Introduction
The extracellular matrix (ECM) is a complex and dynamic entity that drives the formation and development of the cardiovascular system, determines critical aspects of cardiovascular performance, and plays key roles in the initiation and progression of abnormal cardiovascular function with aging and disease. Microscopic studies in the late 1700s identified fibrous structures surrounding cells, which led scientists to conclude that the ECM was primarily a foundational unit to provide support.1 Unfortunately, this historic view that the ECM is a static scaffold is still to this day held by many who are not in the field. Using molecular, cell based, and dynamic imaging systems, however, experts now recognize that the ECM is an ever-changing component that responds to normal and abnormal molecular and biophysical cues and in turn drives changes in overall cardiovascular structure and function.
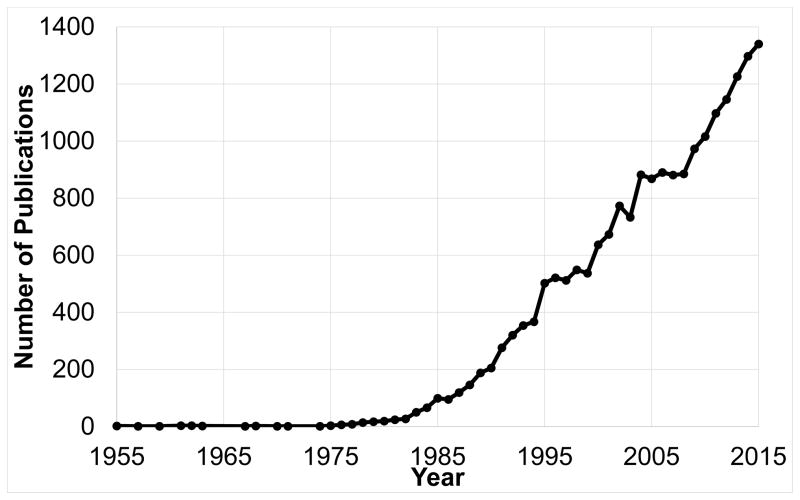
The ECM contains structural and non-structural proteins, interacts dynamically with unique and differentiating cell types, serves as a reservoir and processing site for signaling molecules, and forms communication corridors for both protein and genetic information. Thus, the ECM is a diverse entity that presents a novel and exciting research frontier that could yield improvements in diagnostics, prognostics, therapeutics, and prevention of cardiovascular disease. A recent Pubmed search (accessed August 23, 2016) using the terms ECM and cardiovascular, cardiac, or vascular revealed that this field has been growing annually since the 1990s (Figure 1). Driving forces for the increased interest in ECM research include the recognition of biological and pathophysiological importance, improvements in biochemical, cellular, and molecular techniques by which to study this complex unit, and advances in the capacity at microscopic and macroscopic levels to provide greater insight into the dynamically changing ECM. Using combinatorial approaches, ECM research can be explored at greater depths than previously possible. This editorial forms a coalescence of discussions by an ad-hoc panel motivated by a call by the AHA to define important cardiovascular ECM research topic areas.
Figure 1.
Cardiac Extracellular Matrix Research is in a growth phase. Source: PubMed
Significance
There is no question that the ECM plays a defining role in the initiation and progression of major cardiovascular diseases, including ischemic heart disease, hypertensive heart disease, valvular disease, pediatric and adult cardiomyopathies and cardiovascular malformations, arteriosclerotic and vascular disease, and cerebrovascular disease. Advances in our understanding of ECM roles will yield new approaches towards the prevention, detection and treatment of cardiovascular disease states.2
ECM abnormalities form a milestone event in aging and increased susceptibility to cardiovascular disease. Thus, new discoveries can yield improvements in approaches to maintain a healthy ECM that contributes to prolonged longevity and health span. Moreover, since an abnormal ECM is a common defining point in a number of diseases including neurodegenerative disorders, cancer, and inflammatory, autoimmune and fibrotic conditions, new ECM discoveries within the setting of the cardiovascular system will transcend to overall health and disease concepts.
Current Knowledge Gaps in ECM roles in Cardiac Physiology and Pathophysiology
Table 1 summarizes a number of areas that require thorough investigation for a full understanding of ECM roles, both in the healthy aging environment as well as following pathology. Note that the areas are synergistic, as defining what the ECM is at the cellular and molecular scales and developing more selective ECM imaging tools will provide the knowledge on which to build computational models. This will in turn generate novel hypotheses regarding how cell and ECM interactions and signaling interact during aging and after pathology such as myocardial infarction (MI; heart attack), chronic pressure overload of the heart (hypertension), or diabetes- all of which will reveal how ECM influences physiology and pathology of the left ventricle (LV).
