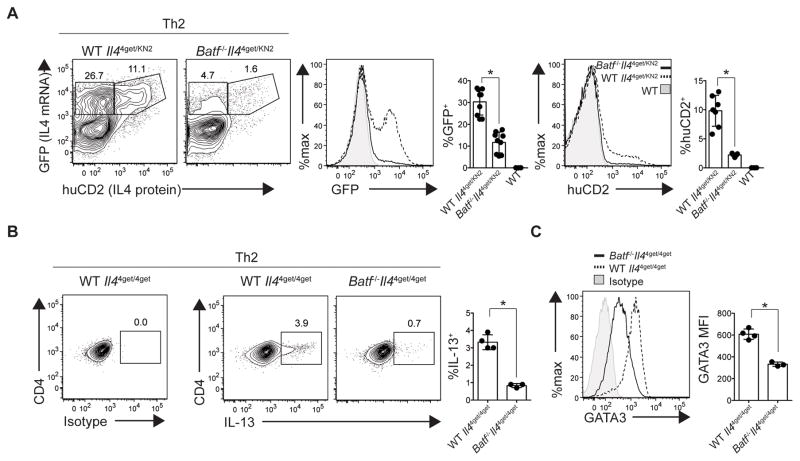
IL-4 positively regulates its own expression in Th2 cells, and thereby stabilizes the Th2 cell fate. Since BATF deficiency resulted in impaired IL-4 expression by CD4+ T cells, low levels of IL-4 in the lymph nodes and lungs of Batf−/− mice may fail to reinforce cytokine expression and Th2 commitment. To determine if providing exogenous IL-4 could rescue IL-4 production in Batf−/− CD4+ T cells, CD4+ T cells were cultured under Th2-inducing conditions. In wild-type cultures, 35–38% of cells acquired IL-4 competency of which a subset secreted IL-4 protein (Fig. 5A). In comparison, Batf−/− CD4+ T cells exhibited negligible IL-4 competency and protein production (Fig. 5A). A similar defect was observed in BALB/c Batf−/− CD4+ T cells cultured under Th2 conditions (Supplemental Fig. 3). Therefore, even under conditions in which IL-4 is in excess, BATF was required for optimal IL-4 production. Similarly, IL-13 expression was also impaired among Batf−/− CD4+ T cells cultured under Th2 conditions (Fig. 5B). Because the threshold of GATA3 expression is highly associated with both immune cell-mediated IL-13 production (35, 36) and cell fate decisions (37), we assessed whether BATF deficiency impacted GATA3 expression in cells cultured under Th2 conditions. Similar to our results in vivo, Batf−/− CD4+ T cells demonstrated decreased GATA3 expression in vitro (Fig. 5C).
Fig. 5. BATF is required for Th2 differentiation and function.
Naïve CD4+ T cells were isolated from indicated IL-4 reporter mice and cultured under Th2 conditions. (A) Representative contour plots of CD4+ T cells. Gates depict the proportion of IL-4 transcript-expressing (GFP; top left) and IL-4 mRNA- and protein-expressing (GFP, huCD2; top right) CD4+ T cells. Histogram overlays and bar graphs depict the relative levels and frequency of IL-4 transcript (GFP; left) and protein (huCD2; right) production in wild-type (reporter negative, filled), wild-type Il44get/KN2 (dashed) and Batf−/−Il44get/KN2 (solid) CD4+ T cells. (B) Th2 cultured CD4+ T cells, were stimulated with PMA/Ionomycin for 5 hours on the day of harvest. Representative contour plots of CD4+ T cells. Gates depict the proportion of IL-13 protein. Bar graphs show the relative frequencies of IL-13 protein production within CD4+ T cells. (C) Histogram overlay and bar graph depict the MFI of GATA-3 among indicated CD4+ T cell populations after Th2 culture. *P<0.001 (two-tailed t-test). Data are representative of 3 (A,C) or 2 (B) independent experiments. (A,B,C) n= 2–4 replicate wells per group. Data are represented as mean +/− SD.
