Abstract
Background
Obesity is associated with chronic low-grade inflammation leading to insulin resistance and diabetes. Adiponectin is an adipokine that regulates inflammatory responses. The aim of our study was to investigate whether any effects of adiponectin against obesity and insulin-resistance may depend on the adaptive immune system.
Methods
We treated high-fat-diet fed Rag1−/− mice lacking mature lymphocytes with adiponectin over 7 weeks and investigated alterations in their metabolic outcome and inflammatory state.
Results
Adiponectin protects from weight gain despite a small compensatory stimulation of energy intake in mice lacking an adaptive immune system. Additionally, adiponectin protects from dysglycemia. Minor alterations in the macrophage phenotype, but not in the circulating cytokine levels, may contribute to the protective role of adiponectin against hyperglycemia and diabetes.
Conclusion
Adiponectin or agents increasing adiponectin may be a promising therapeutic option against obesity and hyperglycemia in immune-deficient populations.
Keywords: adiponectin, obesity, insulin, diabetes, lymphocyte, immunity
INTRODUCTION
Obesity is associated with chronic low-grade inflammation leading to insulin resistance, diabetes and metabolic syndrome (1-4). The entire spectrum of immune cells participates in systemic inflammatory reactions involving predominantly the adipose tissue (5). Secretion of adipokines, cytokines and chemokines leads either to increased infiltration of adipose tissue by macrophages or to their transition from anti-inflammatory M2 to pro-inflammatory M1 phenotype (6). These actions among others are coordinated by the adaptive immune system. CD8+ T cells recruit macrophages by secretion of chemokines, while they stimulate M1 polarization through IFN-γ. Similarly, CD4+ Th1 cells, which secrete pro-inflammatory cytokines (e.g IFN-γ) in visceral adipose tissue, are activated either by macrophages or directly by adipocytes in obesity, joining CD8+T cells as crucial effector lymphocytes (7, 8). On the contrary, regulatory T cells (Tregs), that exert anti-inflammatory effects, are severely reduced in the adipose tissue in the obese and in the insulin-resistant state. Finally, B lymphocytes secrete more pro-inflammatory (e.g. IL-6, IFN-γ) and less anti-inflammatory (e.g. IL-10) cytokines in obesity, while they produce more IgG2 antibodies promoting insulin resistance (6, 9, 10). In this context, we have recently reported that Rag1−/− mice lacking mature T- and B-lymphocytes and consequently adaptive immune response, gain more weight and faster during exposure to high fat diet (HFD) than mice with an intact immune system (11). However, Rag1−/− mice demonstrate only minor defects in their glucose homeostasis after HFD. This supports the notion that the adaptive immune system is a key mediator of the obesity-related glucose and metabolic imbalance.
Adiponectin is an adipocyte-derived hormone that plays a protective role in obesity, insulin resistance, type 2 diabetes and metabolic syndrome (12-15). Administration of recombinant adiponectin in mice fed a HFD leads to weight loss by stimulating fat oxidation and regulating energy expenditure (14). Moreover, it improves the glucose profile primarily by ameliorating insulin sensitivity and secondarily by increasing insulin secretion or by affecting muscle and liver-mediated glucose metabolism (16-18). Many of these functions of adiponectin are achieved by regulation of the inflammatory response during high-caloric diet and obesity. Adiponectin has anti-inflammatory properties by suppressing the levels of TNFα and IFN-γ, and by increasing the expression of IL-10 and IL-1Ra (19). Moreover, it is involved in the accumulation and polarization of macrophages to the M2 anti-inflammatory phenotype in adipose tissue.
Although many studies have demonstrated the anti-inflammatory properties of adiponectin in obesity, it still remains unclear to which extend adiponectin depends on the adaptive immune system to mediate its functions. The aim of this study was to investigate if adiponectin can still exert its regulatory effects on weight and metabolic profile, as well as on inflammatory cascades, in mice lacking an adaptive immune system. For this reason, Rag1−/− mice fed with HFD for a longer period compared to our first study (14 vs 11 weeks) were treated with physiological doses of adiponectin over 7 weeks and alterations in their metabolic outcome (e.g. weight, fat distribution, energy intake, glucose, insulin secretion) and their inflammatory state (macrophage distribution and phenotype, cytokine expression) were investigated.
METHODS
Animals
Male, 3-week-old, Rag1tm1Mom(Rag1−/−) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed in individual cages with unlimited access to water in a room maintained at 22°C on a 12:12-h light-dark cycle. All mice were acclimated to the facility for 3 days while being fed a regular chow diet (Formulab diet 5008, Lab Diet, Richmond, IN) which contains 16.7% calories from fat and water ad libitum. The HFD was a western diet containing 42.2% of calorie from milk fat, 42.8% from carbohydrates and 15% from protein (TD88137, Harlan Teklad, Madison, WI, USA). The macronutrient distribution (saturated vs unsaturated fat) was representative of diets associated with a high-risk cardiovascular disease in humans (Teklad Custom Research Diet data sheet). All animals were handled in accordance with the principles and guidelines of the National Institutes of Health. The animal facilities and protocols reported were approved by the BIDMC Institutional Animal Care and Use Committee.
Experimental Design
Rag1−/− mice were randomly assigned to 3 treatment groups (n=8) as follows: Rag+HFD+adiponectin (HFD-APN), Rag+HFD+Placebo (HFD-PL), Rag+Low fat diet (LFD). Mice in these 3 groups were maintained in the study for 14 weeks. HFD-APN and HFD-PL groups received 5μg of adiponectin per kg body weight per day or phosphate-buffered saline (PBS) respectively from week 7 to the end of the experiment. This was administered continuously via ALZET osmotic pump (Cupertino, CA), implanted under isoflurane anesthesia (IsoFlo; Abbot Laboratories, North Chicago, IL) subcutaneously on the back of the mice, slightly posterior to the scapulae. Recombinant full-length mouse adiponectin (Biovendor, Candler, NC) was diluted in PBS, pH=7.2, one day before implantation of the pump according to the manufacturer's instructions.
Experimental procedures
Physiological characterization
Individual body weights and total amount of food intake were measured weekly on the same day between 8:00 and 10:00 A.M. with an analytical balance. Total body fat mass, lean tissue mass, and water content were accessed by magnetic resonance imaging techniques using Echo MRI (ECHO Medical Systems, Houston, TX). For whole-body composition analysis, anaesthetized mice were placed in a restraint tube and inserted into the nuclear magnetic resonance machine for determination of body adiposity one day before ALZET osmotic pump implantation and one day before sacrifice.
Glucose and insulin tolerance test
To investigate glucose tolerance and insulin sensitivity, intraperitoneal glucose tolerance tests (IPGTTs) and insulin tolerance tests (ITT) were performed. The IPGTTs were conducted in the morning after mice had been fasted overnight and the ITTs were conducted after mice had been fasted for at least 8 hours. Mice were then injected with a standard glucose bolus, as previously described (9). Intraperitoneal insulin tolerance tests were performed by injecting 0.75 units of insulin per kg body weight intraperitoneally.
