Abstract
Germinal centers (GCs) are the site of antibody diversification and affinity maturation, and as such are vitally important for humoral immunity. The study of GC biology has undergone a renaissance in the past 10 years, with a succession of findings that have transformed our understanding of the cellular dynamics of affinity maturation. In this review, we discuss recent developments in the field, with special emphasis on how GC cellular and clonal dynamics shape antibody affinity and diversity during the immune response.
Introduction
One of the hallmarks of the humoral immune response is the progressive increase in the affinity of antibodies over time (Eisen, 2014; Eisen and Siskind, 1964). This increase is the result of activation induced cytidine deaminase (AID)-driven somatic hypermutation (SHM) of the antigen-binding variable regions of immunoglobulin (Ig) genes (Berek and Milstein, 1987; Muramatsu et al., 2000; Weigert et al., 1970), which generates a panel of mutated B cells that are then selected, based on their affinity, to proliferate and differentiate into antibody-secreting plasma cells and memory B cells. This selective process occurs in microanatomical structures known as germinal centers (GCs) (Berek et al., 1991; Jacob et al., 1991b), which emerge in several copies within secondary lymphoid organs upon exposure to antigen by infection or immunization. In these structures, B cells compete for an array of signals that are delivered in an affinity-dependent manner, so that B cells with higher-affinity B cell receptors (BCRs, the complex formed by surface immunoglobulin (sIg) and the Igα and Igβ co-receptors) are expected to progressively outcompete lower-affinity B cells. Differentiation over time of plasma cells and memory cells from this evolving population drives the increase in the overall affinity of serum antibodies during the primary response and upon re-immunization or re-infection (Berek and Milstein, 1987; Eisen and Siskind, 1964).
A fundamental characteristic of the GC reaction is its dynamic nature. At the cellular level, GC B cells constantly migrate between microanatomical compartments as they undergo iterative cycles of SHM and selection and seek to obtain, from other GC-resident cell populations, the signals required for their survival. At the clonal level, the expansion and contraction of clonal populations based on their relative fitness follows a dynamics of its own, much akin to Darwinian selection. In the present review, we provide an overview of our current understanding of cellular and clonal dynamics in the GC, with greater emphasis on findings arising since our last review of the field (Victora and Nussenzweig, 2012). While we briefly touch upon molecular aspects when appropriate, more thorough reviews of these topics are available elsewhere (Basso and Dalla-Favera, 2015; De Silva and Klein, 2015). Likewise, the vast amount of knowledge that has recently been generated on the differentiation and regulation of the Tfh cells that support GC selection has been extensively reviewed in recent years (Crotty, 2014; Vinuesa et al., 2016), and is beyond our present scope.
Functional anatomy of the GC
GCs form in the center of the B cell follicles of secondary lymphoid organs, interspersed within a network of stromal cells known as follicular dendritic cells (FDCs) (Heesters et al., 2014). In follicles that do not contain GCs (primary follicles), FDCs play an organizational role, helping B cells to cluster into compact, well-defined follicles (Wang et al., 2011). In secondary follicles (which contain GCs), FDCs are located within the GC itself, where they perform two key roles. The best characterized of these is the long-term retention of intact antigen within complement-coated immune complexes, in a form that can support affinity-dependent “testing” of SHM-modified BCRs that occurs during GC selection (Heesters et al., 2014). A recent study has shown that antigen in fact recycles between the FDC surface and nondegradative endosomal compartments, suggesting a mechanism by which antigen can be maintained on these cells for the extended periods required for efficient affinity maturation (Heesters et al., 2013). A second role for FDCs is to support GC B cell survival and the overall prolificacy of the GC reaction. This is supported by the finding that preventing FDC activation through TLR4 results in smaller GCs and lower antibody titers in response to immunization (Garin et al., 2010).
GC formation begins with acquisition of antigen by resting B cells (Cyster, 2010; Gonzalez et al., 2011), followed by their migration to the follicle:T-zone (T:B) border, where they receive co-stimulatory signals from CD4+ T cells (Garside et al., 1998; Okada et al., 2005). This interaction triggers a period of intense proliferation in which responding B cells are located preferentially in the outer B cell follicle (Coffey et al., 2009). A fraction these cells will then coalesce into tight clusters in the follicle center, in close apposition with the FDC network, giving rise to the early GC. GC B cells are held together by modulations in the expression of several G-protein coupled receptors (GPCRs). One of these is Ebi2—the receptor for 7α,25-dihydroxycholesterol—which normally attracts naïve B cells towards the outer follicle but is strongly downregulated in GC B cells (Gatto et al., 2009; Gatto et al., 2011; Hannedouche et al., 2011; Kelly et al., 2011; Pereira et al., 2009). A second GPCR that contributes to GC B cell clustering is S1P2, one of five receptors for the signaling lipid sphingosine-1-phosphate (S1P) (Cyster and Schwab, 2012; Green and Cyster, 2012). S1P2 acts through inhibitory G-protein subunits Gα12 and Gα13 to prevent GC B cells—and T follicular helper (Tfh) cells—from migrating towards S1P–rich areas in the outer follicle (Green et al., 2011; Moriyama et al., 2014). In humans, S1P2 acts in concert with the orphan GPCR P2YR8, which also signals through Gα13. Deletion of Gna13 or its downstream effector Arhgef1 in mice allows GC B cells to leave their tight clusters and disseminate into lymph and blood (Muppidi et al., 2014). Interestingly, when GC B cells are made resistant to apoptosis by expression of a Bcl2 transgene, ablation of the FDC network itself causes GC B cells to disperse, suggesting that FDCs may help maintain low concentrations of S1P, and possibly of a putative P2YR8 ligand, in the follicle center (Muppidi et al., 2015; Wang et al., 2011).
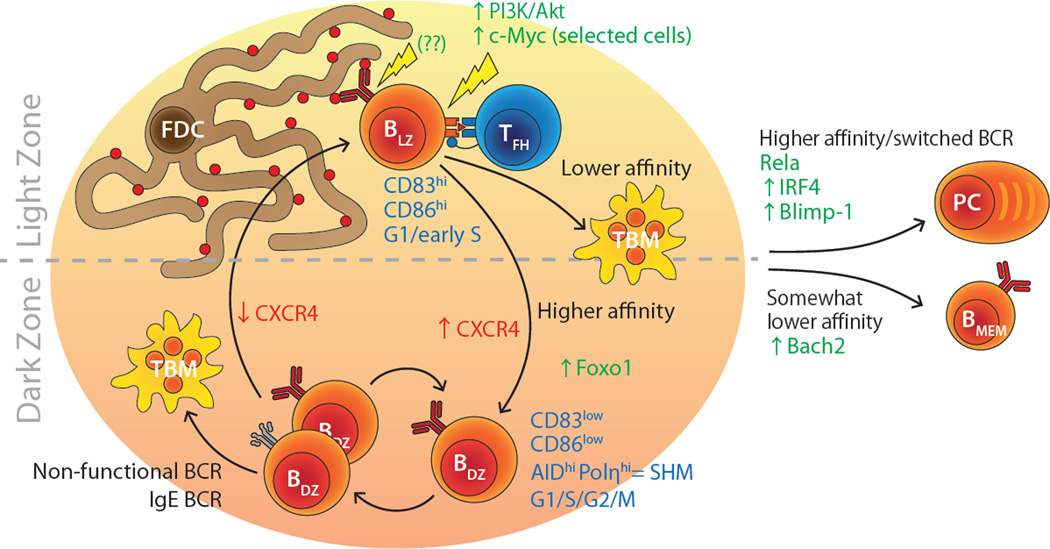
A defining feature of the mature GC is its division into two compartments, or “zones” (Fig. 1). The pole of the GC closest to the T cell zone in lymph nodes and spleen, and from which FDC are largely absent, is referred to as the dark zone (DZ). The pole distal to the T zone, closest to the capsule in LN or marginal zone in spleen, and rich in FDCs, is referred to as the light zone (LZ). The origin of these names is historical: the LZ appears “lighter” in histology using traditional DNA stains due to the sparser distribution of lymphocyte nuclei among a more abundant stromal network (Allen et al., 2007a).
Figure 1. Overview of the GC reaction.
The GC is divided into two anatomical compartments, LZ and DZ, which are the sites of antigen-driven selection, and B cell proliferation and hypermutation, respectively. Affinity maturation happens in cycles of proliferation and SHM in the DZ followed by antigen-driven selection in the LZ. See text for further details. TBM, tingible body macrophage.
The DZ consists primarily of a tight cluster of highly proliferative B cells, known classically as “centroblasts,” which strongly express the chemokine receptor CXCR4 (Allen et al., 2004; Allen et al., 2007b; Victora et al., 2012; Victora et al., 2010). The DZ stroma consists of a delicate meshwork of CXCL12-expressing Reticular Cells (CRCs) that are thought to be the source of the CXCL12 chemokine (the ligand for CXCR4) that holds DZ B cells away from the FDC network (Bannard et al., 2013; Rodda et al., 2015). DZ B cells have high expression of AID and the error-prone DNA polymerase eta (Polη, which introduces point mutations into DNA when repairing AID-induced lesions) (McHeyzer-Williams et al., 2015; Victora et al., 2012; Victora et al., 2010). This expression pattern suggests that the DZ is the site of Ig SHM, and therefore where clonal variants with different affinities for antigen are generated (Victora et al., 2012; Victora et al., 2010).