We next sought to determine whether BATF was also required for Th1 differentiation and function. To do this we used an IFN-gamma cytokine reporter strain (IfnγGreat) to assess cytokine production. IfnγGreat reporter mice utilize a bicistronic reporter to generate both IFN-gamma and yellow fluorescent protein (YFP) expression from the same transcript (3, 38). In these mice, cells that produce IFN-gamma concomitantly express YFP. Both wild-type and Batf-deficient, ifnγGreat CD4+ T cells cultured under Th1 conditions readily expressed YFP and IFN-gamma protein (Fig. 6A). These results were consistent with previous findings showing that Th1 differentiation is uncompromised in Batf−/− CD4+ T cells cultured in vitro (13, 18, 19). To examine whether this trend held true in vivo, we exposed wild-type and Batf−/− IfngGreat mice to N. brasiliensis and quantified YFP expression in the lung 8 days later. Again, Batf-deficient mice showed no defect in IFN-gamma transcript expression among CD4+ T cells (Fig. 6B). In fact, a higher percentage of CD4+ T cells expressed IFN-gamma in Batf−/− mice relative to wild-type, a finding consistent with ovalbumin models of allergic lung inflammation (22). However, in the helminth model we did not observe a significant increase in the number of IFN-gamma-producing CD4+ T cells in the lung relative to wild-type animals. Together, these results demonstrate that BATF has a fundamental role in Th2 cell differentiation, but is not necessary for Th1 cell differentiation in vitro or in vivo.
Fig. 6. BATF is not required for Th1 differentiation or function.
(A) Th1 cultured CD4+ T cells, were stimulated with PMA/Ionomycin for 5 hours on the day of harvest. Representative contour plots of total CD4+ T cells from wild-type- and Batf−/−- IfngGreat mice with gates depicting the proportion of IFNg transcript (YFP). Histogram overlays and bar graphs depict the relative levels and frequency of IFNg transcript (YFP) and protein production. (B) Mice were exposed to N. brasiliensis and the lung (left lobe) was assessed 8 days later. Representative contour plots of isolated CD4+ T cells. Gate represents the percentage of IFNg transcript (YFP+) producing CD4+ T cells. Histogram overlay and bar graph depict the levels of IFNg transcript (YFP+) among indicated CD4+ T cell populations and the total number of IFNg transcript (YFP) producing cells, respectively. Ns: P>0.235 (two-tailed t-test). Data are representative of 3–4 (A) and cumulative of 2 (B) independent experiments. (A) n=2–3 replicate wells per group; (B) n=4–5 per group. Data are represented as mean +/− SD.
BATF deficiency limits permissive epigenetic modifications at the Th2 LCR and Th2 cytokine loci
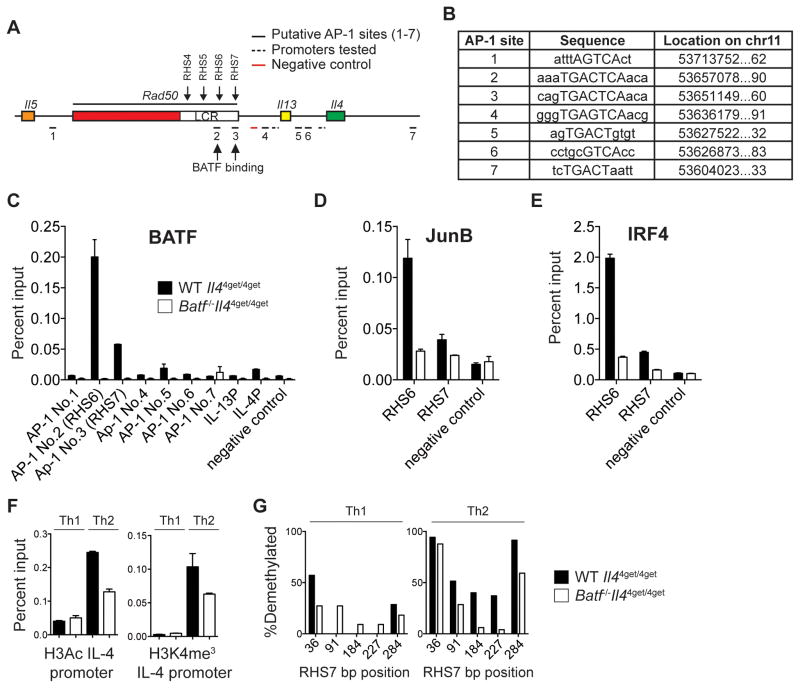
BATF binds directly to the Il17 locus to facilitate transcription factor recruitment and Il17 transcription early during Th17 differentiation (39). Similarly, BATF has been shown to bind directly to the Il9 promoter during Th9 differentiation (20). To test whether BATF regulated Il4 and Il13 in a similar fashion, we performed BATF Chromatin Immunoprecipitation (ChIP) in wild-type Th2 cells at putative conserved AP-1 binding sites across Il4 and Il13 loci and their promoters (Fig. 7A, B). We detected no enrichment of BATF binding among these sites (relative to the negative control region in wild-type Th2 cells) (Fig. 7C). Instead, BATF binding within the Th2 locus was enriched