Tissue collection and biochemical analyses
Mice were fasted for at least 4 hours prior to each blood draw. Blood was collected in chilled BD Microtainer (Becton, Dickinson and Company, Franklin Lakes, NJ). Serum or plasma was isolated by centrifugation at 1,800 g for 15 min at 4°C and was stored at −80°C until measurements of hormones and markers of inflammation were performed. Plasma adiponectin, leptin, and insulin were measured by enzyme-linked immunosorbent assay (ELISA, Millipore, Billerica, MA; ALPCO Diagnostics, Salem, NH). Blood glucose concentrations were measured using the LifeScan One Touch Ultra glucose meter (Johnson & Johnson, New Brunswick, NJ). Markers of inflammation, interferon-γ (IFN-γ), interleukin (IL)-1β, IL-2, IL-4, IL-5, keratinocyte-derived chemokine/growth related oncogene (KC/GRO), IL-10, IL-12-total, and tumor necrosis factor-α (TNF-α), were determined using a mouse TH1/TH2 9-plex assay ultra-sensitive kit (Meso Scale Discovery (MSD) multi-spot Assay System, Gaithersburg, MD) according to manufacturer's instruction.
After 14 weeks, mice were anesthetized with isoflurane and sacrificed by exsanguination (Figure 1). Tissues, including heart, liver, kidney, pancreas, spleen, intestine, hypothalamus, muscle and epididymal, perirenal, mesenteric, subcutaneous and brown adipose tissue, were collected, immediately frozen in liquid nitrogen, and stored at −80°C.
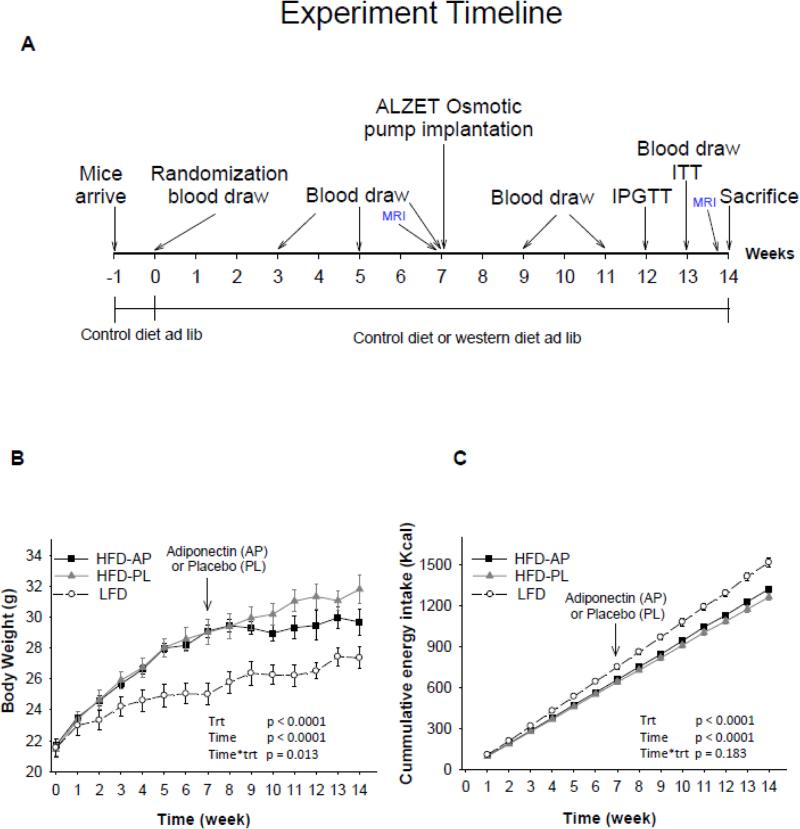
Figure 1.
Experimental timeline (A), body weight (B) and energy intake (C) in Rag1−/− mice maintained on low fat diet (LFD) or high fat diet (HFD) and treated either with adiponectin or placebo for 14 weeks. LFD= Rag1−/− mice maintained with LDF. HFD-PL= Rag1−/− mice maintained with HFD treated with placebo. HFD-AP= Rag1−/− mice maintained with HFD treated with adiponectin. P treatment (Trt) was considered the p-overall. Post hoc tests for body weight: HFD-AP vs HFD-PL p=0.039, HFD-AP vs LFD p<0.0001, HFD-PL vs LFD p<0.0001. For energy intake: HFD-AP vs HFD-PL p=0.001, HFD-AP vs LFD p<0.0001, HFD-PL vs LFD p<0.0001. Values are means ± SE; n= 7 for LFD, 8 HFD-PL and 6 HFD-APN.
Total RNA was extracted from hypothalamus, liver, muscle, and five areas of adipose tissue and measured using the same procedure/equipment described in our prior study (11). Briefly, total RNA was extracted using TRIZOL (Invitrogen, Grand Island, NY) and quantified spectrophotometrically at 260 nm. Integrity was confirmed by visualization of 18S and 28S rRNA on the Flash-Gel system (Lonza, Rockland, ME). cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantification of mRNA expression was done in a 2-step Reverse Transcriptase-Real Time PCR(RT-qPCR) using mouse-specific TaqMan® Gene Expression Assays((Assay ID: Adiponectin Receptor1 (AdipoR1), Mm01291331_m1; Adiponectin Receptor2 (AdipoR1), Mm00815950_m1; uncoupling protein 1 (UCP1), Mm01244861_m1; uncoupling protein 2 (UCP2), Mm00627597_m1; uncoupling protein 3 (UPC3), Mm00494077_m1; TNF-a, Mm99999068_m1; IL-1a, Mm99999060_m1; IL-1b, Mm01336189_m1; IL-4, Mm00445259_m1; IL-17a, Mm00439618_m1; neuropeptide Y(NPY), Mm03048253_m1; orexigenic agouti related protein(AgRP), Mm00475829_g1; anorexigenic pro-opiomelanocortin (POMC), Mm00435874_m1; melanocortin receptor 4 (MC4R), Mm00457483_s1; M1 marker (integrin alpha X), Mm00498698_m1; M2 marker (mannose receptor, C type 1), Mm00485148_m1; Macrophage (F4/80), Mm00802529_m1; Applied Biosystems, Foster City, CA) Applied Biosystems, Foster City, CA) in 7500 Fast Real-Time PCR system using Standard real-time 7500 protocol. Data were analyzed using 7500 system software (Applied Biosystems, Foster City, CA) and relative quantification was done using ΔΔCt method. Flow cytometry data were acquired using BD FACScalibur (BD Biosciences) and analyzed with FlowJo software 6.4 (Tree Star Inc., Ashland, OR). Of note, due to limited sample size, for one mice in the LFD group and two in the HFD-APN group the measurement of some parameters was not possible.
Statistical Analyses
Changes in body weight, energy intake, leptin, blood glucose, ipGTT glucose, ipITT glucose were analyzed using on treatment linear mixed models (Figures 1,2). P values for treatment (HFD-AP, HFD-PL, LFD), time (14 timepoints, 1/week) and treatment*time were calculated initially among the three groups (HFD-AP vs HFD-PL vs LFD). The p value for treatment was considered the p-overall and in the parameters where p for treatment was <0.05, we performed post-hoc analysis between the groups with adjustment for multiple comparisons with Bonferroni (SPSS version 24; IBM corporation, New York, USA) . Similarly, circulating inflammation markers were assessed by repeated measurements followed by post-hoc analysis with the least significance-differences technique, only when p overall was <0.05. Tissue weight and body compositions, mRNAs of hypothalamic hormones, UCP in muscle and brown adipose tissue, adiponectin receptors, macrophage and cytokines in different tissues (Tables 1-4) were assessed by one-way analysis of variance followed by the protected least significant-differences technique (SAS version 9.2; SAS Institute, Cary, NC). All data are presented as means ± standard error. P< 0.05 was considered statistically significant.