Table 1.
Fundamental Knowledge Gaps
We need to:
|
Collagen Production as a Driver of Remodeling
Fibrosis is defined as the excessive accumulation of ECM. In cardiac pathology, fibrosis is a generic term that encompasses different types of ECM deposition, all of which impair or even obliterate heart function, for example, by replacing cardiomyocytes causing dilation and suppressing heart pumping, and disrupting electrical transmission between cardiomyocytes. In fibrosis in response to hypertension, collagen fibers interweave between viable cardiomyocytes and surrounding coronary arteries, and this pattern is characterized by interstitial and perivascular ECM accumulation that slowly impairs cardiac function over time, and is known as reactive fibrosis. Cardiac aging generates an ECM environment that is characterized by replacement interstitial fibrosis.3 Fibrosis in response to MI is scar ECM that replaces dead myocytes (reparative fibrosis), and this post-infarction scar displays notable variability in collagen content and arrangement.4
Collagen accounts for a majority of the cardiac ECM protein volume, yet our fundamental understanding of how collagen is produced, assembled, and aligned is not well understood.5 Outside of the fibrillar assembly and mechanical properties provided by collagenous ECM, understanding how an initially isotropic ECM can develop regional variation due to local cell-ECM interactions and influences how other ECM proteins with structural, signaling and accessory functions play critical roles in disease states remain to be elucidated.6, 7 While fibrosis is often considered a negative event, the absence of an ECM scar in the setting of MI would obviously be disastrous, resulting in a significant loss of myocardial structural integrity and increased risk for rupture. We need to understand how to guide myocardial cells to generate a scar that has the right balance of structural support but also one that does not contribute to continuous abnormalities in local biomechanics to induce feed-forward processes of MI expansion, LV dilation, and pump dysfunction. Moreover, understanding what critical structural proteins and cell types drive favorable remodeling will also guide new avenues of research using injectable bioengineered biomaterials and ECM scaffolds strong enough to prevent rupture and stiff enough to minimize systolic dyskinesis, without impairing filling or the function of remaining viable myocardium.
The adult mammalian myocardium has negligible endogenous regenerative capacity, and MI can result in death of up to a billion cardiomyocytes, overwhelming the limited cardiac regenerative reserve. Inflammatory leukocytes, vascular cells and fibroblasts control deposition and remodeling of ECM needed to repair the heart and to form scar.8, 9 Matricellular proteins transduce signals to inflammatory and reparative cells.10 These cells are enmeshed within a dynamic network of ECM proteins that modify their phenotype and function and may modulate cell differentiation and survival. Whether ECM modulation and activation of specific matricellular pathways can activate a regenerative program in the myocardium has not been investigated.
ECM Degradation and Turnover
An extensive body of information on inhibition of ECM degradation as well as ECM production has been made available from studying compiled data from MI studies – especially related to degrading collagen by inhibiting matrix metalloproteinase (MMP) activity.11, 12 These studies, despite their overall failure to ameliorate post-MI outcomes, taught us that small molecules can be deployed to modify the ECM.13, 14 Understanding what the optimum ECM is to allow repair without promoting further development of dysfunction will provide insight into the new homeostatic balance that is achieved between ECM degradation and production following injury.
Of note, over half of the MMPs have not been evaluated in the heart.13 Further, other protease families such as the disintegrin and metalloproteinase domain-containing proteins (ADAMs) and ADAMs with thrombospondin motifs (ADAM-TS) are understudied.15 How the different protease families compete or work in concert to degrade ECM, both in physiological and pathological conditions, remains to be clarified. Likely different portfolios of enzymes are produced or activated dependent on the stimulus. How proteases work with their endogenous inhibitors, the tissue inhibitors of metalloproteinases (TIMPs), remains to be fully evaluated.16 While the imbalance in MMP/TIMP ratio is an important factor in ECM remodeling, the complex in vivo protease and anti-protease system is likely more complicated. The Cardiac Metalloproteinase Actions Postulates have been developed to serve as a template for exploring protease roles in the post-MI setting.13
In addition to enzymes that contribute to ECM remodeling by degradation, other protein classes such as transglutaminases, lysyl oxidases, lysyl oxidase-like enzymes, and lysyl hydroxylases control the crosslinking degree of the ECM. Crosslinking provides higher mechanical stability (stiffness), but excess cross-linking could render ECM less susceptible to controlled degradation in an irreversible manner.17 While proteolytic enzymes and their cell sources represent attractive targets for therapeutic strategies, we need to understand which cells, and in which activation state, promote beneficial or detrimental heart repair.