significantly upstream of Il4 and Il13 within the Th2 locus control region (LCR) (Fig. 7A, C). Within the LCR, there are several Rad50 hypersensitivity sites (RHS) that individually contribute to LCR hub activity and type-2 cytokine regulation (40, 41). We found that BATF binds specifically at RHS6 and RHS7 (Fig. 7C), two regions of the LCR critical for type-2 cytokine production (27, 40, 42). As BATF forms a heterodimer with JUN family members and these complexes cooperate with IRF4 to promote Th17 differentiation (43, 44), we assessed whether JunB and IRF4 binding was also enriched at RHS6 and RHS7. Indeed, both JunB and IRF4 bound at the same AP-1 sites within RHS6 and RHS7 that were enriched for BATF binding (Fig. 7D and E). Together, these findings indicate that BATF-JunB-IRF4 complexes are likely involved in the regulation of type-2 cytokine expression through the modulation of LCR activity. This mechanism is independent of BATF binding at proximal promoter and enhancer regions as described for other cytokine loci.
Fig. 7. BATF binds to and modulates the activity of the Th2 LCR.
(A) Diagram of the Th2 locus with conserved AP-1 bindings sites underscored throughout. BATF binding is identified with arrows at RHS6 and RHS7. (B) List of conserved AP-1 motifs as labeled in (A), capitalized letters indicate consensus sequences. (C, D, E) Wild-type (filled bar) and Batf−/− (open bar) cells were cultured under Th2 conditions and prepared for ChIP analysis. BATF (C), JunB (D), and IRF4 (E) binding was evaluated at designated AP-1 sites along the Th2 locus relative to a non-AP1 site. (F) Bar graph represents ChIP analyses of permissive Histone 3 modifications at the IL-4 promoter. Bar graphs depict relative levels of H3Ac (left) and H3K4me3 (right) in wild-type (filled bar) and Batf−/− (open bar) cells. (G) Percent demethylation of wild-type (filled bar) and Batf−/− (open bar) Th1 (left) and Th2 (right) cells at indicated sites within the RHS7 amplicon. Data are representative of 2 (C, D, E) or 3 (F, G) independent experiments. Data are represented as mean +/− SD.
To investigate a possible connection between BATF and the LCR, we focused on RHS6 and RHS7. RHS6 is required for permissive epigenetic modifications at the Th2 locus (27). As a result, RHS6-deficient mice are substantially impaired in the production of Th2 cytokines both from CD4+ T cells cultured in vitro and after OVA-induced allergic inflammation (27). Consistent with these results, we found decreased levels of permissive modifications, histone 3 pan-acetylation (H3Ac) (Fig. 7F, left) and H3 lysine 4 tri-methylation (H3K4me3) (Fig. 7F, right) at the IL-4 promoter in Batf−/− CD4+ T cells cultured under Th2 conditions.
Demethylation of RHS7 occurs early during Th2 differentiation, but not during Th1 differentiation (40, 45). As expected, both wild-type and Batf−/− Th1 cells demonstrated a highly methylated RHS7 region (Fig. 7G, left). However, in contrast to the demethylated state of RHS7 in wild-type Th2 cells, RHS7 remained largely methylated in Batf−/− CD4+ T cells cultured under Th2 conditions (Fig. 7G, right). Given the importance of both RHS6 and RHS7 in LCR-mediated regulation of type-2 cytokine production, these data suggest that BATF works early to establish permissive epigenetic changes at the LCR and Th2 cytokine loci. Such epigenetic changes are critical to achieve type-2 cytokine competency in CD4+ T cells.