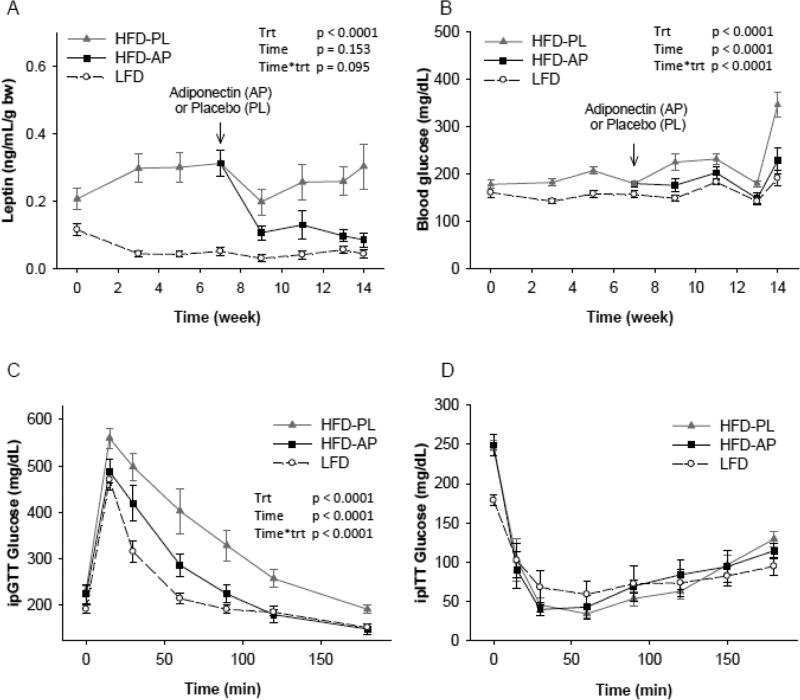
Figure 2.
Plasma concentration of leptin (A) and blood glucose (B) in Rag1−/− mice maintained on low fat diet (LFD) or high fat diet (HFD) and treated either with adiponectin or placebo for 14 weeks, ipGTT (C), and ipITT (D) in all groups of Rag1−/− mice at week 12 and week 13 respectively. LFD= Rag1−/− mice maintained with LFD. HFD-PL= Rag1−/− mice maintained with HFD treated with placebo. HFD-AP= Rag1−/− mice maintained with HFD treated with adiponectin. P treatment (Trt) was considered p overall. Post-hoc tests for leptin (A): HFD-AP vs HFD-PL p<0.0001, HFD-AP vs LFD p<0.0001, HFD-PL vs LFD p<0.0001. For Glucose levels (B) : HFD-AP vs HFD-PL p<0.0001, HFD-AP vs LFD p<0.0001, HFD-PL vs LFD p<0.0001. For ipGTT (C): HFD-AP vs HFD-PL p<0.0001, HFD-AP vs LFD p<0.006, HFD-PL vs LFD p<0.0001. After adjustment for baseline glucose (0 min), p treatment = 0.091. ipITT comparisons among the three groups, p treatment p = 0.652. After adjustment for baseline glucose (0 min), p treatment <0.0001, Post-hoc: HFD-AP vs HFD-PL, p=1, HFD-AP vs LFD p <0.0001, HFD-PL vs LFD p <0.0001. Values are means ± SE
Table 1.
Tissue weight and body composition at week 7 before APN treatment, and at week 14 after APN treatment.
| Rag−/− |
|||||
|---|---|---|---|---|---|
| LFD |
HFD |
p-overall | |||
| (n=7) | PL (n=8) |
APN (n=6) |
|||
| Variable | week | Mean ± SE | Mean ± SE | Mean ± SE | |
| Fat Mass (g)** | 7 | 0.89 ± 0.11 b | 5.25 ± 0.72 a | 5.23 ± 0.37 a | 0.006 |
| 14 | 1.55 ± 0.24 | 6.01 ± 0.95 | 3.82 ± 0.15 | 0.24 | |
| change | 0.72 ± 0.21 a | 1.14 ± 0.64 a | −1.41 ± 0.53 b | 0.005 | |
| Fat Mass (%)** | 7 | 3.48 ± 0.41 b | 18.05 ± 2.01 a | 18.00 ± 1.21 a | 0.001 |
| 14 | 5.78 ± 0.81 | 17.93 ± 2.20 | 12.34 ± 0.34 | 0.09 | |
| change | 2.39 ± 0.71 a | 0.97 ± 1.50 a | −5.55 ± 1.40 b | 0.0001 | |
| Lean Mass (g)** | 7 | 18.39 ± 0.46 a | 17.86 ± 0.32 b | 18.55 ± 0.23 a | 0.001 |
| 14 | 19.22 ± 0.51 | 19.86 ± 0.37 | 20.28 ± 0.44 | 0.15 | |
| change | 0.83 ± 0.09 b | 2.00 ± 0.19 a | 1.73 ± 0.30 a | 0.03 | |
| Lean Mass (%)** | 7 | 73.55 ± 0.48 a | 62.28 ± 1.05 b | 64.51 ± 1.25 b | 0.0003 |
| 14 | 71.74 ± 0.49 a | 60.89 ± 1.69 c | 65.62 ± 0.73 b | 0.03 | |
| change | −1.81 ± 0.61 b | −1.40 ± 1.16 b | 1.10 ± 1.65 a | 0.0004 | |
| Body Weight (g) | 14 | 27.4 ± 0.7 b | 31.8 ± 0.9 a | 29.6 ± 0.8 ab | 0.005 |
| Heart (g) | 14 | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.00 | 0.99 |
| (%) | 14 | 0.44 ± 0.02 | 0.41 ± 0.01 | 0.43 ± 0.02 | 0.98 |
| Spleen (g) | 14 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.05 ± 0.01 | 0.08 |
| (%) | 14 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.17 ± 0.05 | 0.07 |
| Pancreas (g) | 14 | 0.39 ± 0.10 | 0.27 ± 0.04 | 0.24 ± 0.01 | 0.48 |
| (%) | 14 | 1.03 ± 0.07 | 0.86 ± 0.13 | 0.79 ± 0.03 | 0.42 |
| Liver (g) | 14 | 1.04 ± 0.04 | 1.35 ± 0.06 | 1.14 ± 0.02 | 0.06 |
| (%) | 14 | 3.79 ± 0.12 | 4.24 ± 0.11 | 3.86 ± 0.11 | 0.06 |
| Kidney (g) | 14 | 1.21 ± 0.04 | 1.14 ± 0.04 | 1.26 ± 0.05 | 0.22 |
| (%) | 14 | 0.33 ± 0.01 | 0.36 ± 0.01 | 0.37 ± 0.01 | 0.21 |
| Intestine | |||||
| Duodenum (g) | 14 | 0.24 ± 0.01 | 0.23 ± 0.02 | 0.20 ± 0.03 | 0.49 |
| (%) | 14 | 0.87 ± 0.05 | 0.73 ± 0.07 | 0.68 ± 0.11 | 0.43 |
| Jejunum (g) | 14 | 0.65 ± 0.06 | 0.59 ± 0.03 | 0.52 ± 0.03 | 0.08 |
| (%) | 14 | 2.36 ± 0.20 | 1.85 ± 0.11 | 1.76 ± 0.10 | 0.08 |
| Ileum (g) | 14 | 0.19 ± 0.02 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.19 |
| (%) | 14 | 0.71 ± 0.07 | 0.48 ± 0.04 | 0.50 ± 0.05 | 0.17 |
| Colon (g) | 14 | 0.17 ± 0.01 a | 0.12 ± 0.01 b | 0.12 ± 0.01 b | 0.001 |
| (%) | 14 | 0.62 ± 0.03 a | 0.39 ± 0.02 b | 0.41 ± 0.03 b | 0.001 |
| Adipose Tissue | |||||
| Epididymal (g) | 14 | 0.49 ± 0.06 | 1.30 ± 0.16 | 0.94 ± 0.07 | 0.06 |
| (%) | 14 | 1.80 ± 0.28 b | 4.05 ± 0.37 a | 3.17 ± 0.19 a | 0.02 |
| Perirenal (g) | 14 | 0.09 ± 0.01 | 0.37 ± 0.07 | 0.21 ± 0.02 | 0.18 |
| (%) | 14 | 0.34 ± 0.05 c | 1.15 ± 0.16 a | 0.71 ± 0.04 b | 0.05 |
| Mesenteric (g) | 14 | 0.25 ± 0.06 | 0.38 ± 0.06 | 0.25 ± 0.02 | 0.58 |
| (%) | 14 | 0.91 ± 0.20 | 1.18 ± 0.16 | 0.85 ± 0.07 | 0.61 |
| Subcutaneous (g) | 14 | 0.25 ± 0.02 b | 0.72 ± 0.10 a | 0.39 ± 0.02 b | 0.03 |
| (%) | 14 | 0.90 ± 0.07 b | 2.24 ± 0.23 a | 1.34 ± 0.08 b | 0.01 |
| Brown (g) | 14 | 0.08 ± 0.01 | 0.13 ± 0.01 | 0.09 ± 0.01 | 0.32 |
| (%) | 14 | 0.28 ± 0.05 | 0.42 ± 0.03 | 0.31 ± 0.03 | 0.33 |
Abbreviations: LFD, low fat diet; HFD, high fat diet; PL, placebo; APN, adiponectin; The p-overall refers to the p value after performing a one-way analysis of variance for the investigated parameter. For the parameters showing a p-overall <0.05, a post-hoc analysis between the different groups was performed (Means with different letters a,b,c are significantly different, p <0.05). P-overall values were adjusted for body weight.
