Preface
Δ9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, is a pressing concern to global mental health. Patterns of use are changing drastically due to legalisation, availability of synthetic analogues (‘spice’), cannavaping and aggrandizements in the purported therapeutic effects of cannabis. Many of THC’s reinforcing effects are mediated by the dopamine system. Due to complex cannabinoid-dopamine interactions there is conflicting evidence from human and animal research fields. Acute THC causes increased dopamine release and neuron activity, whilst long-term use is associated with blunting of the dopamine system. Future research must examine the long-term and developmental dopaminergic effects of the drug.
Introduction
Cannabis is a widely used recreational drug. Over half of young Americans have used the drug1. In Europe cannabis has now overtaken heroin as the most widely reported illegal drug used amongst people entering specialist addiction services2. At the same time, political debates about changes to the legal status of the drug continue internationally. Although causality has not been conclusively demonstrated, heavy cannabis use is associated with increased risk of mental disorder3 including psychosis4, addiction5, depression6, suicidality7, cognitive impairment8 and amotivation9.
Δ9-Tetrahydrocannabinol (THC), cannabis’ main psychoactive component10, elicits its acute psychoactive effects via the endocannabinoid type 1 (CB1) receptor (CB1R)11. THC has been linked to the rewarding aspects of cannabis and the induction of symptoms of mental illnesses and cognitive impairment. Lately the THC content of cannabis has been increasing12, synthetic THC analogues (potent cannabinoid agonists; termed ‘spice’) are now widely used13. The future consumption of cannabinoids through electronic cigarettes (‘cannavaping’) and edible products14 changes the landscape further15. Given the widespread use of cannabinoids, and the links between THC exposure and adverse outcomes, it is imperative to understand the neurobiological effects of THC. Recently, we and others have found that heavy cannabis use is associated with reductions in dopaminergic function. Since the rewarding and psychotogenic effects of THC and its analogues are thought to be mediated by the dopaminergic system, demonstrating dopaminergic alterations in vivo in human users is of clinical relevance for the prevention and treatment of cannabis use disorders and psychoses. Therefore, we review the animal and human literature on the complex effects of acute and longer-term THC on dopamine synthesis, release, and its receptors, critically analysing the factors that contribute to effects, and variations between studies, before finally providing a framework for future research including pharmacologically dissecting these effects, especially in the developing brain.
THC receptor binding in the brain
THC is a CB1R and endocannabinoid type 2 receptor (CB2R) partial agonist11. The psychoactive effects of THC are blocked by the CB1R antagonist rimonabant16,17 indicating that these are mediated through activating G-protein-coupled CB1R receptors which reduce cyclic adenosine monophosphate (cAMP) levels by inhibiting adenylate cyclase18. THC disrupts finely-tuned endocannabinoid retrograde signalling systems due to the temporal and neuronal specificity of endocannabinoids over THC. Under conditions of low CB1R density, THC antagonises endogenous agonists possessing greater receptor efficacy than THC19. THC also allosterically modulates opioid receptors20, which may provide additional indirect routes for altering dopamine transmission21. Furthermore, THC has psychoactive metabolites with CB1R affinity, further complicating the analyses of receptor binding studies22.
CB1 receptors and dopamine
Early animal studies described the interactions of amphetamine, which increases dopamine release, and THC23. These reported that amphetamine’s behavioural effects were potentiated or antagonised depending on the dose of THC leading researchers24 to propose that dopamine was “a prime candidate for…the mode of action of Δ9-tetrahydrocannabinol”. Indeed, THC produces complex effects on the dopamine system, contributing to the drug’s recreational and harmful effects. However, there are inconsistencies between the preclinical and clinical findings which challenge the field. It is thus timely to review the evidence and provide a framework for understanding the inconsistencies between the preclinical and clinical findings.