Discussion
Using a mouse model of intestinal helminth infection, we show that BATF is an essential regulator of Tfh cell generation and IgE production in non-allergic settings of type-2 immunity. In addition, BATF is required for canonical Th2 generation and Th2-mediated hallmarks of type-2 inflammation such as mucus production and innate cell recruitment to peripheral sites of inflammation. Thus, BATF serves as a central regulator of both Tfh (humoral) and Th2 (peripheral) cell-mediated hallmarks of type-2 immunity. This is the first evidence that BATF plays an important role in non-allergic settings of type-2 inflammation and highlights its necessity in establishing productive anti-helminth immunity and immunologic memory.
Although findings in allergic models also support the importance of BATF in type-2 inflammation, no consensus has been reached regarding the mechanisms involved (15, 20, 22). One possibility may be through the expression of IL-9 by Th9 cells. Indeed, BATF has been described as a critical regulator of allergic inflammation through its collaboration with IRF4 in Th9 differentiation (20, 46). Although IL-9 is induced in response to N. brasiliensis, its functional contribution is overshadowed by IL-4 and IL-13 (2, 47, 48). Indeed, our data generated from Batf−/− mice more closely resemble that of helminth-infected Il4−/−Il13−/− animals than of helminth-infected Il9−/− mice (47). As such, BATF appears to be important for both Th9-dependent and -independent hallmarks of type-2 inflammation (49, 50).
A second mechanism proposed to explain the reduced allergic inflammation observed in Batf−/− mice involved a specific defect in Tfh-driven IL-4 production, as Th2-derived IL-4 production was thought to be normal in this model (15). Our data suggests that this mechanism does not play a significant role during helminth-induced type-2 inflamamtion for three reasons. First, we observed a significant defect in Th2 cell generation and IL-4 production in helminth-exposed Batf−/− mice. This finding is consistent with the idea that Th2 cells are the predominant orchestrators of type-2 inflammation in mucosal tissues (51) and thus required for peripheral hallmarks of type-2 inflammation. Second, we found a complete absence of Tfh cells in helminth-exposed Batf−/− mice, consistent with the requirement for BATF in Tfh cell generation (13, 14). Thus, during helminth infection, defects attributed to Tfh cells likely result from their absence rather than their inability to express IL-4. Lastly, IL-13-producing cells are required to establish the hallmarks of type-2 inflammation observed during N. brasiliensis infection, and Tfh cells do not produce IL-13 in this model (2). Together, these findings make a specific-defect in Tfh-derived IL-4 an unlikely mechanism to explain the reduced type-2 inflammation observed after helminth infection in Batf−/− mice.
Additional evidence that BATF deficiency likely impacts type-2 airway inflammation by affecting Th2 cells rather than Tfh cytokine production, comes from studies using mice that are deficient in the CNS2 enhancer of the Il4 locus. BATF binds to the CNS2 region and is critical for IL-4 production by Tfh cells (15). However, deletion of the CNS2 region ultimately has little impact on airway responsiveness to OVA (16, 52). Thus, BATF binding to the CNS2 region to promote IL-4 production in Tfh cells is neither sufficient nor necessary to mediate OVA-induced allergic airway inflammation. We believe the data is most consistent with a model where BATF deficiency results in a failure to generate both Tfh and Th2 cells during allergic and non-allergic settings of type-2 inflammation.