The values measured by MRI.
Table 4.
Adipose tissue macrophage and cytokine mRNA expression level
| LFD (n=7) |
HFD-PL (n=8) |
HFD-APN (n=6) |
p-overall | |
|---|---|---|---|---|
| Variable | Mean ± SE | Mean ± SE | Mean ± SE | |
| Macrophage (F4/80) | ||||
| Epididymal | 0.67 ± 0.06 | 0.41 ± 0.08 | 0.86 ± 0.11 | 0.17 |
| Mesenteric | 0.80 ± 0.43 | 1.09 ± 0.18 | 2.16 ± 0.31 | 0.37 |
| Perirenal | 0.77 ± 0.06 b | 1.19 ± 0.09 a | 1.11 ± 0.08 a | 0.03 |
| Subcutaneous | 1.69 ± 0.28 b | 1.78 ± 0.50 b | 3.06 ± 0.59 a | 0.02 |
| Brown | 0.87 ± 0.04 | 1.31 ± 0.13 | 1.29 ± 0.03 | 0.65 |
| TNFα | ||||
| Epididymal | 0.53 ± 0.10 | 0.26 ± 0.06 | 0.80 ± 0.24 | 0.08 |
| Mesenteric | 1.10 ± 0.81 | 1.17 ± 0.51 | 1.92 ± 0.74 | 0.34 |
| Perirenal | 0.57 ± 0.11 | 1.17 ± 0.34 | 0.89 ± 0.12 | 0.10 |
| Subcutaneous | 5.39 ± 1.52 | 1.13 ± 0.46 | 3.44 ± 1.47 | 0.28 |
| Brown | 0.64 ± 0.07 b | 1.23 ± 0.25 a | 0.79 ± 0.07 ab | 0.05 |
| IFNγ | ||||
| Epididymal | 1.07 ± 0.14 ab | 0.87 ± 0.13 b | 1.75 ± 0.38 a | 0.09 |
| Mesenteric | 0.89 ± 0.15 | 1.29 ± 0.48 | 4.18 ± 1.85 | 0.12 |
| Perirenal | 0.94 ± 0.07 b | 1.34 ± 0.16 a | 1.13 ± 0.08 ab | 0.05 |
| Subcutaneous | 6.21 ± 1.75 a | 0.67 ± 0.12 b | 3.11 ± 1.29 ab | 0.03 |
| Brown | 1.51 ± 0.13 | 1.34 ± 0.22 | 1.47 ± 0.30 | 0.10 |
| IL-1α | ||||
| Epididymal | 0.51 ± 0.19 ab | 0.18 ± 0.03 b | 0.97 ± 0.47 a | 0.05 |
| Mesenteric | 0.79 ± 0.55 | 1.47 ± 0.78 | 2.17 ± 0.92 | |
| Perirenal | 1.35 ± 0.57 b | 12.71 ± 4.73 a | 18.21 ± 12.83 a | 0.04 |
| Subcutaneous | 4.75 ± 1.26 | 0.75 ± 0.28 | 3.40 ± 1.48 | 0.06 |
| Brown | 0.51 ± 0.10 | 0.79 ± 0.30 | 0.51 ± 0.09 | 0.33 |
| IL-1β | ||||
| Epididymal | 0.68 ± 0.06 b | 0.51 ± 0.07 b | 1.17 ± 0.25 a | 0.02 |
| Mesenteric | 0.93 ± 0.44 | 1.33 ± 0.47 | 2.45 ± 0.97 | 0.30 |
| Perirenal | 0.63 ± 0.08 b | 2.23 ± 0.50 a | 2.60 ± 0.70 a | 0.004 |
| Subcutaneous | 3.08 ± 0.92 ab | 1.46 ± 0.42 b | 6.25 ± 1.83 a | 0.07 |
| Brown | 0.99 ± 0.09 b | 1.72 ± 0.30 a | 2.05 ± 0.38 a | 0.002 |
| IL-4 | ||||
| Epididymal | 0.26 ± 0.17 | 0.19 ± 0.13 | 1.36 ± 0.78 | 0.83 |
| Mesenteric | 1.08 ± 0.53 | 0.83 ± 0.20 | 4.38 ± 2.90 | 0.26 |
| Perirenal | 0.50 ± 0.50 | 0.89 ± 0.39 | 0.67 ± 0.31 | 0.77 |
| Subcutaneous | 3.14 ± 2.08 | 0.36 ± 0.13 | 3.05 ± 1.14 | 0.26 |
| Brown | 2.30 ± 0.89 | 2.64 ± 0.65 | 1.90 ± 0.91 | 0.50 |
| IL-17a | ||||
| Epididymal | 0.58 ± 0.58 | 0.24 ± 0.08 | 0.25 ± 0.19 | 0.32 |
| Mesenteric | 1.08 ± 0.52 | 0.16 ± 0.09 | 1.43 ± 0.78 | 0.42 |
| Perirenal | Non-detectable | |||
| Subcutaneous | Non-detectable | |||
| Brown | Non-detectable | |||
Abbreviations: LFD, low fat diet; HFD, high fat diet; PL, placebo; AP, adiponectin; TNFα, tumor necrosis factor alpha; IFNγ, interferon γ; The p-overall refers to the p value after performing a one-way analysis of variance for the investigated parameter. For the parameters showing a p-overall <0.05, a post-hoc analysis between the different groups was performed (Means with different letters a,b,c are significantly different, p <0.05). P-overall values were adjusted for body weight
RESULTS
Effect of adiponectin on body weight and body composition
Consistent with our previous study (11), HFD Rag1−/− mice weighed more than LFD Rag1−/− mice at baseline (Week 7). HFD Rag1−/− mice treated with placebo (HFD-PL) gained further weight, while HFD Rag1−/− mice treated with adiponectin (HFD-APN) maintained stable body weight during the 7 weeks of treatment (week 7-14) (Figure 1B). Body composition measured by MRI demonstrated that HFD-APN mice significantly reduced their fat mass during treatment (Table 1). Regarding the different tissues, HFD-APN mice had lower mass of subcutaneous and perirenal adipose tissue compared to HFD-PL mice, while no differences were observed in epididymal, mesenteric and brown adipose tissue (BAT), as well as in the mass of other organs (Table 1).