The LZ is less compact and more diverse than the DZ. In addition to GC B cells and FDCs, the LZ contains a large proportion of infiltrating naïve B cells (Schwickert et al., 2007), and a smaller but crucially important population of T follicular helper (Tfh) cells. As we will see below, Tfh cells play a fundamental role in the positive selection of higher-affinity B cells, driving their proliferation and differentiation into the plasma cell fate (Allen et al., 2007b; Meyer-Hermann et al., 2006; Victora et al., 2010). There has been a dramatic increase in our knowledge of Tfh cell biology in recent years, and there are excellent reviews on the subject (Crotty, 2014; Vinuesa et al., 2016). A recent development has been the discovery that a subset of GC-resident CD4+ T cells express the regulatory T (Treg) cell master regulator Foxp3, while sharing many phenotypic characteristics of Tfh cells (Chung et al., 2011; Linterman et al., 2011; Wollenberg et al., 2011). The precise origin, antigen specificity, and the nature and mechanism of regulation by these “T follicular regulatory” (Tfr) cells is currently under intense scrutiny (Ramiscal and Vinuesa, 2013; Sage and Sharpe, 2015). LZ B cells themselves are traditionally referred to as “centrocytes.” These cells display an activated phenotype, including higher expression of activation markers CD86 and CD83 (Victora et al., 2012; Victora et al., 2010). Other features such as the overexpression of gene signatures associated with activation of the CD40 and BCR pathways and of Myc are also indicative of an activated state (Dominguez-Sola et al., 2012; Victora et al., 2012; Victora et al., 2010). Together, the presence of antigen and Tfh cell help and the activated phenotype of its resident B suggest that the LZ is the site of selection of high-affinity SHM variants.
The segregation of proliferation and hypermutation in the DZ from antigen-driven selection in the LZ requires that cells migrate between these two zones for affinity maturation to occur. Early mathematical modeling studies propose that iterative cycles of SHM followed by selection (in DZ and LZ, respectively) would be required to achieve the degree of affinity maturation observed in vivo, a framework known as cyclic re-entry (Kepler and Perelson, 1993; Oprea and Perelson, 1997). According to this model, now widely regarded as accurate, a fraction of LZ B cells with improved affinity is allowed to re-enter the DZ for further rounds of proliferation and SHM. Recent studies have provided ample experimental evidence for the re-entry of selected B cells from LZ to DZ upon antigen-driven selection (Allen et al., 2007b; Gitlin et al., 2014; Schwickert et al., 2007; Victora et al., 2010).
More recent work has focused on the molecular mechanisms that control the transition of B cells between the GC zones and their associated phenotypes. The initial finding that many of the genes upregulated in LZ B cells could be linked to B cell activation by antigen or T cells (Victora et al., 2012; Victora et al., 2010) led us to speculate that, rather than being cell-intrinsic, much of the LZ phenotype was a consequence of exposure to the signals present in the LZ (Victora and Nussenzweig, 2012). However, forcing DZ B cells into the LZ by deletion of the DZ-homing receptor CXCR4 is not sufficient to turn on a LZ program in DZ cells, implying that DZ cells must have a cell-intrinsic “timer” that restricts the emergence of a LZ phenotype even when these cells are physically located in the LZ (Bannard et al., 2013). Later work showing that, upon arrival to the DZ, GC B cells execute a pre-programmed number of divisions before being allowed to return to the LZ suggested that, rather than (or in addition to) a timer, there may be a cell-intrinsic “division counter” restricting the DZ to LZ transition (Gitlin et al., 2014). Further insight into these dynamics comes from two parallel studies of the role in GC B cells of transcription factor Foxo1 (Dominguez-Sola et al., 2015; Sander et al., 2015). Deletion of a loxP-flanked allele of Foxo1 in GC B cells using an Ighgh1-cre (also known as γ1-cre) driver results in “DZ-less” GCs, in which all B cells reside in the LZ and display the LZ surface phenotype (CXCR4lowCD86hi), although certain essential features of DZ B cells—such as rapid proliferation and SHM—are maintained to different extents. Interestingly, gene expression profiling and ChIP-seq experiments show that part of the role of Foxo1 in the DZ is to suppress activation-related genes, including genes involved in BCR signaling and T cell-mediated activation. Therefore, one possibility is that Foxo1 may help maintain a non-activated phenotype in DZ B cells, even when these are physically forced into the LZ by deletion of CXCR4.
Still unresolved is the role of the physical segregation of affinity-based selection from proliferation and SHM. This segregation is conserved across species, and the gene expression programs that distinguish LZ and DZ B cells in mice and humans are largely overlapping (Victora et al., 2012). Surprisingly, however, disruption of LZ-DZ polarity by deletion of CXCR4 has little if any effect on the efficiency of affinity maturation towards a hapten antigen (Nie et al., 2004), and leads only to a moderate decrease in the number of mutations accrued by B cells in influenza-induced and Peyer’s patch GCs (Bannard et al., 2013). Whether LZ-DZ segregation plays a role in preventing potentially deleterious outcomes of GC selection, such as autoimmunity and B cell transformation, has not been investigated.
Positive selection of high-affinity clones in the GC
Affinity maturation relies on the ability of the GC to select B cells based on the affinity of their BCRs for antigen. The dynamics of this selection were initially quantified in intravital photoactivation experiments, revealing that while the LZ is constantly being repopulated by massive immigration from the DZ (at a rate of 50% of DZ cells transitioning to the LZ over a period of ~4 hours), less than 10% of LZ cells return to the DZ over a period of 6 hours. Simple mathematical analysis of these rates based on a cyclic re-entry framework (Victora et al., 2010), later refined using more sophisticated models (Meyer-Hermann et al., 2012), estimates that in the order of 10–30% of B cells that arrive to the LZ are selected to re-enter the DZ (the remainder either dying by apoptosis or exiting the GC). The question of how GCs select for high-affinity B cells can thus be rephrased as how the best cells are selected to re-enter from LZ to DZ.
Two candidate antigen-based signals are available to B cells in the LZ, arising from the dual role of the BCR as a signaling and an endocytic receptor (Allen et al., 2007a; Victora and Nussenzweig, 2012). The first signal is from antigen itself, which is held on FDCs in a form that can bind to and signal through the BCR. Although signaling through the BCR is required during the initiation of the B cell response, the role of such signaling in GC B cells is less clear. A surprising finding is that most GC B cells appear unresponsive to BCR stimulation by antigens in solution, with the exception of a brief period of Syk phosphorylation that coincides with the G2/M phase of the cell cycle in the DZ (Khalil et al., 2012). (Victora et al., 2010) This unresponsiveness has been confirmed by experiments using a Nur77-GFP transgenic mouse, which in B cells is specifically activated by BCR engagement (Mueller et al., 2015; Zikherman et al., 2012). Despite an overall low signal, a small fraction of GC B cells, mostly located in the LZ, strongly express GFP. These cells upregulate certain genes related to positive selection (such as Myc, Ccnd2, and Irf4), suggesting that these may indeed represent cells undergoing antigen-driven selection (Mueller et al., 2015). However, a recent study in which GC B cells are exposed to antigen displayed on immobilized plasma membrane sheets (PMSs) shows that, differently from antigens in solution, PMS-bound antigen can efficiently activate signaling downstream of the BCR in GC B cells, with the notable exception of nuclear translocation of NF-κB p50, which required CD40 ligation (Nowosad et al., 2016). Thus, while antigen stimulation can under certain circumstances trigger signaling in GC B cells, the key step of NF-κB activation appears to require additional input from T cells.
Further insight into the role of BCR signaling in GC selection comes from studies of the fate of cells that have undergone CSR, which have different signaling capabilities due to the extended cytoplasmic tails of switched isotypes. A recent report in which CSR to IgG1 is induced by cre recombination in the absence of SHM shows that, although IgG1+ GC B cells display lower expression of Nur77-GFP than their IgM+ counterparts, neither isotype appears to be favored (or disfavored) during GC selection (Gitlin et al., 2016). Thus the different signaling capabilities of IgG1 do not strongly affect GC B cell competitiveness. However, switching to IgE strongly disfavors permanence in the GC, such that IgE cells correspond to at best a minor population within GCs, even when a strong serum IgE response is induced (Erazo et al., 2007; He et al., 2013; Talay et al., 2012; Xiong et al., 2012; Yang et al., 2012). A detailed study of the dynamics of IgE+ GC B cells show that these cells are mostly restricted to the DZ, are more likely to be apoptotic, express markedly lower amounts of sIg, and are impaired in their ability to phosphorylate downstream mediators in response to soluble antigen (He et al., 2013). Thus, a possibility is that IgE-expressing B cells may fail a BCR-dependent checkpoint required for DZ to LZ transition (He et al., 2015), possibly related to the G2/M BCR signal described by Khalil et al. (Khalil et al., 2012).