Dopaminergic neurons are modulated by the endocannabinoid system (eCBS)25. CB1Rs and the endocannabinoid ligands anandamide and 2-arachidonoylglycerol (2-AG) are abundant in dopaminergic pathways including the striatum26 where they act as a retrograde feedback system on presynaptic glutamatergic and γ-aminobutyric acid (GABA) nerve terminals (Fig. 1) to modulate dopamine transmission. Anandamide27 and 2-AG28 stimulate dopamine release in the nucleus accumbens (NAc) shell. This effect is blocked by the CB1 antagonist rimonabant, indicating that dopaminergic effects of endocannabinoids involve CB1 receptors. The rewarding properties of THC via increased dopamine release and dopaminergic neuron firing are underpinned by biased signal transduction mechanisms from the CB1R16. There is evidence of differential effects of acute vs. chronic THC exposure on the dopaminergic system. Therefore, we will treat these separately and describe the effects on different neurobiological components of the dopaminergic system including neuron firing, synthesis, release, reuptake and receptors.
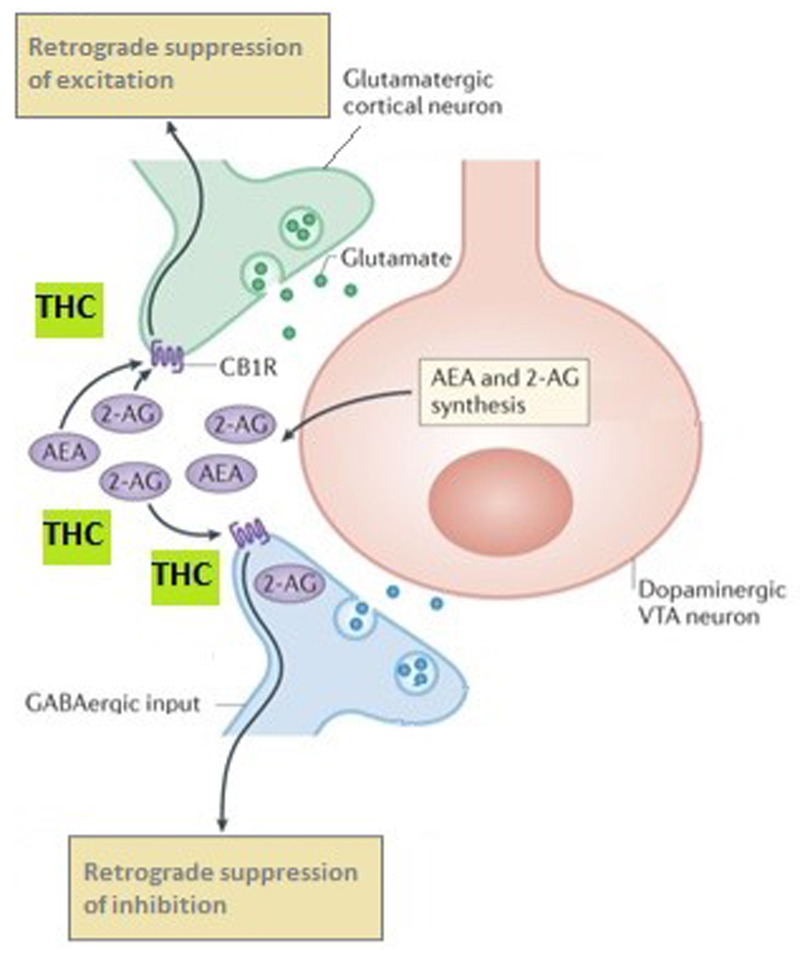
Fig. 1. THC binds to CB1 receptors on glutamatergic and GABAergic neurons disrupting normal endocannabinoid retrograde signalling from dopaminergic neurons137.