Effect of adiponectin on energy intake, appetite regulation and thermogenesis
In contrast to the observed significant weight gain and in line to our previous report (11), HFD-PL mice had significantly lower energy intake compared to LFD mice (Figure 1C). HFDAPN mice demonstrated a small increase in energy intake compared to HFD-PL but had still significantly lower energy intake compared to LFD mice. The changes in energy intake led us to investigate the expression of neuropeptides involved in appetite regulation. The levels of the orexigenic Agouti-related peptide (AgRP), as we have previously reported, were reduced in HFD-PL mice compared to LFD mice, but interestingly they were almost completely restored in HFD-APN mice (Table 2). We did not observe any significant difference between levels of other neuropeptides. Furthermore, HFD-APN mice demonstrated significantly lower levels of the anorexigenic hormone leptin compared to HFD-PL (Figure 2A). In conclusion, the reduced energy intake observed in Rag1−/− mice fed HFD was increased approx.. 5% after adiponectin treatment. This increase in energy intake was probably due to an increase in the secretion of the most important orexigenic peptide (AgRP) and a decrease in the circulating levels of the anorexigenic hormone leptin. Since energy intake was rather increased than reduced despite maintaining body weight under HFD after adiponectin treatment, we searched for increased energy expenditure by thermogenesis. The expression of the thermogenic genes UCP1, UCP2 were neither elevated in muscle nor in BAT and there was a trend of higher UCP3 expression levels in BAT (p=0.08) (Table 2).
Table 2.
AgRP, POMC, NPY and MC4R mRNA expression in hypothalamus, UCP mRNA expression in muscle and brown adipose tissue
| LFD (n=7) |
HFD-PL (n=8) |
HFD-APN (n=6) |
p-overall | |
|---|---|---|---|---|
| Variable | Mean ± SE | Mean ± SE | Mean ± SE | |
| Hypothalamus | ||||
| AgRP | 0.55 ± 0.06 a | 0.28 ± 0.04 b | 0.46 ± 0.07 a | 0.0094 |
| POMC | 0.68 ± 0.04 | 0.83 ± 0.11 | 1.09 ± 0.15 | 0.15 |
| NPY | 0.85 ± 0.04 | 0.75 ± 0.08 | 0.92 ± 0.09 | 0.35 |
| MC4R | 0.73 ± 0.07 | 0.78 ± 0.09 | 0.81 ± 0.09 | 0.88 |
| Muscle | ||||
| UCP1 | 1.01 ± 0.50 | 2.15 ± 1.45 | 1.02 ± 0.44 | 0.85 |
| UCP2 | 0.67 ± 0.11 | 0.71 ± 0.07 | 0.97 ± 0.25 | 0.43 |
| UCP3 | 1.36 ± 0.08 | 1.16 ± 0.12 | 1.39 ± 0.16 | 0.15 |
| Brown Adipose Tissue | ||||
| UCP1 | 0.85 ± 0.05 | 0.81 ± 0.05 | 0.86 ± 0.05 | 0.89 |
| UCP2 | 0.97 ± 0.15 | 0.89 ± 0.02 | 0.94 ± 0.03 | 0.82 |
| UCP3 | 1.05 ± 0.04 | 0.86 ± 0.05 | 0.94 ± 0.05 | 0.08 |
Abbreviations: LFD, low fat diet; HFD, high fat diet; PL, placebo; AP, adiponectin; UCP, Uncoupling protein; AgRP, agouti-related peptide; POMC, proopiomelanocortin; NPY, neuropeptide Y; MC4R, melanocortin 4 receptor; The p-overall refers to the p value after performing a one-way analysis of variance for the investigated parameter. For the parameters showing a p-overall <0.05, a post-hoc analysis between the different groups was performed (Means with different letters a,b,c are significantly different, p <0.05). P-overall values were adjusted for body weight.
Effect of adiponectin on metabolic profile
Glucose levels were elevated in HFD-PL compared to LFD mice from the third week of HFD, but a severe hyperglycemia was observed only at the last week of the study (week 14) (Figure 2B). Interestingly, HFD-APN mice maintained their glucose levels stable after the initiation of the adiponectin treatment and did not develop severe hyperglycemia at the last week (Figure 2B). The ipGTT demonstrated significantly lower glucose levels in the adiponectin-treated mice compared to placebo (Figure 2C). When corrected though for the fasting glucose levels (baseline), this significant difference was reduced and achieved significance only by one tailed statistical tests. The improved glucose profile in adiponectin treated mice was achieved despite the similar level of insulin sensitivity between HFD-APN and HFD-PL mice, as shown by ipITTs. (Figure 2D).
Effect of adiponectin on the expression of the adiponectin receptors R1 and R2
Since adiponectin protects from weight gain and hyperglycemia in an insulin-resistance-independent manner, we searched for changes in the expression of adiponectin receptors in the different organs, in order to identify the most probable adiponectin targets (Table 3). HFD-PL mice demonstrated compared to LFD mice significantly lower expression of adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2) in SAT, lower AdipoR1 in the liver and a trend to lower AdipoR1 and AdipoR2 in epididymal tissue. Finally, they demonstrated higher AdipoR1 in perirenal tissue. When compared to HFD-PL, HFD-APN mice demonstrated compared to HFD-PL higher expression of AdipoR1 and AdipoR2 in the epididymal tissue, higher AdipoR1 in the liver and hypothalamus, and a trend to higher AdipoR1 and AdipoR2 expression in the SAT. Taken together, the reduction mainly of AdipoR1 and secondarily of AdipoR2 expression in the different tissues, which was induced by HFD, was partially restored after adiponectin treatment (Table 3).
Table 3.
Adiponectin receptor R1 and R2 mRNA expression.