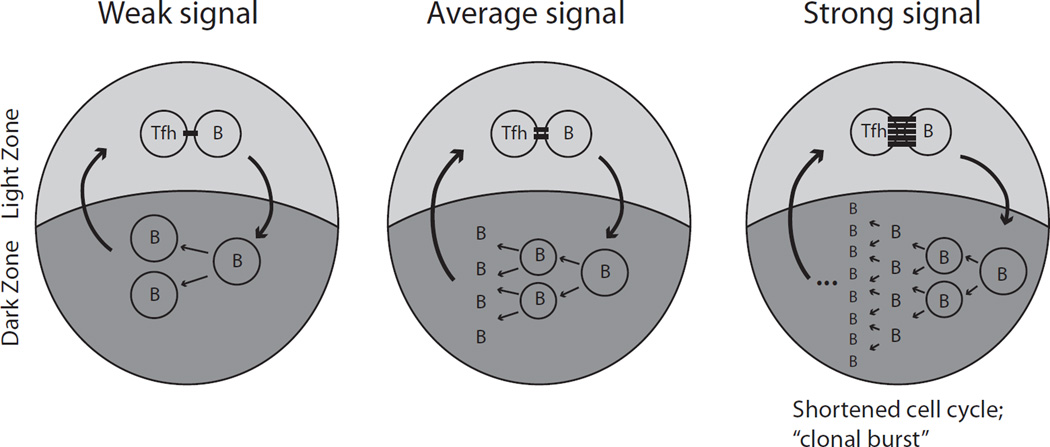
The second antigen-dependent signal available to GC B cells is delivered by cognate interaction with Tfh cells. B cells use the BCR to retrieve antigen deposited on the membranes of other cells (FDCs in the case of the GC) in an affinity-dependent manner (Batista and Neuberger, 2000). By applying tensile force to the antigen-antibody bond, B cells can readily discriminate between ligands with different affinities (Natkanski et al., 2013), and this process appears to be especially efficient in GC B cells due to their distinctive synapse configuration (Nowosad et al., 2016). Antigen retrieved in this fashion can be presented to helper T cells, providing a mechanism whereby T cells can sense B cell affinity indirectly. Competition between GC B cells displaying different surface densities of peptide-MHCII for a limited number of Tfh cells would then drive GC selection. This Tfh cell-centric model was conceived through a combination of mathematical modeling (Meyer-Hermann et al., 2006; Radmacher et al., 1998), in vitro studies (Batista and Neuberger, 2000), and intravital imaging (Allen et al., 2007b), and later confirmed experimentally in vivo (Gitlin et al., 2015; Gitlin et al., 2014; Shulman et al., 2013; Victora et al., 2010). A useful experimental system in this sense has been the targeted loading of T cell antigen onto class II MHC on GC B cells by fusing it to an antibody to the surface lectin DEC-205 (Victora et al., 2010). Acutely increasing pMHC concentration in a subset of DEC-205-expressing GC B cells [within a majority DEC-205− (Ly75−/−) population] forces these cells to interact strongly with Tfh cells, triggering them to migrate from LZ to DZ, proliferate extensively, and differentiate into PC. Thus, T cell help is sufficient to drive cyclic re-entry and GC selection. Further in vivo studies have shown that T cell help not only triggers cyclic re-entry, but also increases the number of divisions and the cell cycle speed of selected B cells in a manner proportional to the intensity of the B cell-Tfh cell interaction (Gitlin et al., 2015; Gitlin et al., 2014). Under steady-state, B cells divide on average twice per LZ-DZ cycle: the “typical” selected B cell will initiate one cell cycle while still in the LZ, then migrate to the DZ during S-phase. There it will divide into two daughters, each of which will undergo another full cell cycle before returning to the LZ (Fig. 2). The number of consecutive DZ cycles, and therefore DZ dwell time, is increased dramatically by DEC-205 antigen targeting (Gitlin et al., 2014). The strength of the T cell signal thus controls not only the odds of a GC B cell surviving in the LZ, but also the number of divisions this cell will undergo upon transitioning to the DZ. Therefore, a simple model where T cell signals rescue GC B cells from apoptosis (Liu et al., 1989) cannot fully explain the process of GC selection. These findings also implicate Tfh cell help as being responsible for setting the “timer” or “counter” (Bannard et al., 2013) that determines the length of residency of a GC B cell in the DZ.
Figure 2. Dynamic control of B cell proliferation.
The intensity of T cell help received in the LZ determines the number of division cycles a B cell will undergo in the DZ. On average, there are 2 S-phase initiation events in the LZ for each one in the DZ, indicating a ratio of 2 cell cycles per LZ-DZ cycle [(Gitlin et al., 2015), center panel]. A B cell that receives the minimum amount of help necessary for survival (left panel) would undergo only one cell cycle per LZ/DZ cycle, whereas a B cell that receives a strong Tfh cell-signal [either due to DEC-205 targeting or prior to a “clonal burst” (Tas et al., 2016); right panel] can undergo multiple cell cycles in the DZ without returning to the LZ.
Some mechanistic insight has also been gained into how Tfh cells drive B cell selection. At the cellular level, Tfh cells can be readily observed to engage in cell-cell contacts with GC B cells in GCs, although these are generally of short duration (Allen et al., 2007b). An interesting finding is that GC T:B interactions are prone to feed-forward loops: ICOSL on B cells promotes upregulation of CD40L on T cells, which in turn further upregulates ICOSL on the B cell, and so forth. Such loops promote a mode of intimate contact in which a large area of the B and T cell surfaces are juxtaposed, which was described as “entanglement” (Liu et al., 2015). Entanglement and contact duration are greatly increased by antigen delivery to B cells through DEC-205 (Shulman et al., 2014), which also enhances T cell production of B cell-helper cytokines IL-4 and IL-21. In addition, Tfh cells are the major source of the pro-survival cytokine BAFF (also known as BLyS) in the GC, and BAFF produced by Tfh cells was shown to promote the survival of B cells that have acquired high-affinity mutations (Goenka et al., 2014). Thus, small differences in pMHC density between B cells of different affinities can be nonlinearly amplified into large gains in signaling that would allow for more efficient selection of higher B affinity cells, and possibly trigger the multiple rounds of DZ proliferation seen after DEC-205 targeting (Gitlin et al., 2014). Interestingly, class II MHC molecules are rapidly turned over on DZ B cells by ubiquitin-mediated degradation, ensuring that peptide-MHCII density is “reset” after every LZ-DZ cycle (Bannard et al., 2016). Such resetting may prevent carryover of peptide-MHC molecules from previous selection rounds from interfering with a T cell’s sensing of the affinity of a B cell’s current BCR.
At the molecular level, the proto-oncogene Myc—previously thought to be silenced in GC B cells—has been shown to be briefly but strongly induced in LZ B that are undergoing positive selection (Calado et al., 2012; Dominguez-Sola et al., 2012). Antigen targeting to GC B cells through DEC-205 induces strong upregulation of c-Myc in targeted cells, implicating Tfh cell signals in the induction of c-Myc under physiological conditions. Interestingly, c-Myc is shut down in the DZ, where most B cell proliferation takes place (Calado et al., 2012; Dominguez-Sola et al., 2012). A recent study shows that the transcription factor AP4 is induced by c-Myc in a delayed fashion in positively-selected GC B cells, so that its expression is sustained in the DZ. Ablation of AP4 decreases the ability of B cells to undergo additional rounds of proliferation in the DZ, suggesting that AP4 may perform a similar role in the DZ as Myc does in the LZ. Thus, AP4 could be an essential component of the DZ “timer” or “counter” (Chou et al., 2016).
A final mechanism that may contribute to GC selection is antibody-mediated feedback, whereby soluble antibody “masks” antigen held on FDCs, effectively competing with B cells bearing BCRs of overlapping specificities (Zhang et al., 2013). This mechanism could explain how clones of widely different affinities can coexist within the same reaction [(Kuraoka et al., 2016; Tas et al., 2016), see discussion below], since low-affinity clones would be expected to survive if they are specific for epitopes against which little secreted antibody exists. Importantly, such a mechanism would be compatible with both a BCR and T cell-driven selection models, given that greater antigen availability would allow for stronger BCR signaling as well as for more antigen retrieval for presentation to T cells.