Endocannabinoids (eCBs) influence ventral tegmental area (VTA) synaptic signalling. 2-Arachidonoylglycerol (2-AG) is synthesised by diacylglycerol lipase (DAGL) in dopaminergic VTA neurons and, once released, retroactively acts on endocannabinoid type 1 receptors (CB1Rs) on nearby glutamatergic and γ-aminobutyric acid (GABA)-ergic terminals. CB1Rs mediate robust inhibition of GABA inputs onto VTA dopamine cells29, termed retrograde suppression of inhibition. CB1Rs are also localized on glutamatergic terminals synapsing on VTA dopamine neurons30 where eCBs mediate retrograde suppression of excitation. Thus, eCBs fine-tune the activity of the mesolimbic dopamine projections through modulating both excitatory and inhibitory signalling. THC disrupts this finely tuned system.
Acute THC and presynaptic dopamine in animals
From the outset it was clear that THC exerts complex effects on the dopamine system. Early in vitro studies in rodents using radiolabelled dopamine in synaptosomes found that THC caused increased dopamine synthesis31 and release24 (Fig. 2). However, the effects on dopamine uptake yielded conflicting results, with evidence of both increases32 and dose-dependent decreases24. Subsequently biphasic and triphasic effects of THC were discovered, whereby low doses of THC produced increases in the conversion of tyrosine to dopamine, but high doses of THC resulted in decreased dopamine synthesis33. Likewise, complicated temporal relationships between THC administration and changes in dopamine levels were observed34, such that repeated dosing results in behavioural and neurochemical tolerance –highly pertinent to the mechanisms of dependence to the drug. The complex dose-specific effects of THC in rodents were thought to be due to dose-related decreases in precursor uptake32 and dopamine-opioid interactions via μ-opioid receptors31.
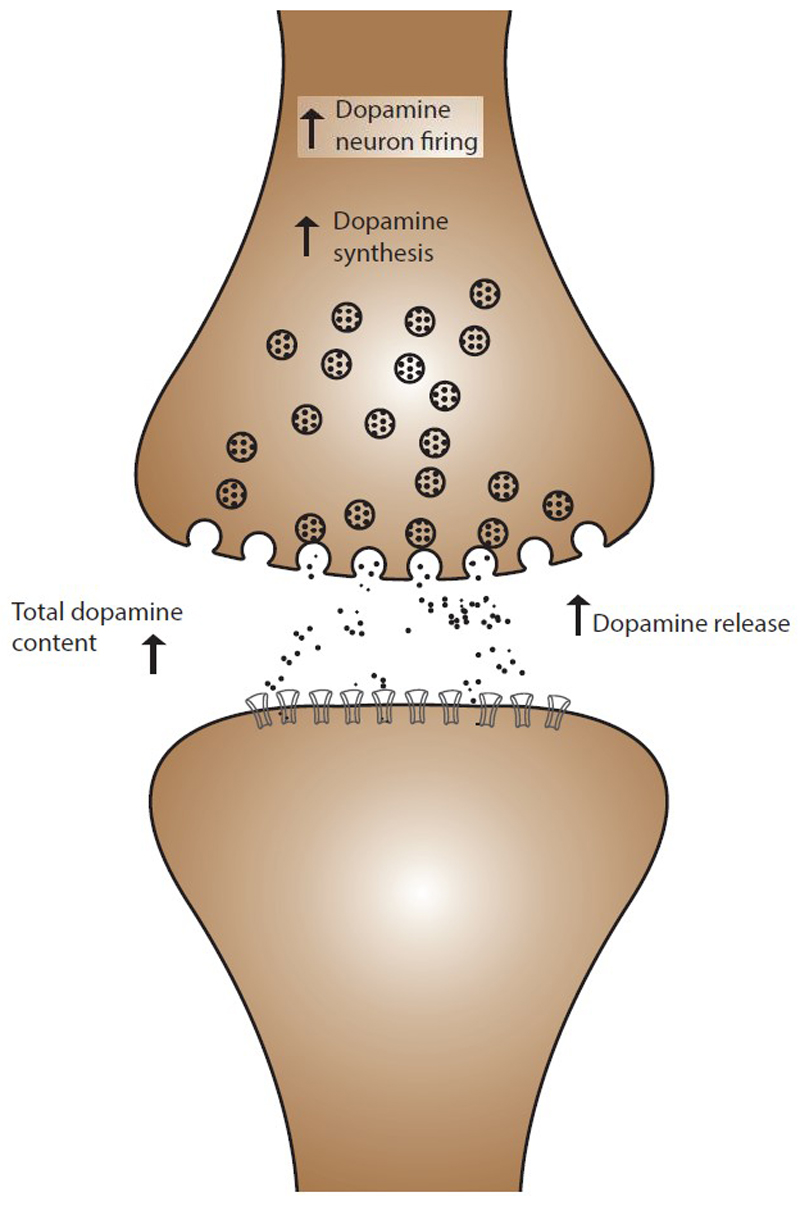
Fig. 2. Summary of the acute effects of THC on dopaminergic function.