| Rag−/− |
p-overall | |||
|---|---|---|---|---|
| LFD |
HFD |
|||
| (n=7) | PL (n=8) |
APN (n=7) |
||
| Variable | Mean ± SE | Mean ± SE | Mean ± SE | |
| Hypothalamus | ||||
| AdipoR1 | 0.83 ± 0.02 a | 0.88 ± 0.02 a | 0.99 ± 0.05 b | 0.01 |
| AdipoR2 | 0.90 ± 0.05 | 0.95 ± 0.09 | 1.16 ± 0.14 | 0.042 |
| Liver | ||||
| AdipoR1 | 1.01 ± 0.05 a | 0.71 ± 0.02 b | 0.87 ± 0.06 a | 0.004 |
| AdipoR2 | 1.23 ± 0.17 | 1.24 ± 0.05 a | 1.30 ± 0.10 | 0.71 |
| Muscle | ||||
| AdipoR1 | 0.96 ± 0.06 | 1.09 ± 0.10 | 1.02 ± 0.13 | 0.38 |
| AdipoR2 | 1.44 ± 0.14 | 1.75 ± 0.40 | 1.57 ± 0.37 | 0.79 |
| Adipose Tissue | ||||
| Epididymal | ||||
| AdipoR1 | 1.00 ± 0.36 ab | 0.45 ± 0.28 b | 1.08 ± 0.37 a | 0.03 |
| AdipoR2 | 1.18 ± 0.15 ab | 0.39 ± 0.14 b | 1.67 ± 0.55 a | 0.01 |
| Mesenteric | ||||
| AdipoR1 | 2.53 ± 0.83 | 1.06 ± 0.15 | 2.17 ± 0.69 | 0.13 |
| AdipoR2 | 2.12 ± 0.40 | 1.46 ± 0.58 | 1.89 ± 0.68 | 0.56 |
| AdipoR2/R1 | 10.31 ± 1.66 | 8.72 ± 0.74 | 8.68 ± 0.95 | 0.91 |
| Perirenal | ||||
| AdipoR1 | 0.74 ± 0.10 b | 1.26 ± 0.21 a | 1.19 ± 0.13 a | 0.02 |
| AdipoR2 | 0.89 ± 0.07 | 1.03 ± 0.14 | 1.11 ± 0.20 | 0.63 |
| AdipoR2/R1 | 29.46 ± 0.85 | 26.16 ± 2.37 | 24.62 ± 1.75 | 0.29 |
| Subcutaneous | ||||
| AdipoR1 | 4.90 ± 0.99 a | 1.03 ± 0.16 b | 2.83 ± 1.25 b | 0.03 |
| AdipoR2 | 3.62 ± 0.61 a | 0.56 ± 0.11 b | 1.73 ± 0.89 b | 0.01 |
| Brown | ||||
| AdipoR1 | 0.79 ± 0.05 | 0.88 ± 0.13 a | 0.87 ± 0.05 | 0.5 |
| AdipoR2 | 0.73 ± 0.04 a | 0.66 ± 0.08 b | 0.73 ± 0.06 a | 0.05 |
Abbreviations: LFD, low fat diet; HFD, high fat diet; PL, placebo; AP, adiponectin; AdipoR1, adiponectin receptor 1; AdipoR2, adiponectin receptor 2. The p-overall refers to the p value after performing a one-way analysis of variance for the investigated parameter. For the parameters showing a p-overall <0.05, a post-hoc analysis between the different groups was performed (Means with different letters a,b,c are significantly different, p <0.05). P-overall values were adjusted for body weight
Effect of adiponectin on inflammation
In order to evaluate if adiponectin treatment can affect inflammatory markers in HFD Rag1−/− mice lacking adaptive immune system, we have investigated for changes in macrophage infiltration and expression of important cytokines in tissues. HFD-PL mice showed higher mRNA levels of F4/80 (marker of macrophages) in perirenal adipose tissue and a trend to higher levels in SAT, BAT and mesenteric adipose tissue compared to LFD mice (Table 4). The levels of F4/80-mRNA were similar in HFD-APN mice compared to HFD-PL mice, with the exception of SAT, where they were increased (Table 4). To further evaluate this result, we investigated the expression of M1 and M2 macrophages in SAT and epididymal adipose tissue, which were the two tissues that restored their adiponectin receptor expression during adiponectin treatment. Consistent with our previous results, HFD-PL mice showed significantly (37,7%) lower M2 macrophage expression levels in the SAT compared to LFD mice. This was however completely restored in mice treated with adiponectin (HFD-APN), which demonstrated similar M1 (84,3%) and M2 (95,4%) infiltration with the LFD mice (Relative expression of M1 macrophages adjusted to LFD, LFD 1±0.18 vs HFD-PL 1.24±0.3 vs HFD-AP 0.73±0.11, p-overall > 0.05, Relative expression of M2 macrophages adjusted to LFD, LFD 1±0.26 vs HFD-PL 0.62±0.29 vs HFD-AP 0.95±0.38, p-overall=0.04, HFD-PL vs HFD-AP and HFD-PL vs LFD, p< 0.05). The same was also observed in epididymal tissue, although the differences in M1 and M2 infiltration between LFD, HFD-PL and HFD-APN were much less pronounced and non-significant in this tissue (data not shown). Finally, regarding the different cytokines, we confirm the higher expression of TNFα, IFN-γ, IL-1α and IL-1β in the perirenal adipose tissue of HFD-PL mice compared to LFD mice shown in our first study (11) (Table 4). However, treatment of the HFD mice with adiponectin did not significantly affect the expression of these cytokines in the perirenal adipose tissue. On the contrary, HFD-APN mice demonstrate significantly higher levels of IL-1α and IL-1β in epididymal tissue compared to HFD-PL mice.
Finally, in order to investigate the effect of adiponectin on systemic inflammation in Rag1−/− mice fed HFD, plasma cytokines were also measured at different time points during treatment with adiponectin vs placebo (Supplementary Figure 1). Adiponectin treatment did not affect the levels of circulating cytokines over the time-period of this experiment.
DISCUSSION
We demonstrate herein that adiponectin can prevent HFD-induced weight gain and improve glucose profile independently of any deficiencies in the adaptive immune system, only in part, by regulating inflammation through innate immunity in the adipose tissue.
In our prior study, we showed that Rag1−/− mice, which lack mature lymphocytes and therefore have a defect in their adaptive immune response, gain weight faster during HFD than C57 wild type mice (WT mice) with an intact immune system (11). Body composition analysis demonstrated that this weight gain was due to adipocyte hypertrophy observed predominantly in epididymal and SAT (11). Here we show that treatment of Rag1−/− mice fed HFD with a physiological dose of adiponectin limits weight gain, while it restores body composition by reducing SAT and perirenal adipose tissue. Previous studies in rodents having an intact immune system have reported contradictory results regarding the effect of adiponectin on weight, ranging from significant weight loss (in most of the cases) to no effect at all (rarely) (12, 14, 20-23). These discrepancies may be explained by the variations in the experimental settings used, since the dose and duration of adiponectin treatment as well as the metabolic status of the animals at treatment-initiation significantly differed among the studies. Additionally, results from studies concentrating on knocking out adiponectin cannot be easily compared with findings from studies focusing on increasing adiponectin levels. Regarding fat distribution, most reports agree that transgenic overexpression or application of adiponectin reduces visceral, SAT and consequently whole body fat (14, 20, 24, 25). Based on our results, adiponectin can regulate body weight and adipose tissue mass without the support of the adaptive immune system.
In order to clarify how the adiponectin-treated Rag1−/− mice manage to maintain their weight despite HFD, we investigated for changes in energy intake, appetite and thermogenesis during adiponectin treatment. Interestingly, instead of a reduction, we observed an approx. 4-5% increase in energy intake in the Rag1−/− mice fed HFD during adiponectin treatment, which apparently represented a compensatory increase. Additionally, we measured an increased secretion of the orexigenic peptide AgRP accompanied with an increased expression of the AdipoR1 in hypothalamus. Moreover, we observed a reduction in the levels of the anorexigenic hormone leptin. Previous studies have demonstrated that circulating adiponectin levels and expression of AdipoR1 are elevated in the fasting state and decline after feeding (26, 27). Moreover, leptin stimulates satiety by activating the anorexigenic peptides POMC and CART and inhibiting the orexigenic AgRP and neuropeptide Y (28, 29). In our study, the increase of AgRP and reduction of leptin, both of which would be expected to induce appetite, were probably responsible for this small increase in energy intake.