Negative selection of self-reactive clones in the GC
A risk associated with random SHM is the introduction of mutations that lead to recognition of self-antigens, which can generate autoreactive antibodies and potentially lead to autoimmune disease (Shlomchik et al., 1987; Tiller et al., 2010). Thus, in addition to selecting for cells with the highest affinity for the immunizing antigen, GCs are thought to rely on autoreactivity “checkpoints” to eliminate B cells that acquire the ability to bind self. Recently, Brink and colleagues have introduced a sophisticated genetic system in which adoptively transferred monoclonal B cells specific for hen egg lysozyme (SWHEL) are allowed to affinity-mature in GCs elicited by immunization with an HEL triple mutant (HEL3X), to which SWHEL cells bind with low affinity (Chan et al., 2012). Affinity maturation selects for SHM variants with increased affinity for HEL3X but which now also bind a quadruple mutant HEL4X. When this experiment is performed in hosts that express HEL4X as a pseudo-self antigen in different tissues, appearance of these highest-affinity variants (and thus, the failure of GC autoreactivity checkpoints) can be precisely monitored. These studies show that self-antigens can censor GC output only when expressed within or near the GC, but not when expressed as tissue-specific antigens in the liver or kidneys (Chan et al., 2012). A follow-up study has shown that the pro-apoptotic receptor FAS, highly expressed in GC B cells, is not necessary for negative selection of HEL4X-binding cells. Rather, FAS is required to prevent the generation of “rogue” GC B cells that escape normal antigen-driven selection and differentiate into plasma cells that secrete copious amounts of autoreactive (HEL4X-binding) IgG and IgE (Butt et al., 2015). Additionally, under certain conditions, GCs are able to use SHM to eliminate pre-existing self-reactivity to ubiquitous antigens, essentially “redeeming” formerly autoreactive clones to promote their participation in immune responses to foreign antigens, even if this means selecting for B cells with lower rather than higher affinity (Sabouri et al., 2014). Likewise, unusual human antibodies with broad reactivity against Plasmodium falciparum-infected erythrocytes and that carry a gene insertion between the IGH V and DJ segments derived from the gene encoding collagen binding protein LAIR1 show somatic mutations that decrease binding to collagen, again suggesting selection for loss of autoreactivity in GCs (Tan et al., 2016). Thus, multiple checkpoints appear to prevent the emergence of autoreactivity to widely available antigens; however, checkpoints against reactivity to tissue-specific self-antigens appear to be absent. Nonetheless, polyreactivity (reactivity to several unrelated antigens, which can be self or nonself) is a common consequence of SHM, in that polyreactive B cells are frequent within the memory B cell population in mice and humans (Gitlin et al., 2016; Tiller et al., 2007). Interestingly, polyreactive memory B cells appear to be shorter-lived than their non-polyreactive counterparts, suggesting that autoreactive B cells generated by SHM may be held at bay by a post-GC control mechanism that eliminates such cells from the memory pool (Gitlin et al., 2016).
Clonal diversity and evolution in the GC
In order to enter a GC, antigen-binding B cells must first receive cognate signals from T helper cells activated by the same or a linked antigen, delivered at the follicle:T-zone (T:B) border (Garside et al., 1998; Okada et al., 2005). Access to the GC is competitive, in that even B cell clones with very low affinity for antigen are allowed to colonize GCs in the absence of competition from other clones that bind the same antigen (Dal Porto et al., 1998; Schwickert et al., 2011). A commonly held view is that such pre-GC competition is highly restrictive, so that only a handful of “founder” clones seed each individual GC. This view stems from a series of reports that inferred total clonal diversity from the prevalence of homogeneous GCs in responses containing two distinguishable populations of B cells (Jacob et al., 1991a; Kroese et al., 1987; Liu et al., 1991), but was questioned by a study that directly measured clonal diversity by sequencing Ig genes from single human GC B cells picked from histological sections (Kuppers et al., 1993).
More recently, we have used in situ photoactivation followed by flow cytometry (Shulman et al., 2013; Victora et al., 2010) to obtain large numbers of single cells from GCs at around the peak of clonal diversity (6 days after immunization with antigen in alum) (Tas et al., 2016). This approach shows that clonal diversity in individual GCs can be quite high—reaching over 100 clones per GC for immunization with chicken gamma globulin (CGG, a model antigen consisting of a mixture of chicken immunoglobulins of different isotypes)—but can also vary substantially depending on the antigen used. Thus, while no strict bottleneck limits the number of founder cells in a GCs, the full extent of early GC clonal diversity appears to be determined by antigen-related properties, possibly including frequency of specific naïve precursors or the immunodominance relationships between the antigen’s epitopes. A further possibility is that polyclonal recruitment of B cells that have no specificity towards the immunizing antigen—presumably through direct adjuvant effects on B cells or through noncognate T cell help—could add to the initial diversity of GCs. Indeed, several studies have reported that antibodies cloned from GC (or other activated) B cells do not always bind the immunizing antigen by ELISA or other assays (Di Niro et al., 2015; Kuraoka et al., 2016; Tas et al., 2016). However, transgenic B cells with monoclonal specificity for antigens other than those used for infection or immunization fail to participate in the antibody response, indicating that BCR sequence—and, presumably, antigen specificity—is a determining factor in GC recruitment (Di Niro et al., 2015; Tas et al., 2016). Accordingly, analysis of the unmutated ancestor of a potent broadly-neutralizing antibody (bNAb) to influenza shows limited or no antigen binding by ELISA, although this same antibody could trigger a calcium flux when expressed on the surface of a B cell exposed to antigen (Lingwood et al., 2012). Thus, it is likely that many GC B cells bind antigen with affinities that are not detectable by standard binding assays, but which are nonetheless biologically relevant.
Once the GC structure is formed, bona fide GC competition begins to take place. This consists of a combination of interclonal competition (between clones bearing different V(D)J rearrangements, and therefore including a variety of epitope specificities and starting affinities) and intraclonal competition (between SHM variants of the same clone, and thus for the same epitope). Early studies show that hapten-driven GCs tend to become more clonally homogeneous over time (Jacob et al., 1993), and can contain variable-sized expansions of particular SHM variants (Ziegner et al., 1994), thus providing evidence of both interclonal and intraclonal competition. A caveat of these studies is that hapten-protein conjugates generate antibody responses that are strongly focused on the hapten, and heavily dominated by stereotypical V genes. Therefore, even though many clones with distinct V(D)J rearrangements may participate in the response, competition occurs mostly between B cells that bind a single epitope, and is thus perhaps better described as being intraclonal (Kuraoka et al., 2016).
Two recent studies (Kuraoka et al., 2016; Tas et al., 2016) have attempted to circumvent hapten-related issues by using technologies that allow examination of the more diverse GC responses to unhaptenated protein antigens. In one of these studies, we applied multicolor fate-mapping using a “Brainbow” allele to estimate the rate at which individual GCs became clonally homogeneous over time (Tas et al., 2016). Our findings show that most GCs retain substantial clonal diversity within the 3-week lifetime of the GC response to CGG in alum. However, rapid and massive expansion of higher-affinity SHM variants (which we refer to as “clonal bursts”) leads to substantial loss of diversity in a subset of GCs. Affinity maturation was observed regardless of the presence of clonal bursts. Thus, while bursts allow for rapid diversification of single SHM mutants at the expense of clonal diversity, affinity maturation of several clones in parallel can occur within more diverse GCs, at least over a period of a few weeks. An independent study by Kelsoe and colleagues also provides evidence that affinity can increase without narrowing the diversity of the responding repertoire in GCs arising in response to immunization with influenza hemagglutinin (HA) or anthrax protective antigen (rPA) (Kuraoka et al., 2016). Using “Nojima” cultures (Nojima et al., 2011), to obtain workable amounts of antibody from single-sorted GC B cells, this study has measured the affinities of a large number of cells from the same responding LN. While affinity maturation within GCs is readily observable, there was no evidence of constriction of repertoire diversity as the GC reaction progressed. Thus, affinity maturation does not necessarily involve radical loss of diversity (Fig. 3A), which is teleologically satisfying for a system that must generate antibodies that are protective not only against re-challenge with the exact same strain of pathogen, but also with other variant strains.
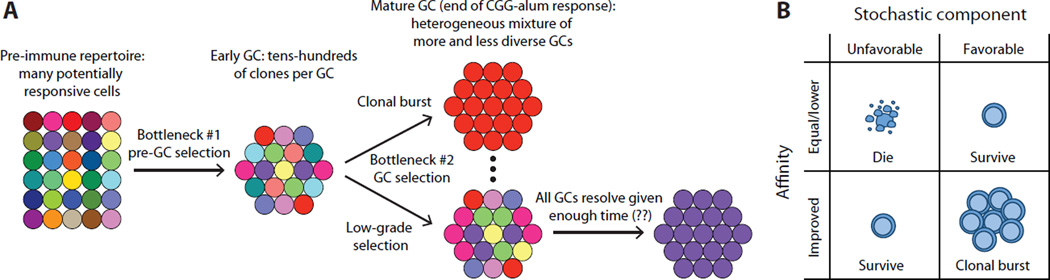
Figure 3. Clonal dynamics in GCs.
(A) Evolution of clonal diversity in GCs. Each color corresponds to a different clone (a distinct V(D)J rearrangement). Many potentially responsive cells are present in the pre-immune repertoire. Priming involves a competitive bottleneck, which prevents many lower-affinity B cells from entering the GC reaction. Despite this bottleneck, early GCs can still contain tens to hundreds of distinct clones, depending on the antigen used for immunization. Clonal diversity is lost at different rates of in different GCs, so that, by the end of the response to a protein antigen in alum, GCs are distributed across a wide range of clonal diversity. It is not yet known if all GCs would eventually become monoclonal were the response to last indefinitely. (B) Speculative model for the influence of stochastic “noise” (i.e., any factor not directly related to BCR affinity) on GC selection. B cells that fail to gain affinity upon SHM can still be rescued by stochastic factors (e.g., fortuitous encounters with antigen-rich FDC patches or with high-avidity Tfh cells). If the same positive stochastic factors act on a B cell whose affinity increased by SHM, a clonal burst may ensue.