In animal models acute THC challenge is associated with increased dopaminergic cell firing, increased dopamine synthesis and increased dopamine release.
Subsequent work investigated THC-induced increases in dopamine synthesis in vivo. THC increased [3H]-dopamine synthesis35,36, tyrosine hydroxylase37 and messenger ribonucleic acid (mRNA) expression38, the rate-limiting step in the dopamine synthesis pathway. Similarly, increases in dopamine metabolism, measured with the dihydroxphenylacetic acid/dopamine (DOPAC/DA) ratio, were reported in most39 but not all37 rodent studies. However, the majority of early studies using spectrophotofluorimetry were inconsistent due to technical limitations in detecting the rapid changes in extracellular dopamine concentration detectable by microdialysis techniques used more recently40.
In vivo microdialysis shows that acute THC increases dopamine efflux in the prefrontal cortex (PFC)41, striatum42 and nucleus accumbens (NAc)43. Only one study did not find THC–induced increases in dopamine efflux44, which may have been related to route of administration since that study used a THC gavage whereas the other studies used intravenous injection which produces a rapid increase in THC which reaches the brain promptly compared to gavage which favours sequestration in lipid compartments due to the very high lipid solubility of THC45. Differences in microdialysis results are associated with the strain of experimental rat46. Electrophysiological studies in rats have categorically demonstrated that THC dose-dependently increases firing rates in ascending midbrain dopaminergic projections via CB1R agonism47,48. Taken together, these findings suggest THC increases the firing rates of dopamine neurons which leads to increased dopamine synthesis and release in terminal fields.
Acute THC and post-synaptic dopamine in animals
Acute THC did not alter dopamine receptor proteins levels in rhesus monkeys49. In the rat limbic forebrain, one study reported increased dopamine type 1 receptor (D1R) availability50 whilst other studies reported decreases51. In the striatum, dopamine type 2 receptor (D2R) density showed either a decrease51 or no significant changes, whilst decreases in D1R have been reported50. Taken together, the findings of no change in receptor protein levels together with a tendency for reduced receptor availability most likely reflects THC-induced changes in synaptic dopamine levels. .
Acute THC and dopamine in humans
Studies of metabolic brain activity in humans using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) provide indirect measures of dopaminergic function through changes in cerebral blood flow and glucose metabolism. These serve as surrogate markers of brain activity in areas with dopaminergic projections. In humans, acute THC is associated with increased activity in frontal and sub-cortical regions52. However, as the CB1R has the highest concentrations in these regions53 these findings may be due to direct endocannabinoid effects rather than reflecting dopamine-mediated processes. When studies of acute THC on resting brain activity have focussed on regions with dense dopaminergic innervation, such as the striatum, there have been inconsistent effects, with reports of both increased and reduced activity52. However, certain cognitive tasks are modulated by dopaminergic signalling and may provide a more robust proxy for THC-induced changes in dopaminergic transmission. For example, motor response inhibition is associated with cortical dopamine release and an fMRI study in healthy humans with previous exposure to cannabis found that THC attenuates activation in the right inferior frontal cortex and the anterior cingulate cortex (ACC) during suppression of motor inhibition54. Further indirect evidence of blunted dopaminergic processing comes from a study in healthy humans with previous exposure to cannabis using a verbal working memory task whereby THC attenuated striatal activation55 and a study of reward function in occasional cannabis users, which found that THC induced a widespread attenuation of the brain response to feedback in reward trials56 but not under reward anticipation conditions56.