Since energy intake was rather increased than reduced, the preserved weight despite HFD in the Rag1−/− mice treated with adiponectin in our study may be explained by an increase in energy expenditure. Of note, as shown in our first report, Rag1−/− mice demonstrate generally lower energy expenditure compared to WT mice due to reduced thermogenesis and locomotor activity (11). In the current study, treatment with adiponectin did not increase the expression of UCP1, UCP2, and there was only a trend (p=0.08 by two tailed test and p=0.04 on the basis of a one tailed test based on our a priori hypothesis of expected increased UCP3) towards higher UCP3 in BAT in the Rag1−/− mice fed HFD. Despite the lack of major changes in the expression of thermogenic genes in BAT and muscle, we cannot exclude an increased thermogenesis from the SAT or visceral adipose tissue in the adiponectin treated Rag1−/− mice fed HFD. Finally, we cannot exclude a possible recovery of the reduced locomotor activity after adiponectin treatment, which could have also contributed to increased energy expenditure and could have prevented further weight gain. Although energy expenditure and locomotor activity were not directly assessed herein, it is known that adiponectin promotes free fatty acid oxidation in mitochondria and increases energy expenditure (30). Especially in muscle, adiponectin stimulates mitochondrial biogenesis and palmitate oxidation by activation of AMPK and PGC-1a signaling pathways (31, 32). Moreover, peripheral adiponectin administration increases body energy expenditure leading to weight loss without affecting energy intake.
Another aim of our study was to investigate the importance of the adaptive immune system in the adiponectin-mediated effects on glucose homeostasis, insulin sensitivity and inflammation in the Rag1−/− mice. We have demonstrated in our first report that Rag1−/− mice, which lack mature lymphocytes, do not develop diabetes after 11 weeks of HFD. In contrast, WT mice, which have an intact immune system, are diabetic after an 11-week HFD despite their lower weight compared to Rag1−/− mice. This shows that the lack of adaptive immune system in Rag1−/− mice may protect them from inflammatory reactions mediated by lymphocytes that lead to hyperglycemia. Similarly, in the current study, we did not observe after 11 weeks of HFD elevated glucose levels in the Rag1−/− mice. However, after 14 weeks of HFD, the Rag1−/− mice become diabetic. Taking into consideration the results of both of our studies, the lack of innate immune system may delay but not completely abolish the development of hyperglycemia in Rag1−/− mice. In other words, lymphocytes accelerate the inflammatory reactions which are primarily controlled by innate immune system in obesity, but at the end the presence of lymphocytes is probably not decisive for the development of hyperglycemia and diabetes.
Treatment of the Rag1−/− mice fed HFD with physiological low dose of adiponectin in our study prevented severe hyperglycemia, although it did not affect insulin sensitivity. Previous reports have demonstrated that overexpression of the adiponectin gene or direct administration of adiponectin protein significantly reduces glucose levels in obese mouse models with intact immune system and this is primarily achieved by increasing insulin sensitivity (14, 18, 20-22). Here, we did not observe a change in insulin sensitivity, possibly due to the lack of CD4+ T cells that are crucial for the reduction of the inflammatory-mediated insulin resistance in obesity (33). Therapy with aCD3 and F(ab'2) can restore CD4+Foxp3+T cell pools in VAT and lead to a severe enhancement of insulin-sensitivity and improvement of glucose homeostasis (33).
Since adiponectin did not significantly affect insulin sensitivity in Rag1−/− mice, we propose that it probably protects them from diabetes through insulin-resistance-independent pathways and/or increased insulin secretion from pancreas. According to previous reports, adiponectin decreases hepatic glucose output in the liver, while it stimulates glucose uptake in muscle (17). In order to mediate these glucose-regulatory-effects, adiponectin binds to the AdipoR1 and AdipoR2 receptors in the different organs. Disruption of the adiponectin receptors leads to increased triglyceride content, inflammation, oxidative stress and diabetes (27). In our study, we did not observe a significant change in the expression of adiponectin receptors in the muscle of the Rag1−/− mice fed HFD after treatment with adiponectin, which speaks against an increased function of adiponectin in this organ. On the contrary, in the liver we observed a significant upregulation of AdipoR1 expression, which may indicate an increased function of adiponectin in this organ with consequent reduction of glucose output and amelioration of glucose profile.
Furthermore, adiponectin can regulate glucose homeostasis by inhibiting both the systemic and the adipose-tissue related chronic low inflammation in obesity. According to previous reports, adiponectin reduces the secretion of pro-inflammatory and stimulates the secretion of anti-inflammatory cytokines, and also induces the polarization of macrophages from pro-inflammatory (M1) to an anti-inflammatory (M2) phenotype in rodents with intact immune system (5, 17, 19, 27). In our study, the plasma levels of the pro- and anti-inflammatory cytokines remained stable. Similarly, with the exception of an increase of IL-1α and IL-1β mRNA in the epididymal adipose tissue, the levels of all the other pro- and anti-inflammatory cytokines in the visceral adipose tissue as well as in SAT and BAT did not change after treatment of the Rag1−/− mice fed HFD with adiponectin.
Regarding macrophage infiltration and phenotype, we searched for changes in the SAT and epididymal adipose tissue of the Rag1−/− mice fed HFD, since these tissues demonstrated an upregulation of AdipoR1 and AdipoR2 expression after adiponectin treatment. We observed increased levels of macrophages in SAT and a trend to higher levels in the epididymal adipose tissue after adiponectin treatment. However, the phenotype of macrophages changed from pro-inflammatory-M1 to anti-inflammatory-M2 state, consistent to the findings from previous studies on mice with intact immune system. Such changes can significantly reduce obesity-induced inflammation and the development of insulin resistance. Depletion of M1 macrophages in humans and mice has been associated with improvement of insulin resistance and glucose profile (34). In conclusion, adiponectin can still exert anti-inflammatory actions to macrophages independently of lymphocyte status and despite minor changes in cytokine profile.
Finally, we recognize some limitations in our current work that need to be addressed in future studies. First of all, we did not investigate for changes in body energy expenditure or locomotor activity during adiponectin treatment, which could have explained the protective effect of adiponectin against weight gain despite the small increase in energy intake. Moreover, we did not investigate for stimulation of insulin secretion from pancreas or increased glucose uptake in the liver during adiponectin treatment, that may participate in the improved glucose profile of the adiponectin treated mice. Nevertheless, this study was performed according to an established experimental protocol that we have previously fully described (9) and the investigators were blinded to the type of treatment. Finally, the continuous administration of adiponectin by a subcutaneous osmotic pump in a similar (to other studies) dose helped so that the reported differences are robust despite performing the measurements at the trough levels of adiponectin.
In summary, we demonstrated that adiponectin protects from weight gain despite a compensatory stimulation of energy intake in mice lacking an adaptive immune system. Additionally, adiponectin protects from glucose imbalance, although it does not improve insulin sensitivity. Alterations in the phenotype of macrophages but not in secreted cytokines may contribute to the protective role of adiponectin against hyperglycemia in the absence of the adaptive immune system. Beyond the physiological implications of the data presented herein in terms of adiponectin actions, these data point to the possibility that adiponectin or agents that either increase adiponectin levels or mimic its actions could prove to be a promising therapeutic option against obesity and diabetes in immune-deficient populations, including but not limited to HIV positive individuals who develop insulin resistance and central obesity (35-39).
Supplementary Material
ACKNOWLEDGEMENTS
X.L. and C.S.M. were responsible for the study conception and design.
X.L. was responsible for the acquisition and interpretation of the data.
N.P. contributed to the analysis and interpretation of the data and in drafting of the manuscript.
H.G, J.P. C., M.T. B., participated in the study performance and coordination.
X.L., N.P. and C.S.M. were responsible for the critical revision of the manuscript.
X.L. is the guarantor of the study.
This work was supported by Institutional funds.