Both studies also provide evidence that B cells of widely disparate affinities can co-exist within the same GC, suggesting that GC selection is much less strict than generally appreciated (Kuraoka et al., 2016; Tas et al., 2016). Mechanistically, the factors underlying this permissiveness are unclear, but may include a strong component of stochastic “noise” (defined as any parameter unrelated to BCR affinity) that is superimposed upon affinity-based selection. The finding that Brainbow GCs can deviate substantially from baseline color distribution even when all cells have identical affinity (Tas et al., 2016) provides experimental evidence of the degree of noise involved in GC selection. The prominent role of Tfh cells in driving GC selection within a framework that is highly dynamic and prone to strong feed-forward loops (Gitlin et al., 2015; Gitlin et al., 2014; Liu et al., 2015; Shulman et al., 2014): and see Fig. 2) suggests a cellular mechanism for this noise. We propose a speculative model (Fig. 3B) in which stochasticity cooperates with changes in affinity in determining the fate of a mutant GC B cell: while a lower affinity cell can be rescued from death by stochastic factors (such as finding a large portion of antigen on a FDC, or engaging an available, high-affinity T cell), a higher-affinity B cell favored by these same factors may become the source of a clonal burst. This stochastic component would ensure the maintenance of some degree of polyclonality while still allowing for strong SHM of a subset selected clones through clonal bursts (Tas et al., 2016).
Post-GC B cell fates
In order to exert their effector or memory functions, B cells must differentiate into plasma cells (PC) or memory B cells (Bmem), respectively. The choice between these fates can happen at two distinct stages of the antibody response: at the T:B border, recently activated B cells choose between entering the GC or differentiating into PC or Bmem; later, within the GC itself, positively-selected GC B cells interrupt the LZ-DZ re-entry cycle to exit the GC, again differentiating into PC or Bmem. Due to technical constraints, differentiation from naïve progenitors at the T:B border has been more widely studied than differentiation from within the GC. How similar or different the rules of differentiation are at these two stages is not clear. A number of studies have shown that acquisition of a PC phenotype is facilitated by higher affinity, both in the early (T:B border) response and within ongoing GCs (Paus et al., 2006; Phan et al., 2006; Smith et al., 1997), whereas a recent study suggests that lower-affinity B cells are preferentially recruited into the Bmem pool (Shinnakasu et al., 2016). The mechanism by which affinity may promote PC or Bmem differentiation is not fully understood and, as with selection for cyclic re-entry, could depend on signals from both BCR and T cell help. Whereas the T-independent response to repetitive antigens argues that, at least in some cases, PC differentiation can be triggered by strong BCR signaling alone (Shih et al., 2002), acutely inducing strong BCR signals triggers apoptosis in GC B cells (Han et al., 1995; Pulendran et al., 1995; Shokat and Goodnow, 1995). Alternatively, targeting antigen to B cells through DEC-205 simultaneously triggers massive differentiation of plasmablasts and GC entry or re-entry, both at the T:B border and from within ongoing GCs (Schwickert et al., 2011; Victora et al., 2010). Thus, enhanced T cell help alone is sufficient to trigger PC differentiation, at least under manipulated experimental settings.
An interesting question is whether B cell fate decision occurs prior to or after proliferation (i.e., at the level of the B cell that received the proliferative signal or independently for each daughter cell). In the pre-GC response, the answer is most likely the latter. Early studies show that B cells in extrafollicular foci (predominantly PC or plasmablasts) and GCs share a common clonal origin (Jacob et al., 1991a; Jacob et al., 1991b); thus, in agreement with Burnet’s original predictions (Burnet, 1957), a single naïve lymphocyte can generate distinct progenies. More recently, an elegant study tracing the ability of single antigen-specific B cells to differentiate into the PC, GC, Bmem, or “activated precursor” fates after T:B border interactions reported that, whereas just under half of the clones analyzed contained cells of only one type, clones choosing multiple fates were common, with about 10% of clones including members of all four cell types (Taylor et al., 2015). Multiplicity of fates correlate strongly with clonal size (which is associated with ability to resist apoptosis rather than with proliferation), so that clones that included all 4 fates comprise over thirty-fold more cells on average than clones that followed only one fate. This is consistent with a previous study showing that increased propensity to generate PC is likely secondary to increased survival and proliferation of B cells that previously chose the PC fate (Chan et al., 2009). Thus, at least in early stages, B cell differentiation appears to be acting subsequently to proliferation and at the level of each daughter cell. In the GC, this question can be rephrased as an anatomical one: does differentiation occur in the LZ (prior to proliferation, since selected B cells move from LZ to DZ during S phase) or in the DZ (during or after one or more rounds of proliferation)? Only DZ differentiation would be compatible with models that require asymmetric cell division (see below) or re-setting of cell state by proliferation (Duffy et al., 2012), and a theoretical problem would be the chance of an effector cell acquiring a deleterious or self-reactive mutation which would remain untested (since the mutated cell does not go through a LZ phase prior to exit). The available data on the site of GC B cell fate choice is ambiguous. While most studies suggest that this process initiates in the LZ—for example, Blimp1 and Irf4 are found to be expressed by a minority of cells in this zone (Angelin-Duclos et al., 2000; Falini et al., 2000)—other studies have proposed a DZ origin for PCs based on cell migration dynamics (Meyer-Hermann et al., 2012).
In addition to affinity, other factors have been found to contribute to B cell fate choice. The propensity of B cells to differentiate into Bmem or PC has been shown to change over the course of the antibody response, so that Bmem are generated mostly in the pre-GC and early GC periods, after which long-lived PC differentiation becomes more pronounced (Weisel et al., 2016). Because the affinity of GC B cells for antigen also increases over time, the temporal hypothesis is not incompatible with a role for affinity in promoting the PC fate. Asymmetric cell division, in which one daughter B cell retains most of the endocytosed antigen (Thaunat et al., 2012) or a higher concentration of key molecules for the GC phenotype such as Bcl-6 and the IL-21 receptor (Barnett et al., 2012) may also be involved in post-GC fate choices. Additionally, BCR isotype may play a role in B cell fate choice. Enhancement of PC differentiation by IgG expression in memory B cells has been widely documented [reviewed in (Xu et al., 2014)]. However, the distinction between the role of isotype-specific signaling capabilities and other factors such as changes in cell state due to prior antigen experience (Kometani et al., 2013) or affinity for antigen (which may be higher in switched cells) is not always clear. A recent study separating the effects of SHM and class switching demonstrated that switching to IgG1 strongly biased B cells towards the PC and against the Bmem fates, independently of any other cell-intrinsic factors, suggesting an instructive role for the IgG1 signaling tail (Gitlin et al., 2016). Likewise, activated B cells that switch to IgE during the early B cell response preferentially adopt the PC over the GC fate (Erazo et al., 2007). This appears to be related to the expression of IgE itself, since in vitro-activated B cells that have switched to IgE are more likely to differentiate into PC or plasmablasts than those that switched to IgG1, even in the absence of antigen (Yang et al., 2012). Finally, B cells from a mouse in which the human sIgA constant region was inserted upstream of the Cμ gene preferentially develop into PCs both in vitro and in vivo (Duchez et al., 2010). Thus, the skewing of fate decisions noted in cells expressing non-IgM receptors is most likely the direct result of differences in the signaling capability of IgM and non-IgM BCRs.
The molecular mechanism underlying GC B cell differentiation into Bmem cells of distinct phenotypes remain unclear (Kurosaki et al., 2015; Tarlinton and Good-Jacobson, 2013). Whereas no single deterministic transcription factor acting as a “master regulator” of the Bmem fate has yet been identified, higher expression of Bach2 in lower-affinity GC B cells can favor Bmem differentiation (Shinnakasu et al., 2016). The molecular drivers of B cell differentiation into the PC fate are better understood, and have been recently reviewed (Kometani et al., 2013; Nutt et al., 2015). Key in this process is the activation of the PC master-regulator Blimp1 (Prdm1) (Turner et al., 1994), which, among many other functions (Minnich et al., 2016; Tellier et al., 2016) helps shut down expression of transcription factors such as Pax5 and Bcl6, responsible for maintenance of B cell and GC identity, respectively [reviewed in (Nutt et al., 2015)]. What signals underlie the decision of activated or GC B cells to switch on Blimp1 is an active area of investigation. Dialing up expression of Irf4 (which at high concentrations represses Bcl-6) and shutting down expression of Bach2 (which can act as a repressor of Blimp1) appear to be crucial to establish the PC program (Muto et al., 2010; Sciammas et al., 2006). Interestingly, the NF-κB subunit RelA is specifically required for differentiation of GC B cells into the PC fate, likely by directly promoting Blimp1 expression independently of Irf4 (Heise et al., 2014). Understanding what signals induce activation of RelA in vivo may provide valuable clues to the cellular processes involved in PC differentiation. Other recent developments have included the notions that epigenetic modifiers such as EZH2 and MOZ may influence the balance between PC and Bmem differentiation (Beguelin et al., 2013; Caganova et al., 2013; Good-Jacobson et al., 2014) and that mitochondrial function may provide instructive signals that can skew activation-induced B-cell fates (Jang et al., 2015).