The Cardiac Fibroblast
The cardiac fibroblast is the key cell that produces and organizes the ECM, yet this cell type has been understudied both in vivo and in vitro due to technical limitations (e.g., limited ability to define this cell type and its heterogeneity) and variability introduced by cell type examined (e.g., neonatal vs. adult) and culture conditions used (e.g., passage and plate coating).18 Activated fibroblasts have enhanced ECM production and have been termed myofibroblasts based on their high ECM-contracting capacity due to increased α–smooth muscle actin synthesis.19, 20 Fibroblast and myofibroblast are generic labels, however, for a heterogeneous groups of cells that have limited molecular and phenotypic characterization. While the fibroblast cell family has common features among its members, these cells likely derive from a variety of sources and respond to a diverse range and combination of stimuli that are present in the heart under physiological and pathological conditions, including differences in mechanical loading.19, 21 New genetic models that trace cell origins in the heart coupled with genomic and proteomic evaluations provide the means for more accurately cataloguing the fibroblast subtypes, which will provide the ability to specifically distinguish and target cells with detrimental roles without affecting cells that promote repair. Using the lineage tracing approach, periostin and periostin-positive myofibroblasts were recently shown to be important in post-MI ECM synthesis and scar formation, indicating the importance of the expression and action of locally active biomolecules to drive fibroblast activation in fibrotic remodeling of the infarcted LV.22
Following MI, ischemic myocytes release factors that convert fibroblasts to a pro-inflammatory state, while at the later stage macrophages release factors that convert fibroblasts to a proliferative and secretory state.23 Understanding how cell-cell interactions fluctuate over the time continuum will reveal new targets for therapeutic intervention.9 This level of complexity in phenotypic shifts over the time continuum of cardiac remodeling lends itself to computational approaches.7 Studying the positive and negative feedback loops between the fibroblast and ECM will be essential to advance our understanding of the balance between physiological and pathologic heart remodeling.17
The ECM as a Storage and Regulatory Center
Growth factors are stored in and released from the ECM. For example, vascular endothelial growth factor A and transforming growth factor β1 are factors stored within the ECM that modulate a wide range of tissue responses, including angiogenesis, hypertrophy, and fibrosis.24, 25 Mechanisms of growth factor storage and release are not fully understood. Growth factors do not simply adhere to ECM to be picked up by receptors of any cell that shares the same space. Rather, the biology of growth factor availability is complex and can depend on the maturation state of the ECM, its mechanical properties, the chemical environment, and specific proteins on the cell surface that are required to release the growth factor for receptor binding.26–28
There are a number of accessory matricellular proteins that reside in the ECM to assist in both normal and pathological turnover, including secreted protein acidic and rich in cysteine, thrombospondins, CCN2 (formerly known as connective tissue growth factor), periostin, and osteopontin.10 Matricellular proteins support inflammation, which is a fundamental response to injury with common trans-organ applicability. Even proteins that are considered as being primarily structural, such as collagen and fibronectin, contain signaling domains that are controlled by glycation, proteolytic processing, and mechanical strain.29, 30 These elements may combine to provide intersection points for cell and ECM therapies once the molecular mechanisms are understood.
Maintaining a Healthy Cardiac ECM Over the Life and Health span
In healthy individuals, the assumption has been that there is a uniform ECM connected to cardiomyocytes via integrins, and that the frequency and distribution of integrin connections are uniform. While cardiac ECM changes with aging have been evaluated for individual proteins such as collagen, fibronectin, and MMP-9,31, 32 a systems level evaluation is not available. How ECM components globally modify the myocardium, in terms of ECM production (ECM gene stimulating factors), deposition (matricellular proteins and cross-linking enzymes), and removal (MMPs and ADAMs), is unknown. Multi-omic approaches that evaluate temporal and spatial events over the course of aging will show the kinetics of aging. In aging and accelerated aging models (e.g., diabetes), increased myocardial stiffness due to alterations in the ECM network are proposed but poorly understood mechanisms for the development of heart failure with preserved ejection fraction (HFpEF).2, 33
Common and Distinct Mechanisms between Adult and Pediatric Cardiac Disease
Comparative studies are needed to provide insights into common denominator and disparate mechanisms and signaling pathways, including evaluations of groups across age and pathology spectrums. In patients with congenital heart disease (CHD), abnormal ECM accumulation is prevalent but not well understood. Malformations in ECM organization may be more detrimental than abnormalities in ECM proteins.34 For CHD patients, a change in disease status or re-emergence of disease may be expressed as ECM malformations.