It is also possible to directly measure the dopamine system using molecular imaging. These studies have examined the effect of acute THC on dopamine in humans with previous exposure to cannabis in vivo. Using PET, a combined analysis57 of two previous studies has shown that THC does indeed cause dopamine release in the ventral striatum in the human brain57. Likewise, acute THC challenge elicited dopamine release in fronto-temporal cortical brain regions58, although this finding requires replication with radiotracers that show higher affinity for cortical dopamine receptors. However, in a separate study using single photon emission computed tomography (SPECT) no significant THC-induced dopamine release was observed,59 which may be due to a relatively small sample size (n=9). This is because THC-induced dopamine release in the striatum appears to be of a lower magnitude than that caused by other drugs such as amphetamine and methylphenidate60, combined with difficulties in imaging dopamine changes that are comparatively small61.
Repeated THC and pre-synaptic dopamine
Studies of repeated THC dosing have yielded complex, regionally-specific effects including increased total striatal dopamine levels62 in rats but reduced hippocampal63 dopamine levels in mice. Reduced dopamine metabolism was found in the medial PFC in two rat studies62, an effect which was not observed in the striatum or the NAc64. THC administration for 21 days down-regulated tyrosine hydroxylase mRNA expression in the substantia nigra and ventral tegmental area (VTA) midbrain nuclei, but not in the cortex or striatum65. In the NAc, several studies have reported that multiple THC doses do not significantly change dopamine release66 in rats. However, this may be due to differential dopaminergic responses within the NAc, as in one rat study repeated THC doses led to increased dopamine release in the NAc core but decreased release in the NAc shell67. The picture is further complicated by genetic strain effects whereby increased dopamine release in the NAc was observed in Lewis but not Fischer 344 rat strains68. Taken together, these studies indicate that repeated THC dosing produces regionally-specific effects on dopamine function.
Human studies in cannabis users
Several molecular imaging studies of dopaminergic function have been conducted in human cannabis users. Using PET, dopamine synthesis capacity was reduced in cannabis users69. Importantly, this reduction was driven by users meeting clinical criteria for abuse or dependence and was related to the severity of cannabis use (Fig. 3). Likewise, in two separate studies, cannabis users displayed reduced dopamine release to a stimulant challenge which was inversely related to severity of cannabis use70 and cognitive deficits including poor working memory71. Since no alteration in amphetamine-induced dopamine release was seen in recently abstinent cannabis users72, this effect is likely related to active use of the drug. While chronic use was not associated with altered stress-induced dopamine release73, there was evidence of a positive relationship between duration of cannabis use and stress-induced dopamine release in the limbic striatum73. Likewise, there is recent data showing that cannabis users have an attenuated metabolic response to methylphenidate challenge in the striatum, with a negative relationship between methylphenidate-induced metabolic increases and severity of cannabis use74. There is also evidence of reduced dopamine transporter (DAT) density in chronic cannabis users75. Whilst the interpretation of some of these studies is complicated by cannabis users also smoking tobacco, a recent experiment has addressed this by studying cannabis users without comorbid substance dependence, to show that cannabis users do indeed have reduced dopamine release71. Overall there is converging evidence for reduced presynaptic dopaminergic function in cannabis users.