Long-lived and chronic GCs
GC life span can vary greatly depending on the nature of the immune stimulus. Whereas protein model antigens adsorbed in alum usually induce short-lived GCs that collapse within the first month after immunization, certain synthetic vaccines, viral infections or the gut microbiota can induce GCs that remain active for much longer periods, if not indefinitely (Adachi et al., 2015; Bachmann et al., 1996; Kasturi et al., 2011; Sutherland et al., 2016). Prolonged GC reactions may allow B cells to reach a higher degree of affinity maturation (Klein et al., 2013; Pappas et al., 2014) and may represent a means to cope with the antigenic drift associated with chronic viral infection (Bonsignori et al., 2016; Liao et al., 2013). Alternatively, extended SHM in chronic GCs may cause the appearance of self-reactive variants that eventually escape negative selection, leading to autoimmunity (Vinuesa et al., 2005), and may also promote genomic instability and the emergence of AID-dependent B cell lymphomas (Robbiani et al., 2015). Despite the importance of chronic GC reactions, specific requirements for long-term GC maintenance and the rules governing the dynamics of B cell selection under such conditions are poorly defined. Persistence of antigen on FDCs is likely to be necessary, since axiomatically GC selection cannot proceed in its absence. However, whether antigen depletion from FDCs is the cause of GC involution in vivo has not been established, and FDCs can retain antigen for periods much longer than the duration of most immunization-induced GCs (Tew and Mandel, 1979). In addition to antigen, innate immune signals seem to play a pivotal role in sustaining the GC reaction. For example, immunization of mice and non-human primates with influenza HA nanoparticles in combination with TLR4 and TLR7 agonists generates GCs that persist for several months (Kasturi et al., 2011). Whether the activity of TLRs is required in GC B cells themselves or in other cell types remains to be determined.
An interesting question is whether a long-lived GC response consists predominantly of GCs that are themselves long-lived, or of a multiplicity of short-lived GCs that wax and wane with unsynchronized kinetics, generating an apparently long-lived steady state. An intermediate scenario is the constant reseeding of ongoing GC reactions by invasion of recently primed B and T cells (Schwickert et al., 2009; Schwickert et al., 2007; Shulman et al., 2013), which seems to be especially prevalent in PP GCs (Bergqvist et al., 2013). The presence of apparently monoclonal GCs in mesenteric LN and even PPs (Tas et al., 2016) suggests that such invasion is not constantly ongoing, but may occur only sporadically. Sporadic re-seeding raises the possibility that GC longevity could correlate with the extent to which new B cells are recruited. Importantly, it has been shown that memory B cells can re-enter GCs and further diversify their BCRs (Dogan et al., 2009; McHeyzer-Williams et al., 2015). Therefore, it is reasonable to speculate that the chronic responses necessary for the generation of highly mutated B cell variants such as those that produce HIV bNAbs may involve sequential “hopping” of B cells between long-lived GCs, likely through a Bmem intermediate.
Conclusions
In the past decade, we have learned great deal about the basic cellular and molecular dynamics of B cell selection in the GC, in large part thanks to technical innovations in intravital imaging and antibody cloning. We are now in a position to re-visit aspects of GC selection related to clonal dynamics, which were placed on hold since the late 1990’s awaiting the development of appropriate technologies. The realization that bNAbs to influenza, plasmodium and, more strikingly, HIV require extensive and unconventional histories of SHM and selection, and the puzzling finding that highly mutated (and presumably extensively selected) bNAbs often arise from very low-affinity precursors, exemplify how little is known regarding the pace and stringency of GC selection, especially during long-lived responses (Klein et al., 2013; Liao et al., 2013; Lingwood et al., 2012; Pappas et al., 2014; Tan et al., 2016). The contribution of GC selection to phenomena such as immunodominance and original antigenic sin has also not been established, and is likely to be of considerable interest. Answering these questions will be crucial to our quest to produce broadly protective vaccines against influenza and HIV (Burton et al., 2012; Victora and Wilson, 2015), and will undoubtedly bring novel insights into the general functioning of GC, as well as of the immune system in general.
Acknowledgments
We thank O. Bannard (Oxford University) and D. Dominguez-Sola (Mount Sinai School of Medicine) for helpful comments and suggestions. This work is supported by NIH grant DP5OD012146 to G.D.V.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi Y, Onodera T, Yamada Y, Daio R, Tsuiji M, Inoue T, Kobayashi K, Kurosaki T, Ato M, Takahashi Y. Distinct germinal center selection at local sites shapes memory B cell response to viral escape. The Journal of experimental medicine. 2015;212:1709–1723. doi: 10.1084/jem.20142284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007a;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007b;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol. 2000;165:5462–5471. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Odermatt B, Hengartner H, Zinkernagel RM. Induction of long-lived germinal centers associated with persisting antigen after viral infection. The Journal of experimental medicine. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannard O, Horton RM, Allen CD, An J, Nagasawa T, Cyster JG. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity. 2013;39:912–924. doi: 10.1016/j.immuni.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannard O, McGowan SJ, Ersching J, Ishido S, Victora GD, Shin JS, Cyster JG. Ubiquitin-mediated fluctuations in MHC class II facilitate efficient germinal center B cell responses. The Journal of experimental medicine. 2016;213:993–1009. doi: 10.1084/jem.20151682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett BE, Ciocca ML, Goenka R, Barnett LG, Wu J, Laufer TM, Burkhardt JK, Cancro MP, Reiner SL. Asymmetric B cell division in the germinal center reaction. Science. 2012;335:342–344. doi: 10.1126/science.1213495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso K, Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nature reviews. Immunology. 2015;15:172–184. doi: 10.1038/nri3814. [DOI] [PubMed] [Google Scholar]
- Batista FD, Neuberger MS. B cells extract and present immobilized antigen: implications for affinity discrimination. The EMBO journal. 2000;19:513–520. doi: 10.1093/emboj/19.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguelin W, Popovic R, Teater M, Jiang Y, Bunting KL, Rosen M, Shen H, Yang SN, Wang L, Ezponda T, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23:677–692. doi: 10.1016/j.ccr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Berek C, Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Bergqvist P, Stensson A, Hazanov L, Holmberg A, Mattsson J, Mehr R, Bemark M, Lycke NY. Re-utilization of germinal centers in multiple Peyer’s patches results in highly synchronized, oligoclonal, and affinity-matured gut IgA responses. Mucosal immunology. 2013;6:122–135. doi: 10.1038/mi.2012.56. [DOI] [PubMed] [Google Scholar]
- Bonsignori M, Zhou T, Sheng Z, Chen L, Gao F, Joyce MG, Ozorowski G, Chuang GY, Schramm CA, Wiehe K, et al. Maturation Pathway from Germline to Broad HIV-1 Neutralizer of a CD4-Mimic Antibody. Cell. 2016;165:449–463. doi: 10.1016/j.cell.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet FM. A modification of Jerne’s theory of antibody production using the concept of clonal selection. Aust. J. Sci. 1957;20:67–68. doi: 10.3322/canjclin.26.2.119. [DOI] [PubMed] [Google Scholar]
- Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, et al. A Blueprint for HIV Vaccine Discovery. Cell host & microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt D, Chan TD, Bourne K, Hermes JR, Nguyen A, Statham A, O’Reilly LA, Strasser A, Price S, Schofield P, et al. FAS Inactivation Releases Unconventional Germinal Center B Cells that Escape Antigen Control and Drive IgE and Autoantibody Production. Immunity. 2015;42:890–902. doi: 10.1016/j.immuni.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Caganova M, Carrisi C, Varano G, Mainoldi F, Zanardi F, Germain PL, George L, Alberghini F, Ferrarini L, Talukder AK, et al. Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. The Journal of clinical investigation. 2013;123:5009–5022. doi: 10.1172/JCI70626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado DP, Sasaki Y, Godinho SA, Pellerin A, Kochert K, Sleckman BP, de Alboran IM, Janz M, Rodig S, Rajewsky K. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nature immunology. 2012;13:1092–1100. doi: 10.1038/ni.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. Journal of immunology. 2009;183:3139–3149. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- Chan TD, Wood K, Hermes JR, Butt D, Jolly CJ, Basten A, Brink R. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity. 2012;37:893–904. doi: 10.1016/j.immuni.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey F, Alabyev B, Manser T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity. 2009;30:599–609. doi: 10.1016/j.immuni.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annual review of immunology. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. Journal of immunology. 1998;161:5373–5381. [PubMed] [Google Scholar]