Advances in Approaches to Understand the Cardiac ECM
As described below, a wide array of molecular and biological tools now exist that can be harnessed and turned towards new discoveries in the physiology of cardiac ECM.
Multi-omic evaluations
Cardiac ECM research, and ECM research in general, has not been easily amenable to genomic, proteomic, and metabolomics approaches in the past, due to a number of logistical issues. These include solubility issues that make sampling inconsistent and a relative lower abundance of ECM compared to easily soluble intracellular proteins in the myocardium. Recent advances have provided research templates on which cardiac ECM omics studies are now highly feasible and informative.35 ECM is regulated primarily at the post-translational level, which means that proteomics is more informative for understanding ECM than genomic approaches. For example, in the absence of pathology, ECM proteins have relatively long half-lives, which increases their chances of being post-translationally modified (e.g., advanced glycation end product accumulation). Following MI, the robust increase in proteases exposes ECM proteins to increased enzymatic processing.11, 12, 14
Computational Approaches
Bringing big data into the cardiac ECM community serves two purposes. First, this technology allows the integration of results from multiple dimensions, which provides a template for novel hypothesis generation and allows a deeper understanding at the systems level. Consolidation of vast amounts of information to distill most informative components (big data to knowledge) streamlines future data collection for more efficient application.36 Second, generating computational models that can be used to predict outcomes has implications for both basic science and clinical translation. Computational tools have been applied to various fields including cardiac electrophysiology and to cardiac fibroblasts to identify potential mechanisms driving a phenotype (e.g., electrical conduction or tissue remodeling) or potential therapeutic targets against a detrimental phenotype.7 The construction of fibroblast signaling networks will provide insight into fundamental molecular pathway changes translate to cell physiology. Similar approaches will be needed to understand the complex interactions of matrix components, proteases, and growth factors in the extracellular space. Multi-scale models and models that integrate cross-talk amongst cell types provide the ability for in silico evaluations to predict how therapies will affect properties and function at the tissue and organ level.
In vivo dissection of the role of ECM components in cardiac pathophysiology
Development of strategies for genetic targeting in rodents has revolutionized biomedical research providing unique opportunities to test a pathophysiologic paradigm in vivo. Progress in understanding the role of the ECM in cardiac homeostasis and disease is dependent on development of robust in vivo models to dissect specific ECM-dependent actions in the pathogenesis of cardiac repair, remodeling, and fibrosis. In terms of cardiac pathophysiology, this means generating models that modify inflammatory leukocytes and fibroblasts.
Cell Biological Approaches
The development of directed therapeutic intervention strategies focused on one cell type will depend on the identification of molecular targets, which is often done in vitro to reduce system complexity. Validity of these systems will depend on our ability to isolate key individual cell components, namely inflammatory leukocytes (neutrophils, macrophages, and lymphocytes) and fibroblasts that have defined inflammatory and reparative polarization phenotypes. Cell culture systems that support cell growth in a setting that sufficiently resembles the in vivo environment will provide insight into cardiac cell physiology within a mechanically active environment. This may include use of mechanically stimulated engineered constructs with physical and chemical properties comparable to the endogenous microenvironment. With the advent of miniaturized systems with defined mechanical conditions, higher throughput testing of cardiac cells relevant to ECM will soon be available. In addition, protocols that couple cell isolation with flow cytometry provide the ability to determine relative abundance of cell types in the myocardium.37
Imaging
Imaging of ECM has largely been limited to visualizing the fibrotic ECM structure, such that fibrosis can be detected but details on quantity and quality of various proteins within the fibrotic environment cannot be readily assessed. MMP activity can be imaged, particularly in the post-MI setting where MMPs are elevated.38 The emergence of elastography methods may provide important information on ECM construction and stiffness, and the use of biomedical imaging using second and third harmonics, provides some information about the organizational state of collagen in biopsies. Overall, imaging of ECM is an area with great potential that remains to be developed.