Why different mechanisms have arisen to explain the diminished type-2 inflammation observed among Batf-deficient animals is interesting. Apart from the obvious reason that the helminth model is different from that of allergen sensitization, one possibility to explain the different results may lie in how T cell fate and function were determined. In this study, we assessed cytokine potential of Tfh and Th2 cells in vivo by histology or directly ex vivo by flow cytometry using sensitive reporter systems. Ex vivo manipulations were not required. In the ovalbumin model (15), cytokine potential was assessed after spleens were harvested from immunized mice and restimulated ex vivo with antigen for an additional three days. Thus, it is possible that extended culturing ex vivo does not reflect certain aspects of biology in vivo. On the other hand, extended culture conditions may reveal a unique pathway for Tfh generation that is absent during helminth exposure or one that occurs only in settings where prolonged and repeated T cell receptor engagement can occur. In fact, this ex vivo system resembles findings in vivo where Tfh-like cells are generated independently of B cells in mice repeatedly dosed with high concentrations of antigen (53). The use of extended cultures may also help to explain the different conclusions made surrounding the role of BATF in Th2 differentiation using similar OVA-induced airway inflammation models (15, 22). Genetic and histological comparisons between Tfh and Th2 cells isolated directly ex vivo and their cultured counterparts will shed further light on this topic.
As stated earlier, prior literature reached contradictory conclusions about the importance of BATF in Th2 differentiation in vitro (21). The mixed results obtained by different groups investigating the role of BATF during Th2 differentiation do not appear to depend on the Batf−/− animal model as the Batf−/− mice and cells used in this study, which were originally generated at Washington University (18), generate a similar phenotype to that observed from mice generated using a completely different targeting strategy (14, 20). Similarly, the genetic background of the mice used can not fully explain the differences observed. CD4+ T cells from both BALB/c (19, 22) and C57BL/6 (14, 15, 18, 20) backgrounds have given similarly conflicting results. Indeed, no obvious role for the genetic background was observed in our studies as Batf−/− CD4+ T cells on the BALB/c background showed the same impairment in Th2 differentiation and IL-4 production as those on the C57BL/6 background. Thus, similar to the in vivo studies discussed above, antigen dose and cytokine availability may also explain the differences observed during in vitro Th2 differentiation.
Similar to the methods used in this study, Th2 culture periods of 4–5 days have shown that BATF deficiency greatly impairs IL-4 production among T cells cultured under Th2 conditions (14, 20), while other publications report no effect (13, 15). However it should be noted that, when other type-2 cytokines were assessed during this 4–5 day period, a significant defect was still reported (15). Only when Th2 cultures were performed for a period of 7–15 days or when cells were subjected to prolonged restimulation prior to the assessment of cytokine potential did BATF deficiency consistently result in little or no defect in Th2 cell development (15, 18, 19). This suggests that extending the duration of T cell receptor engagement or increasing cytokine availability may be sufficient to bypass the early requirement for BATF in developing Th2 cells. Thus, a Batf-independent pathway for both Tfh and Th2 cell generation may occur in conditions when antigen and/or local type-2 cytokine availability is persistent. Whether such conditions occur naturally in vivo will be an interesting area for future investigation.
The precise mechanism of BATF-mediated cytokine regulation in Th2 cells is still unclear. This study uncovers a unique BATF-dependent mechanism required for optimal Th2 differentiation that works through the Th2 LCR. We used ChIP to show that binding of BATF, JunB, and IRF4 is enriched at RHS6 and RHS7 of the Th2 LCR. This finding supports a model where BATF-JunB heterodimers pair with IRF4 to bind AP-1-IRF composite elements (AICE) at key locations within the LCR. Although BATF-IRF4 complexes are known to influence the expression of Il17 by binding directly at its promoter and enhancer regions (39, 43, 44), our results suggest that these complexes work at a distance to control IL-4 and IL-13 in developing Th2 cells. The results shown herein suggest that in Th2 cells, BATF is required for the binding of key lineage-determining factors to the LCR and Th2 locus. Specifically, BATF binds to essential DNase hypersensitivity sites, RHS6 and RHS7, both required for optimal type-2 cytokine expression in Th2 cells (27, 42, 54).
To further support a role for BATF-mediated regulation of the LCR to coordinate type-2 cytokine production, we found that both IL-4 and IL-13 were impaired in Th2 polarized Batf−/− CD4+ T cells. Batf−/−cells cultured under Th2 conditions revealed an appreciably lower level of permissive histone modifications at the IL-4 promoter than wild-type cells. This defect was accompanied by impaired demethylation of the RHS7 region. In sum, the data presented demonstrate a requirement for BATF to facilitate chromatin remodeling at the Th2 locus early during Th2 differentiation. Dissecting precisely how BATF coordinates with other transcription factors at the LCR to modify the chromatin landscape in Th2 and Tfh cells will broaden our understanding of T cell lineage commitment and plasticity (55). It is also likely to strengthen our understanding of the relationship between Tfh cell and Th2 cell subsets in settings of type-2 inflammation (56, 57).