- De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nature reviews immunology. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Niro R, Lee SJ, Vander Heiden JA, Elsner RA, Trivedi N, Bannock JM, Gupta NT, Kleinstein SH, Vigneault F, Gilbert TJ, et al. Salmonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity. 2015;43:120–131. doi: 10.1016/j.immuni.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, Weill JC. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- Dominguez-Sola D, Kung J, Holmes AB, Wells VA, Mo T, Basso K, Dalla-Favera R. The FOXO1 Transcription Factor Instructs the Germinal Center Dark Zone Program. Immunity. 2015;43:1064–1074. doi: 10.1016/j.immuni.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Dominguez-Sola D, Victora GD, Ying CY, Phan RT, Saito M, Nussenzweig MC, Dalla-Favera R. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nature immunology. 2012;13:1083–1091. doi: 10.1038/ni.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchez S, Amin R, Cogne N, Delpy L, Sirac C, Pascal V, Corthesy B, Cogne M. Premature replacement of mu with alpha immunoglobulin chains impairs lymphopoiesis and mucosal homing but promotes plasma cell maturation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3064–3069. doi: 10.1073/pnas.0912393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KR, Wellard CJ, Markham JF, Zhou JH, Holmberg R, Hawkins ED, Hasbold J, Dowling MR, Hodgkin PD. Activation-induced B cell fates are selected by intracellular stochastic competition. Science. 2012;335:338–341. doi: 10.1126/science.1213230. [DOI] [PubMed] [Google Scholar]
- Eisen HN. Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol Res. 2014;2:381–392. doi: 10.1158/2326-6066.CIR-14-0029. [DOI] [PubMed] [Google Scholar]
- Eisen HN, Siskind GW. Variations in Affinities of Antibodies During the Immune Response. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Erazo A, Kutchukhidze N, Leung M, Christ AP, Urban JF, Jr, Curotto de Lafaille MA, Lafaille JJ. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falini B, Fizzotti M, Pucciarini A, Bigerna B, Marafioti T, Gambacorta M, Pacini R, Alunni C, Natali-Tanci L, Ugolini B, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000;95:2084–2092. [PubMed] [Google Scholar]
- Garin A, Meyer-Hermann M, Contie M, Figge MT, Buatois V, Gunzer M, Toellner KM, Elson G, Kosco-Vilbois MH. Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity. 2010;33:84–95. doi: 10.1016/j.immuni.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B Cells by the Orphan G Protein-Coupled Receptor EBI2 Shapes Humoral Immune Responses. Immunity. 2009 doi: 10.1016/j.immuni.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Gatto D, Wood K, Brink R. EBI2 operates independently of but in cooperation with CXCR5 and CCR7 to direct B cell migration and organization in follicles and the germinal center. Journal of immunology. 2011;187:4621–4628. doi: 10.4049/jimmunol.1101542. [DOI] [PubMed] [Google Scholar]
- Gitlin AD, Mayer CT, Oliveira TY, Shulman Z, Jones MJ, Koren A, Nussenzweig MC. T cell help controls the speed of the cell cycle in germinal center B cells. Science. 2015 doi: 10.1126/science.aac4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509:637–640. doi: 10.1038/nature13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin AD, von Boehmer L, Gazumyan A, Shulman Z, Oliveira TY, Nussenzweig MC. Independent Roles of Switching and Hypermutation in the Development and Persistence of B Lymphocyte Memory. Immunity. 2016;44:769–781. doi: 10.1016/j.immuni.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenka R, Matthews AH, Zhang B, O’Neill PJ, Scholz JL, Migone TS, Leonard WJ, Stohl W, Hershberg U, Cancro MP. Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. The Journal of experimental medicine. 2014;211:45–56. doi: 10.1084/jem.20130505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez SF, Degn SE, Pitcher LA, Woodruff M, Heesters BA, Carroll MC. Trafficking of B cell antigen in lymph nodes. Annual review of immunology. 2011;29:215–233. doi: 10.1146/annurev-immunol-031210-101255. [DOI] [PubMed] [Google Scholar]
- Good-Jacobson KL, Chen Y, Voss AK, Smyth GK, Thomas T, Tarlinton D. Regulation of germinal center responses and B-cell memory by the chromatin modifier MOZ. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9585–9590. doi: 10.1073/pnas.1402485111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Cyster JG. S1PR2 links germinal center confinement and growth regulation. Immunological reviews. 2012;247:36–51. doi: 10.1111/j.1600-065X.2012.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Suzuki K, Cho B, Willison LD, Palmer D, Allen CD, Schmidt TH, Xu Y, Proia RL, Coughlin SR, Cyster JG. The sphingosine 1-phosphate receptor S1P(2) maintains the homeostasis of germinal center B cells and promotes niche confinement. Nature immunology. 2011;12:672–680. doi: 10.1038/ni.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J Exp Med. 1995;182:1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011;475:524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JS, Meyer-Hermann M, Xiangying D, Zuan LY, Jones LA, Ramakrishna L, de Vries VC, Dolpady J, Aina H, Joseph S, et al. The distinctive germinal center phase of IgE+ B lymphocytes limits their contribution to the classical memory response. The Journal of experimental medicine. 2013;210:2755–2771. doi: 10.1084/jem.20131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JS, Narayanan S, Subramaniam S, Ho WQ, Lafaille JJ, Curotto de Lafaille MA. Biology of IgE production: IgE cell differentiation and the memory of IgE responses. Current topics in microbiology and immunology. 2015;388:1–19. doi: 10.1007/978-3-319-13725-4_1. [DOI] [PubMed] [Google Scholar]
- Heesters BA, Chatterjee P, Kim YA, Gonzalez SF, Kuligowski MP, Kirchhausen T, Carroll MC. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity. 2013;38:1164–1175. doi: 10.1016/j.immuni.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesters BA, Myers RC, Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nature reviews. Immunology. 2014;14:495–504. doi: 10.1038/nri3689. [DOI] [PubMed] [Google Scholar]
- Heise N, De Silva NS, Silva K, Carette A, Simonetti G, Pasparakis M, Klein U. Germinal center B cell maintenance and differentiation are controlled by distinct NF-kappaB transcription factor subunits. The Journal of experimental medicine. 2014;211:2103–2118. doi: 10.1084/jem.20132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991a;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991b;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Jacob J, Przylepa J, Miller C, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KJ, Mano H, Aoki K, Hayashi T, Muto A, Nambu Y, Takahashi K, Itoh K, Taketani S, Nutt SL, et al. Mitochondrial function provides instructive signals for activation-induced B-cell fates. Nature communications. 2015;6:6750. doi: 10.1038/ncomms7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly LM, Pereira JP, Yi T, Xu Y, Cyster JG. EBI2 guides serial movements of activated B cells and ligand activity is detectable in lymphoid and nonlymphoid tissues. Journal of immunology. 2011;187:3026–3032. doi: 10.4049/jimmunol.1101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepler TB, Perelson AS. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol Today. 1993;14:412–415. doi: 10.1016/0167-5699(93)90145-B. [DOI] [PubMed] [Google Scholar]
- Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336:1178–1181. doi: 10.1126/science.1213368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometani K, Nakagawa R, Shinnakasu R, Kaji T, Rybouchkin A, Moriyama S, Furukawa K, Koseki H, Takemori T, Kurosaki T. Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity. 2013;39:136–147. doi: 10.1016/j.immuni.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Kroese FG, Wubbena AS, Seijen HG, Nieuwenhuis P. Germinal centers develop oligoclonally. Eur J Immunol. 1987;17:1069–1072. doi: 10.1002/eji.1830170726. [DOI] [PubMed] [Google Scholar]
- Kuppers R, Zhao M, Hansmann ML, Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. Embo J. 1993;12:4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka M, Schmidt AG, Nojima T, Feng F, Watanabe A, Kitamura D, Harrison SC, Kepler TB, Kelsoe G. Complex Antigens Drive Permissive Clonal Selection in Germinal Centers. Immunity. 2016;44:542–552. doi: 10.1016/j.immuni.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T, Kometani K, Ise W. Memory B cells. Nature reviews. Immunology. 2015;15:149–159. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, McTamney PM, Yassine HM, Whittle JR, Guo X, Boyington JC, Wei CJ, Nabel GJ. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature. 2012;489:566–570. doi: 10.1038/nature11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. Foxp3(+) follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, Luo D, Qi H. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2015;517:214–218. doi: 10.1038/nature13803. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, McHeyzer-Williams MG. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nature immunology. 2015;16:296–305. doi: 10.1038/ni.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Hermann M, Mohr E, Pelletier N, Zhang Y, Victora GD, Toellner KM. A theory of germinal center B cell selection, division, and exit. Cell reports. 2012;2:162–174. doi: 10.1016/j.celrep.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Meyer-Hermann ME, Maini PK, Iber D. An analysis of B cell selection mechanisms in germinal centers. Math Med Biol. 2006;23:255–277. doi: 10.1093/imammb/dql012. [DOI] [PubMed] [Google Scholar]
- Minnich M, Tagoh H, Bonelt P, Axelsson E, Fischer M, Cebolla B, Tarakhovsky A, Nutt SL, Jaritz M, Busslinger M. Multifunctional role of the transcription factor Blimp-1 in coordinating plasma cell differentiation. Nature immunology. 2016;17:331–343. doi: 10.1038/ni.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama S, Takahashi N, Green JA, Hori S, Kubo M, Cyster JG, Okada T. Sphingosine-1-phosphate receptor 2 is critical for follicular helper T cell retention in germinal centers. The Journal of experimental medicine. 2014;211:1297–1305. doi: 10.1084/jem.20131666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J, Matloubian M, Zikherman J. Cutting edge: An in vivo reporter reveals active B cell receptor signaling in the germinal center. Journal of immunology. 2015;194:2993–2997. doi: 10.4049/jimmunol.1403086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi JR, Lu E, Cyster JG. The G protein-coupled receptor P2RY8 and follicular dendritic cells promote germinal center confinement of B cells, whereas S1PR3 can contribute to their dissemination. The Journal of experimental medicine. 2015;212:2213–2222. doi: 10.1084/jem.20151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi JR, Schmitz R, Green JA, Xiao W, Larsen AB, Braun SE, An J, Xu Y, Rosenwald A, Ott G, et al. Loss of signalling via Galpha13 in germinal centre B-cell-derived lymphoma. Nature. 2014;516:254–258. doi: 10.1038/nature13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Muto A, Ochiai K, Kimura Y, Itoh-Nakadai A, Calame KL, Ikebe D, Tashiro S, Igarashi K. Bach2 represses plasma cell gene regulatory network in B cells to promote antibody class switch. The EMBO journal. 2010;29:4048–4061. doi: 10.1038/emboj.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natkanski E, Lee WY, Mistry B, Casal A, Molloy JE, Tolar P. B cells use mechanical energy to discriminate antigen affinities. Science. 2013;340:1587–1590. doi: 10.1126/science.1237572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145–1156. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima T, Haniuda K, Moutai T, Matsudaira M, Mizokawa S, Shiratori I, Azuma T, Kitamura D. In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nature communications. 2011;2:465. doi: 10.1038/ncomms1475. [DOI] [PubMed] [Google Scholar]