Conclusions
Insights into ECM roles in cardiovascular disease are highly relevant for understanding the pathophysiology of a wide range of conditions in other systems. Alterations in the ECM network play a central role in a variety of conditions involving many different organs, including idiopathic pulmonary fibrosis, systemic sclerosis, liver cirrhosis, and renal failure. In all conditions, dysregulated expression, or altered function of ECM proteins contribute to organ dysfunction. Moreover, ECM proteins play a crucial role for cell proliferation and invasive potential in cancer, and contribute to the pathogenesis of neurological disorders. Understanding the fundamental effects of the ECM on cellular phenotype in the heart can generate new ideas for understanding and treatment of conditions involving other systems. Therefore, a better understanding of how the cardiac ECM coordinates, and is coordinated, will feed forward to inform and benefit other systems as well.
The cardiac ECM field has made remarkable progress, particularly in the last 20 years. The field is poised to answer significant outstanding questions that are fundamental to cardiovascular research. Advancing our understanding of cardiac ECM has the potential to cross-translate to other diseases, because understanding how normal and abnormal cardiac ECM processes occur informs on a broader scale.
Acknowledgments
Sources of Funding
The authors acknowledge funding support from the following: National Institutes of Health (NIH) for HL075360, HL076246, HL085440, HL111090, HL112519, HL116449, HL127654, HL129823, HL130972, and HL131280; the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award 5I01BX000505 and 5I01BX000168; the Department of Defense Congressionally Directed Medical Research Programs; the Canadian Institutes of Health Research (CIHR grants #210820 and #286920, #286720, #137060, and # 84279), the Collaborative Health Research Programme (CIHR/NSERC grants #1004005 and #413783), the Canada Foundation for Innovation and Ontario Research Fund (CFI/ORF grant #26653), the Heart and Stroke Foundation of Canada (HSF: G-14-0006063 & G-14-0006073); and the Canada Foundation for Innovation (CFI 33751).
Footnotes
Disclosures. None
References
- 1.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiological reviews. 2007;87:1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 2.Spinale FG, Zile MR. Integrating the myocardial matrix into heart failure recognition and management. Circulation research. 2013;113:725–38. doi: 10.1161/CIRCRESAHA.113.300309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yabluchanskiy A, Ma Y, Chiao YA, Lopez EF, Voorhees AP, Toba H, Hall ME, Han HC, Lindsey ML, Jin YF. Cardiac aging is initiated by matrix metalloproteinase-9-mediated endothelial dysfunction. American journal of physiology Heart and circulatory physiology. 2014;306:H1398–407. doi: 10.1152/ajpheart.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takawale A, Sakamuri SS, Kassiri Z. Extracellular matrix communication and turnover in cardiac physiology and pathology. Comprehensive Physiology. 2015;5:687–719. doi: 10.1002/cphy.c140045. [DOI] [PubMed] [Google Scholar]
- 5.Richardson WJ, Holmes JW. Emergence of Collagen Orientation Heterogeneity in Healing Infarcts and an Agent-Based Model. Biophysical journal. 2016;110:2266–77. doi: 10.1016/j.bpj.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson WJ, Clarke SA, Quinn TA, Holmes JW. Physiological Implications of Myocardial Scar Structure. Comprehensive Physiology. 2015;5:1877–909. doi: 10.1002/cphy.c140067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeigler AC, Richardson WJ, Holmes JW, Saucerman JJ. Computational modeling of cardiac fibroblasts and fibrosis. Journal of molecular and cellular cardiology. 2016;93:73–83. doi: 10.1016/j.yjmcc.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagalingam RS, Safi HA, Czubryt MP. Gaining myocytes or losing fibroblasts: Challenges in cardiac fibroblast reprogramming for infarct repair. Journal of molecular and cellular cardiology. 2016;93:108–14. doi: 10.1016/j.yjmcc.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circulation research. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiological reviews. 2012;92:635–88. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circulation research. 2002;90:520–30. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 12.Spinale FG, Villarreal F. Targeting matrix metalloproteinases in heart disease: lessons from endogenous inhibitors. Biochemical pharmacology. 2014;90:7–15. doi: 10.1016/j.bcp.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer RP, de Castro Bras LE, Jin YF, Lindsey ML. Translating Koch’s postulates to identify matrix metalloproteinase roles in postmyocardial infarction remodeling: cardiac metalloproteinase actions (CarMA) postulates. Circulation research. 