Interestingly, basophils and eosinophils, which preferentially produce IL-4 and not IL-13 in vivo (2, 36), did not show a defect in IL-4 competency in Batf-deficient animals. This suggests that these innate cells do not require BATF binding at either the CNS2 region or the LCR to achieve IL-4 production. Indeed, the CNS2 region does not appear critical for IL-4 expression by these cells (16). This may indicate that BATF is required specifically in CD4+ T cells to achieve IL-4 competency, and the Il4 locus in these innate subsets becomes accessible independently from BATF. It is interesting to point out that the lack of IL-13 production in basophils and eosinophils despite IL-4 production further highlights the importance of BATF in the coordinate regulation of type-2 cytokines. Further work investigating the role of BATF and LCR activity in these various type-2 immune cells will provide insight into the specificity of this mechanism.
Although much is currently understood with regard to the pathogenesis of allergic disorders and helminth infection, these diseases continue to impact human health on a global scale. As such, there is a need to identify molecular factors and mechanisms responsible for the initiation of both Tfh- and Th2-driven type-2 inflammation. Of interest, factors common to the development of both IL-4-producing Tfh cells and IL-4- and IL-13-producing Th2 cells are not well characterized, but represent critical targets for the development of therapeutics designed to ameliorate a spectrum of allergic pathologies. Furthermore, these findings have obvious implications among various infectious and allergic diseases that depend on the generation and maintenance of allergen-specific immunoglobulin and memory Tfh and Th2 cells. Taken together, BATF is an early regulator of type-2 inflammation, and works via several distinct mechanisms to regulate type-2 cytokine production. These results provide an intriguing platform to explore new therapies against type-2 cytokine-driven immunopathology.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institute of Health (AI119004)
The authors thank Richard Locksley (UCSF) for providing various reporter strains; Sam Johnson and the Duke Light Microscopy Core Facility; The Duke Pathology core for processing of histological stains; Nancy Martin and Lynn Martinek for flow cytometry and sorting; and Ann Miller for expert technical and general support.
References
- 1.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nature immunology. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao K, Reinhardt RL. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine. 2015 doi: 10.1016/j.cyto.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 8.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 9.Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1993–1998. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 11.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. Journal of immunology. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 12.van Panhuys N, Tang SC, Prout M, Camberis M, Scarlett D, Roberts J, Hu-Li J, Paul WE, Le Gros G. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:12423–12428. doi: 10.1073/pnas.0806372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, Alt FW, Tang J, Oltz EM, Murphy TL, Murphy KM. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nature immunology. 2011;12:536–543. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betz BC, Jordan-Williams KL, Wang C, Kang SG, Liao J, Logan MR, Kim CH, Taparowsky EJ. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. The Journal of experimental medicine. 2010;207:933–942. doi: 10.1084/jem.20091548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahoo A, Alekseev A, Tanaka K, Obertas L, Lerman B, Haymaker C, Clise-Dwyer K, McMurray JS, Nurieva R. Batf is important for IL-4 expression in T follicular helper cells. Nature communications. 2015;6:7997. doi: 10.1038/ncomms8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M, Huang X, Interlandi J, Djuretic IM, Brown DR, Sharpe AH, Rao A, Ansel KM. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity. 2012;36:175–187. doi: 10.1016/j.immuni.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka S, Tsukada J, Suzuki W, Hayashi K, Tanigaki K, Tsuji M, Inoue H, Honjo T, Kubo M. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity. 2006;24:689–701. doi: 10.1016/j.immuni.