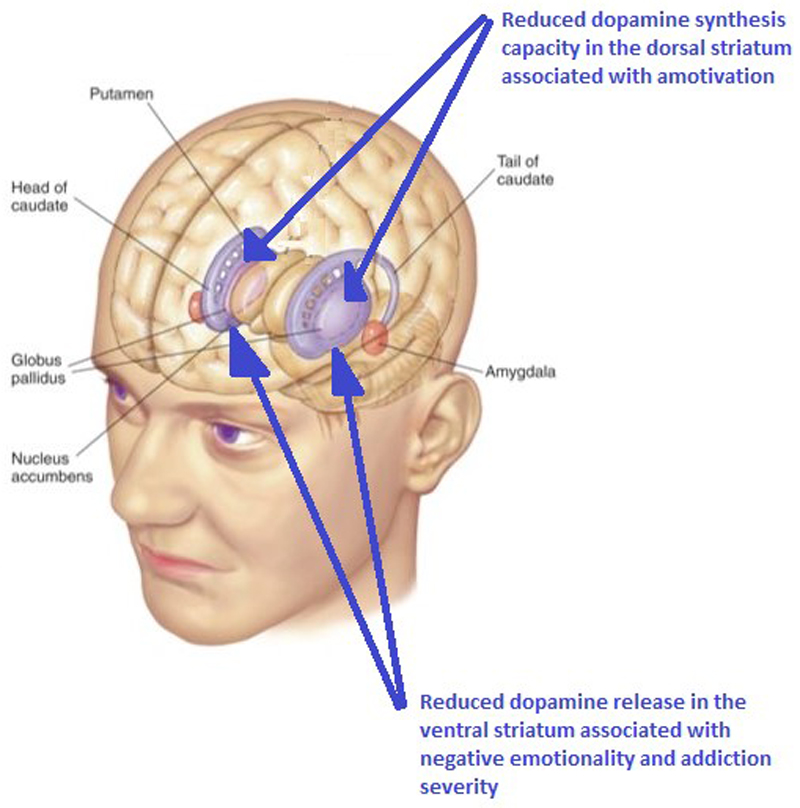
Fig. 3. Cannabis use in humans is associated with reduced dopamine in the striatum. PET studies have shown lower striatal dopamine synthesis and release capacity in cannabis users.
Lower dopamine synthesis capacity in the dorsal striatum is directly associated with reduced motivational levels76 and reduced dopamine release in the ventral striatum is directly associated with negative emotion levels and addiction severity70
Repeated THC and post-synaptic dopamine
Studies in rats have reported that multiple THC dosing results in increased D2R availability in the midbrain, striatum and PFC65,77 and that this is associated with dopamine receptor sensitisation. There is further evidence of downstream dopaminergic effects of THC, as one study in rats78 reported up-regulated postsynaptic dopamine receptor signalling in the NAc via increased adenylyl cyclase activity, which was proposed to underlie THC-induced changes in amphetamine-induced locomotive behaviour. These findings suggest that repeated THC dosing results in altered dopamine receptor signal transduction.
Dose-dependent increases in burst firing in the VTA in response to multiple THC administrations have been reported in nearly all murine electrophysiological studies48, with one exception79. In the substantia nigra pars compacta (SNpc), multiple THC dosing was associated with increased firing, although this was smaller in magnitude than in the VTA80,81. This suggests that VTA and SNpc dopamine neurons develop a differential response to repeated THC exposures. There also appears to be an effect of withdrawal from multiple THC doses whereby decreased firing is elicited by abrupt cessation of repeated THC or administration of the CB1R antagonist SR141716A82.
Recent human studies in current and abstinent ex-cannabis users have found no significant difference in striatal D2R availability compared to individuals with no history of chronic cannabis use70–72,83–85. However, there was an inverse relationship between age of first cannabis use and D2R availability in one study72 and an inverse relationship with current cannabis use in another study83, suggestive of possible dose-effects or susceptibility to drug use.
THC exposure and dopamine cell morphology
In addition to changes in function, there is evidence from studies in rodents that THC exposure causes abnormalities in the structure of dopamine neurons. These morphological effects are region-specific and include reductions across a range of measures of neuronal cell size in the VTA86,87 and increased neuronal arborisation in the NAc shell and frontal cortex88.