- Nowosad CR, Spillane KM, Tolar P. Germinal center B cells recognize antigen through a specialized immune synapse architecture. Nature immunology. 2016 doi: 10.1038/ni.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nature reviews. Immunology. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprea M, Perelson AS. Somatic mutation leads to efficient affinity maturation when centrocytes recycle back to centroblasts. J Immunol. 1997;158:5155–5162. [PubMed] [Google Scholar]
- Pappas L, Foglierini M, Piccoli L, Kallewaard NL, Turrini F, Silacci C, Fernandez-Rodriguez B, Agatic G, Giacchetto-Sasselli I, Pellicciotta G, et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature. 2014;516:418–422. doi: 10.1038/nature13764. [DOI] [PubMed] [Google Scholar]
- Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460:1122–1126. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Kannourakis G, Nouri S, Smith KG, Nossal GJ. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995;375:331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Radmacher MD, Kelsoe G, Kepler TB. Predicted and inferred waiting times for key mutations in the germinal centre reaction: evidence for stochasticity in selection. Immunology and cell biology. 1998;76:373–381. doi: 10.1046/j.1440-1711.1998.00753.x. [DOI] [PubMed] [Google Scholar]
- Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunological reviews. 2013;252:146–155. doi: 10.1111/imr.12031. [DOI] [PubMed] [Google Scholar]
- Robbiani DF, Deroubaix S, Feldhahn N, Oliveira TY, Callen E, Wang Q, Jankovic M, Silva IT, Rommel PC, Bosque D, et al. Plasmodium Infection Promotes Genomic Instability and AID-Dependent B Cell Lymphoma. Cell. 2015;162:727–737. doi: 10.1016/j.cell.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda LB, Bannard O, Ludewig B, Nagasawa T, Cyster JG. Phenotypic and Morphological Properties of Germinal Center Dark Zone Cxcl12-Expressing Reticular Cells. Journal of immunology. 2015;195:4781–4791. doi: 10.4049/jimmunol.1501191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabouri Z, Schofield P, Horikawa K, Spierings E, Kipling D, Randall KL, Langley D, Roome B, Vazquez-Lombardi R, Rouet R, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E2567–E2575. doi: 10.1073/pnas.1406974111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage PT, Sharpe AH. T follicular regulatory cells in the regulation of B cell responses. Trends in immunology. 2015;36:410–418. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander S, Chu VT, Yasuda T, Franklin A, Graf R, Calado DP, Li S, Imami K, Selbach M, Di Virgilio M, et al. PI3 Kinase and FOXO1 Transcription Factor Activity Differentially Control B Cells in the Germinal Center Light and Dark Zones. Immunity. 2015;43:1075–1086. doi: 10.1016/j.immuni.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Schwickert TA, Alabyev B, Manser T, Nussenzweig MC. Germinal center reutilization by newly activated B cells. J Exp Med. 2009;206:2907–2914. doi: 10.1084/jem.20091225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- Schwickert TA, Victora GD, Fooksman DR, Kamphorst AO, Mugnier MR, Gitlin AD, Dustin ML, Nussenzweig MC. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. The Journal of experimental medicine. 2011;208:1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Shih TA, Roederer M, Nussenzweig MC. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nat Immunol. 2002;3:399–406. doi: 10.1038/ni776. [DOI] [PubMed] [Google Scholar]
- Shinnakasu R, Inoue T, Kometani K, Moriyama S, Adachi Y, Nakayama M, Takahashi Y, Fukuyama H, Okada T, Kurosaki T. Regulated selection of germinal-center cells into the memory B cell compartment. Nature immunology. 2016 doi: 10.1038/ni.3460. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, Victora GD. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman Z, Gitlin AD, Weinstein JS, Lainez B, Esplugues E, Flavell RA, Craft JE, Nussenzweig MC. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345:1058–1062. doi: 10.1126/science.1257861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KG, Light A, Nossal GJ, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland DB, Suzuki K, Fagarasan S. Fostering of advanced mutualism with gut microbiota by Immunoglobulin A. Immunological reviews. 2016;270:20–31. doi: 10.1111/imr.12384. [DOI] [PubMed] [Google Scholar]
- Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, Lee WP, Egen JG, Austin CD, Xu M, Wu LC. IgE(+) memory B cells and plasma cells generated through a germinal-center pathway. Nature immunology. 2012;13:396–404. doi: 10.1038/ni.2256. [DOI] [PubMed] [Google Scholar]
- Tan J, Pieper K, Piccoli L, Abdi A, Foglierini M, Geiger R, Tully CM, Jarrossay D, Ndungu FM, Wambua J, et al. A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens. Nature. 2016;529:105–109. doi: 10.1038/nature16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton D, Good-Jacobson K. Diversity among memory B cells: origin, consequences, and utility. Science. 2013;341:1205–1211. doi: 10.1126/science.1241146. [DOI] [PubMed] [Google Scholar]
- Tas JM, Mesin L, Pasqual G, Targ S, Jacobsen JT, Mano YM, Chen CS, Weill JC, Reynaud CA, Browne EP, et al. Visualizing antibody affinity maturation in germinal centers. Science. 2016;351:1048–1054. doi: 10.1126/science.aad3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Pape KA, Steach HR, Jenkins MK. Humoral immunity. Apoptosis and antigen affinity limit effector cell differentiation of a single naive B cell. Science. 2015;347:784–787. doi: 10.1126/science.aaa1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier J, Shi W, Minnich M, Liao Y, Crawford S, Smyth GK, Kallies A, Busslinger M, Nutt SL. Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nature immunology. 2016;17:323–330. doi: 10.1038/ni.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew JG, Mandel TE. Prolonged antigen half-life in the lymphoid follicles of specifically immunized mice. Immunology. 1979;37:69–76. [PMC free article] [PubMed] [Google Scholar]
- Thaunat O, Granja AG, Barral P, Filby A, Montaner B, Collinson L, Martinez-Martin N, Harwood NE, Bruckbauer A, Batista FD. Asymmetric segregation of polarized antigen on B cell division shapes presentation capacity. Science. 2012;335:475–479. doi: 10.1126/science.1214100. [DOI] [PubMed] [Google Scholar]
- Tiller T, Kofer J, Kreschel C, Busse CE, Riebel S, Wickert S, Oden F, Mertes MM, Ehlers M, Wardemann H. Development of self-reactive germinal center B cells and plasma cells in autoimmune Fc gammaRIIB-deficient mice. J Exp Med. 2010;207:2767–2778. doi: 10.1084/jem.20100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Victora GD, Dominguez-Sola D, Holmes AB, Deroubaix S, Dalla-Favera R, Nussenzweig MC. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. Blood. 2012;120:2240–2248. doi: 10.1182/blood-2012-03-415380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. Germinal centers. Annual review of immunology. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal Center Dynamics Revealed by Multiphoton Microscopy with a Photoactivatable Fluorescent Reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Wilson PC. Germinal Center Selection and the Antibody Response to Influenza. Cell. 2015;163:545–548. doi: 10.1016/j.cell.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular Helper T Cells. Annual review of immunology. 2016 doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- Wang X, Cho B, Suzuki K, Xu Y, Green JA, An J, Cyster JG. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. The Journal of experimental medicine. 2011;208:2497–2510. doi: 10.1084/jem.20111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert MG, Cesari IM, Yonkovich SJ, Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970;228:1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity. 2016;44:116–130. doi: 10.1016/j.immuni.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J, Graca L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. Journal of immunology. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- Xiong H, Dolpady J, Wabl M, Curotto de Lafaille MA, Lafaille JJ. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med. 2012;209:353–364. doi: 10.1084/jem.20111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Xu L, Zhao M, Xu C, Fan Y, Pierce SK, Liu W. No receptor stands alone: IgG B-cell receptor intrinsic and extrinsic mechanisms contribute to antibody memory. Cell research. 2014;24:651–664. doi: 10.1038/cr.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Sullivan BM, Allen CD. Fluorescent in vivo detection reveals that IgE(+) B cells are restrained by an intrinsic cell fate predisposition. Immunity. 2012;36:857–872. doi: 10.1016/j.immuni.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Meyer-Hermann M, George LA, Figge MT, Khan M, Goodall M, Young SP, Reynolds A, Falciani F, Waisman A, et al. Germinal center B cells govern their own fate via antibody feedback. The Journal of experimental medicine. 2013;210:457–464. doi: 10.1084/jem.20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegner M, Steinhauser G, Berek C. Development of antibody diversity in single germinal centers: selective expansion of high-affinity variants. European journal of immunology. 1994;24:2393–2400. doi: 10.1002/eji.1830241020. [DOI] [PubMed] [Google Scholar]
- Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]