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, Hatton RD, Stormo GD, Weaver CT, Russell JH, Murphy TL, Murphy KM. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tussiwand R, Lee WL, Murphy TL, Mashayekhi M, Kc W, Albring JC, Satpathy AT, Rotondo JA, Edelson BT, Kretzer NM, Wu X, Weiss LA, Glasmacher E, Li P, Liao W, Behnke M, Lam SS, Aurthur CT, Leonard WJ, Singh H, Stallings CL, Sibley LD, Schreiber RD, Murphy KM. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature. 2012;490:502–507. doi: 10.1038/nature11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabeen R, Goswami R, Awe O, Kulkarni A, Nguyen ET, Attenasio A, Walsh D, Olson MR, Kim MH, Tepper RS, Sun J, Kim CH, Taparowsky EJ, Zhou B, Kaplan MH. Th9 cell development requires a BATF-regulated transcriptional network. The Journal of clinical investigation. 2013;123:4641–4653. doi: 10.1172/JCI69489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sopel N, Graser A, Mousset S, Finotto S. The transcription factor BATF modulates cytokine-mediated responses in T cells. Cytokine Growth Factor Rev. 2016;30:39–45. doi: 10.1016/j.cytogfr.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Ubel C, Sopel N, Graser A, Hildner K, Reinhardt C, Zimmermann T, Rieker RJ, Maier A, Neurath MF, Murphy KM, Finotto S. The activating protein 1 transcription factor basic leucine zipper transcription factor, ATF-like (BATF), regulates lymphocyte- and mast cell-driven immune responses in the setting of allergic asthma. The Journal of allergy and clinical immunology. 2014;133:198–206. e191–199. doi: 10.1016/j.jaci.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 23.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 24.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. The Journal of experimental medicine. 1998;187:225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim ST, Fields PE, Flavell RA. Demethylation of a specific hypersensitive site in the Th2 locus control region. Proc Natl Acad Sci U S A. 2007;104:17052–17057. doi: 10.1073/pnas.0708293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao B, Krangel MS. Long-distance regulation of fetal V(delta) gene segment TRDV4 by the Tcrd enhancer. J Immunol. 2011;187:2484–2491. doi: 10.4049/jimmunol.1100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams A, Lee GR, Spilianakis CG, Hwang SS, Eisenbarth SC, Flavell RA. Hypersensitive site 6 of the Th2 locus control region is essential for Th2 cytokine expression. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6955–6960. doi: 10.1073/pnas.1304720110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urban JF, Jr, Maliszewski CR, Madden KB, Katona IM, Finkelman FD. IL-4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J Immunol. 1995;154:4675–4684. [PubMed] [Google Scholar]
- 29.Katona IM, Urban JF, Jr, Finkelman FD. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol. 1988;140:3206–3211. [PubMed] [Google Scholar]
- 30.Harvie M, Camberis M, Le Gros G. Development of CD4 T Cell Dependent Immunity Against N. brasiliensis Infection. Front Immunol. 2013;4:74. doi: 10.3389/fimmu.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obata-Ninomiya K, Ishiwata K, Tsutsui H, Nei Y, Yoshikawa S, Kawano Y, Minegishi Y, Ohta N, Watanabe N, Kanuka H, Karasuyama H. The skin is an important bulwark of acquired immunity against intestinal helminths. The Journal of experimental medicine. 2013;210:2583–2595. doi: 10.1084/jem.20130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Kreider T, Bowdridge S, Liu Z, Song Y, Gaydo AG, Urban JF, Jr, Gause WC. B cells have distinct roles in host protection against different nematode parasites. J Immunol. 2010;184:5213–5223. doi: 10.4049/jimmunol.0902879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fairfax KC, Everts B, Amiel E, Smith AM, Schramm G, Haas H, Randolph GJ, Taylor JJ, Pearce EJ. IL-4-Secreting Secondary T Follicular Helper (Tfh) Cells Arise from Memory T Cells, Not Persisting Tfh Cells, through a B Cell-Dependent Mechanism. J Immunol. 2015;194:2999–3010. doi: 10.4049/jimmunol.1401225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein Wolterink RG, Serafini N, van Nimwegen M, Vosshenrich CA, de Bruijn MJ, Fonseca Pereira D, Veiga Fernandes H, Hendriks RW, Di Santo JP. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10240–10245. doi: 10.1073/pnas.1217158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien TF, Bao K, Dell'Aringa M, Ang WX, Abraham S, Reinhardt RL. Cytokine expression by invariant natural killer T cells is tightly regulated throughout development and settings of type-2 inflammation. Mucosal Immunol. 2016;9:597–609. doi: 10.1038/mi.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scripture-Adams DD, Damle SS, Li L, Elihu KJ, Qin S, Arias AM, Butler RR, 3rd, Champhekar A, Zhang JA, Rothenberg EV. GATA-3 dose-dependent checkpoints in early T cell commitment. J Immunol. 2014;193:3470–3491. doi: 10.4049/jimmunol.1301663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinhardt RL, Liang HE, Bao K, Price AE, Mohrs M, Kelly BL, Locksley RM. A novel model for IFN-gamma-mediated autoinflammatory syndromes. J Immunol. 2015;194:2358–2368. doi: 10.4049/jimmunol.1401992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkurst CN, Muratet M, Newberry KM, Meadows S, Greenfield A, Yang Y, Jain P, Kirigin FK, Birchmeier C, Wagner EF, Murphy KM, Myers RM, Bonneau R, Littman DR. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fields PE, Lee GR, Kim ST, Bartsevich VV, Flavell RA. Th2-specific chromatin remodeling and enhancer activity in the Th2 cytokine locus control region. Immunity. 2004;21:865–876. doi: 10.1016/j.immuni.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Lee GR, Spilianakis CG, Flavell RA. Hypersensitive site 7 of the TH2 locus control region is essential for expressing TH2 cytokine genes and for long-range intrachromosomal interactions. Nature immunology. 2005;6:42–48. doi: 10.1038/ni1148. [DOI] [PubMed] [Google Scholar]
- 43.Glasmacher E, Agrawal S, Chang AB, Murphy TL, Zeng W, Vander Lugt B, Khan AA, Ciofani M, Spooner CJ, Rutz S, Hackney J, Nurieva R, Escalante CR, Ouyang W, Littman DR, Murphy KM, Singh H. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science. 2012;338:975–980. doi: 10.1126/science.1228309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM, Leonard WJ. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature. 2012;490:543–546. doi: 10.1038/nature11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim ST, Fields PE, Flavell RA. Demethylation of a specific hypersensitive site in the Th2 locus control region. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17052–17057. doi: 10.1073/pnas.0708293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, Dehzad N, Becker M, Stassen M, Steinborn A, Lohoff M, Schild H, Schmitt E, Bopp T. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, McKenzie AN. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17:7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 48.Licona-Limon P, Henao-Mejia J, Temann AU, Gagliani N, Licona-Limon I, Ishigame H, Hao L, Herbert DR, Flavell RA. Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity. 2013;39:744–757. doi: 10.1016/j.immuni.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMillan SJ, Bishop B, Townsend MJ, McKenzie AN, Lloyd CM. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J Exp Med. 2002;195:51–57. doi: 10.1084/jem.20011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stetson DB, Voehringer D, Grogan JL, Xu M, Reinhardt RL, Scheu S, Kelly BL, Locksley RM. Th2 cells: orchestrating barrier immunity. Adv Immunol. 2004;83:163–189. doi: 10.1016/S0065-2776(04)83005-0. [DOI] [PubMed] [Google Scholar]
- 52.Harada Y, Tanaka S, Motomura Y, Ohno S, Yanagi Y, Inoue H, Kubo M. The 3' Enhancer CNS2 Is a Critical Regulator of Interleukin-4-Mediated Humoral Immunity in Follicular Helper T Cells. Immunity. 2012;36:188–200. doi: 10.1016/j.immuni.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koh BH, Hwang SS, Kim JY, Lee W, Kang MJ, Lee CG, Park JW, Flavell RA, Lee GR. Th2 LCR is essential for regulation of Th2 cytokine genes and for pathogenesis of allergic asthma. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10614–10619. doi: 10.1073/pnas.1005383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothenberg EV. The chromatin landscape and transcription factors in T cell programming. Trends in immunology. 2014;35:195–204. doi: 10.1016/j.it.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ballesteros-Tato A, Randall TD, Lund FE, Spolski R, Leonard WJ, Leon B. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity. 2016;44:259–273. doi: 10.1016/j.immuni.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.