FUNDING:
Discretionary grant from BIDMC. This work is also supported in part by NIH DK081913.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have nothing to disclose
REFERENCES
- 1.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117(1):175–84. doi: 10.1172/JCI29881. Epub 2007/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of clinical investigation. 2011;121(6):2111–7. doi: 10.1172/JCI57132. Epub 2011/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaud A, Boulet MM, Veilleux A, Noel S, Paris G, Tchernof A. Abdominal subcutaneous and omental adipocyte morphology and its relation to gene expression, lipolysis and adipocytokine levels in women. Metabolism: clinical and experimental. 2014;63(3):372–81. doi: 10.1016/j.metabol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 4.van Beek L, Lips MA, Visser A, Pijl H, Ioan-Facsinay A, Toes R, et al. Increased systemic and adipose tissue inflammation differentiates obese women with T2DM from obese women with normal glucose tolerance. Metabolism: clinical and experimental. 2014;63(4):492–501. doi: 10.1016/j.metabol.2013.12.002. Epub 2014/01/29. [DOI] [PubMed] [Google Scholar]
- 5.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. Epub 2011/01/12. [DOI] [PubMed] [Google Scholar]
- 6.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nature medicine. 2012;18(3):363–74. doi: 10.1038/nm.2627. Epub 2012/03/08. [DOI] [PubMed] [Google Scholar]
- 7.DiSpirito JR, Mathis D. Immunological contributions to adipose tissue homeostasis. Seminars in immunology. 2015;27(5):315–21. doi: 10.1016/j.smim.2015.10.005. Epub 2015/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathis D. Immunological goings-on in visceral adipose tissue. Cell metabolism. 2013;17(6):851–9. doi: 10.1016/j.cmet.2013.05.008. Epub 2013/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seijkens T, Kusters P, Chatzigeorgiou A, Chavakis T, Lutgens E. Immune cell crosstalk in obesity: a key role for costimulation? Diabetes. 2014;63(12):3982–91. doi: 10.2337/db14-0272. [DOI] [PubMed] [Google Scholar]
- 10.Strissel KJ, Denis GV, Nikolajczyk BS. Immune regulators of inflammation in obesity-associated type 2 diabetes and coronary artery disease. Current opinion in endocrinology, diabetes, and obesity. 2014;21(5):330–8. doi: 10.1097/MED.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Huh JY, Gong H, Chamberland JP, Brinkoetter MT, Hamnvik OP, et al. Lack of mature lymphocytes results in obese but metabolically healthy mice when fed a high-fat diet. International journal of obesity. 2015;39(10):1548–57. doi: 10.1038/ijo.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(4):2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin-Efe A, Katsikeris F, Mantzoros CS. Advances in adipokines. Metabolism: clinical and experimental. 2012;61(12):1659–65. doi: 10.1016/j.metabol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature medicine. 2001;7(8):941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 15.Nakatsuji H, Kishida K, Sekimoto R, Komura N, Kihara S, Funahashi T, et al. Accumulation of adiponectin in inflamed adipose tissues of obese mice. Metabolism: clinical and experimental. 2014;63(4):542–53. doi: 10.1016/j.metabol.2013.12.012. Epub 2014/01/29. [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, Magkos F, Mantzoros CS, Kang ES. Effects of leptin and adiponectin on pancreatic beta-cell function. Metabolism: clinical and experimental. 2011;60(12):1664–72. doi: 10.1016/j.metabol.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–26. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Turdi S, Park T, Morris NJ, Deshaies Y, Xu A, et al. Adiponectin corrects high-fat diet-induced disturbances in muscle metabolomic profile and whole-body glucose homeostasis. Diabetes. 2013;62(3):743–52. doi: 10.2337/db12-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esmaili S, Xu A, George J. The multifaceted and controversial immunometabolic actions of adiponectin. Trends in endocrinology and metabolism: TEM. 2014;25(9):444–51. doi: 10.1016/j.tem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Bauche IB, El Mkadem SA, Pottier AM, Senou M, Many MC, Rezsohazy R, et al. Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinology. 2007;148(4):1539–49. doi: 10.1210/en.2006-0838. [DOI] [PubMed] [Google Scholar]
- 21.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nature medicine. 2001;7(8):947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Liu D. Hydrodynamic delivery of adiponectin and adiponectin receptor 2 gene blocks high-fat diet-induced obesity and insulin resistance. Gene therapy. 2013;20(8):846–52. doi: 10.1038/gt.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tullin S, Sams A, Brandt J, Dahl K, Gong W, Jeppesen CB, et al. Recombinant adiponectin does not lower plasma glucose in animal models of type 2 diabetes. PloS one. 2012;7(10):e44270. doi: 10.1371/journal.pone.0044270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masaki T, Chiba S, Yasuda T, Tsubone T, Kakuma T, Shimomura I, et al. Peripheral, but not central, administration of adiponectin reduces visceral adiposity and upregulates the expression of uncoupling protein in agouti yellow (Ay/a) obese mice. Diabetes. 2003;52(9):2266–73. doi: 10.2337/diabetes.52.9.2266. [DOI] [PubMed] [Google Scholar]
- 25.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, et al. Adiponectin acts in the brain to decrease body weight. Nature medicine. 2004;10(5):524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 26.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell metabolism. 2007;6(1):55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nature medicine. 2007;13(3):332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 28.Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism: clinical and experimental. 2015;64(1):24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sainz N, Barrenetxe J, Moreno-Aliaga MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism: clinical and experimental. 2015;64(1):35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord. 2014;15(2):149–56. doi: 10.1007/s11154-013-9283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao L, Kinney B, Yoo HS, Lee B, Schaack J, Shao J. Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. Diabetes. 2012;61(6):1463–70. doi: 10.2337/db11-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464(7293):1313–9. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 33.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nature medicine. 2009;15(8):921–9. doi: 10.1038/nm.2001. Epub 2009/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell metabolism. 2008;8(4):301–9. doi: 10.1016/j.cmet.2008.08.015. Epub 2008/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Addy CL, Gavrila A, Tsiodras S, Brodovicz K, Karchmer AW, Mantzoros CS. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. The Journal of clinical endocrinology and metabolism. 2003;88(2):627–36. doi: 10.1210/jc.2002-020795. [DOI] [PubMed] [Google Scholar]
- 36.Gavrila A, Hsu W, Tsiodras S, Doweiko J, Gautam S, Martin L, et al. Improvement in highly active antiretroviral therapy-induced metabolic syndrome by treatment with pioglitazone but not with fenofibrate: a 2 x 2 factorial, randomized, double-blinded, placebo-controlled trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;40(5):745–9. doi: 10.1086/427697. [DOI] [PubMed] [Google Scholar]
- 37.Leow MK, Addy CL, Mantzoros CS. Clinical review 159: Human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: clinical presentation, pathophysiology, and therapeutic strategies. The Journal of clinical endocrinology and metabolism. 2003;88(5):1961–76. doi: 10.1210/jc.2002-021704. [DOI] [PubMed] [Google Scholar]
- 38.Magkos F, Mantzoros CS. Body fat redistribution and metabolic abnormalities in HIV-infected patients on highly active antiretroviral therapy: novel insights into pathophysiology and emerging opportunities for treatment. Metabolism: clinical and experimental. 2011;60(6):749–53. doi: 10.1016/j.metabol.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paruthi J, Gill N, Mantzoros CS. Adipokines in the HIV/HAART-associated lipodystrophy syndrome. Metabolism: clinical and experimental. 2013;62(9):1199–205. doi: 10.1016/j.metabol.2013.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.