2014;114:860–71. doi: 10.1161/CIRCRESAHA.114.301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsey ML, Iyer RP, Jung M, DeLeon-Pennell KY, Ma Y. Matrix metalloproteinases as input and output signals for post-myocardial infarction remodeling. Journal of molecular and cellular cardiology. 2016;91:134–40. doi: 10.1016/j.yjmcc.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P, Shen M, Fernandez-Patron C, Kassiri Z. ADAMs family and relatives in cardiovascular physiology and pathology. Journal of molecular and cellular cardiology. 2016;93:186–99. doi: 10.1016/j.yjmcc.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Moore L, Fan D, Basu R, Kandalam V, Kassiri Z. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart failure reviews. 2012;17:693–706. doi: 10.1007/s10741-011-9266-y. [DOI] [PubMed] [Google Scholar]
- 17.Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol. 2013;229:298–309. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac Fibrosis: The Fibroblast Awakens. Circ Res. 2016;118:1021–40. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Hinz B. Myofibroblasts. Exp Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature reviews Molecular cell biology. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 22.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, SCJL, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nature communications. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobaczewski M, de Haan JJ, Frangogiannis NG. The extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium. Journal of cardiovascular translational research. 2012;5:837–47. doi: 10.1007/s12265-012-9406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: a role in inflammation and repair. Journal of molecular and cellular cardiology. 2014;70:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rienks M, Papageorgiou AP, Frangogiannis NG, Heymans S. Myocardial extracellular matrix: an ever-changing and diverse entity. Circulation research. 2014;114:872–88. doi: 10.1161/CIRCRESAHA.114.302533. [DOI] [PubMed] [Google Scholar]
- 26.Hinz B. The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Henderson NC, Sheppard D. Integrin-mediated regulation of TGFbeta in fibrosis. Biochim Biophys Acta. 2013;1832:891–6. doi: 10.1016/j.bbadis.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martino MM, Briquez PS, Guc E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Muller R, Swartz MA, Hubbell JA. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343:885–8. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- 29.McCulloch CA, Coelho NM. Collagen Processing and its Role in Fibrosis, in Cardiac Fibrosis and Heart Failure: Cause or Effect? In: Wigle IMCDaJT., editor. Cardiac Fibrosis and Heart Failure: Cause or Effect? Springer International Publishing; 2015. pp. 261–278. [Google Scholar]
- 30.Vogel V, Sheetz MP. Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways. Curr Opin Cell Biol. 2009;21:38–46. doi: 10.1016/j.ceb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horn MA, Trafford AW. Aging and the cardiac collagen matrix: Novel mediators of fibrotic remodelling. Journal of molecular and cellular cardiology. 2016;93:175–85. doi: 10.1016/j.yjmcc.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Chiao YA, Clark R, Flynn ER, Yabluchanskiy A, Ghasemi O, Zouein F, Lindsey ML, Jin YF. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovascular research. 2015;106:421–31. doi: 10.1093/cvr/cvv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. Journal of molecular and cellular cardiology. 2016;90:84–93. doi: 10.1016/j.yjmcc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsey ML, Mayr M, Gomes AV, Delles C, Arrell DK, Murphy AM, Lange RA, Costello CE, Jin YF, Laskowitz DT, Sam F, Terzic A, Van Eyk J, Srinivas PR. Transformative Impact of Proteomics on Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132:852–72. doi: 10.1161/CIR.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 36.Scruggs SB, Watson K, Su AI, Hermjakob H, Yates JR, 3rd, Lindsey ML, Ping P. Harnessing the heart of big data. Circulation research. 2015;116:1115–9. doi: 10.1161/CIRCRESAHA.115.306013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting Cardiac Cellular Composition. Circulation research. 2016;118:400–9. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahul ZH, Mukherjee R, Song J, McAteer J, Stroud RE, Dione DP, Staib L, Papademetris X, Dobrucki LW, Duncan JS, Spinale FG, Sinusas AJ. Targeted imaging of the spatial and temporal variation of matrix metalloproteinase activity in a porcine model of postinfarct remodeling: relationship to myocardial dysfunction. Circulation Cardiovascular imaging. 2011;4:381–91. doi: 10.1161/CIRCIMAGING.110.961854. [DOI] [PMC free article] [PubMed] [Google Scholar]