Developmental THC exposure and dopamine
Adolescence is an important time for brain development and adolescent cannabinoid exposure has consequences in adulthood89. The eCBS plays an important role in brain development90 and developmental THC exposure produces complex alterations in the dopamine system which are apparent from an early stage. For example, gestational THC exposure is associated with increased foetal brain tyrosine hydroxylase mRNA expression in rats91 and changes in dopamine receptor gene expression. THC exposure in early development was associated with increased cortical D2R availability92. In rats, exposure from gestational day 5 to post-natal day 21 resulted in decreased NAc D2R gene (DRD2) mRNA. Likewise, human maternal cannabis use was associated with decreased foetal NAc DRD2 mRNA93. Early life exposure to THC also blunts the dopaminergic response to stimuli that release dopamine later in life, such as stress and amphetamine. This was seen in the hypothalamus, striatum and limbic forebrain94, and frontal cortex95. In a study of CB1R agonist self-administration in rats96, THC exposure during adolescence enhanced the reinforcing effects of cannabinoids in adulthood, suggesting that exposure to THC during adolescence increased addiction potential during a critical period of development. Importantly, THC-exposed rodents had a reduced capacity for cannabinoid induced increases in firing of dopaminergic neurons, consistent with a blunting of the dopamine response in adulthood.
Inconsistencies between animal and human work
Preclinical evidence shows that acute THC increases in nerve firing rates and animal studies using microdialysis indicate that acute THC challenge causes dopamine release, yet the results of experiments from human studies have not been consistent97,98. There are a number of factors, which may underlie the inconsistencies in the results of the studies presented above.
The animal evidence indicates that there are highly region-specific differences in dopamine activity following THC administration including differential dopaminergic responses in the shell and core of the NAc67. However, whilst human PET imaging provides a reliable measure of dopaminergic function in the striatum61, the spatial resolution of most PET cameras is approximately 4 mm which limits its accuracy for measuring activity in small brain volumes (such as the core and shell of the NAc) due to partial volume and spillover effects99. For the foreseeable future, therefore, human in vivo imaging techniques lack the spatial resolution to detect these complex effects on different parts of the dopamine pathway and research on living complex human brain tissue in vitro raises significant ethical challenges100.
A further possibility underlying the lack of consistent dopamine release in the human studies is that the dose of THC used in human imaging studies has not been sufficient to consistently elicit measurable dopamine release. Human studies all administered 10mg or less of THC because older pharmacological studies indicated that the “standard joint” (defined by the US National Institute on Drug Abuse) delivered an approximate THC dose of 8mg-15mg, equivalent to about 170 micrograms/kg101. These doses are significantly less than those used in animal microdialysis studies, which are typically around 1 mg/kg. Likewise, the THC content of cannabis has increased significantly since early clinical studies102, such that THC doses may be over 40mg per spliff (joint; cannabis cigarette)103, so the doses that were used in the published imaging studies no longer reflects typical THC exposure in cannabis users. The picture is further complicated by animal data which indicate that THC exerts complex dose-dependent effects on the dopamine system33 and the dose-response profile for THC-induced dopamine release has not been investigated in humans so it remains possible that human imaging is missing peak dopamine changes. Likewise, heterogeneity in the animal data may be a reflection of time of sampling in relation to THC treatment. For example, repeated daily THC administration led to an initial decrease in dopamine levels followed by a gradual return to baseline in brains that were studied one hour after THC dosing, but increases were subsequently observed two hours post-dose34. This time period is consistent with acute release and diffusion away, followed by up-regulation of synthesis to increase levels at 2 hours. Alternatively, factors that may contribute to the complex temporal course of dopaminergic effects include intricate changes in dopamine synthesis and metabolism. These comprise CB1R-mediated increases in tyrosine hydroxylase activity38 along with acute increases and longer-term decreases in monoamine-oxidase activity104, such that THC may exert differential effects on the time courses of dopamine synthesis and degradation. Equally, the partial agonist properties of THC on the CB1R only serve to obfuscate its dopaminergic effects since THC can both activate and block cannabinoid receptors, in a regionally specific and species-dependent manner based on the density and efficiency of populations of receptors and the concentrations of endogenous agonists19.
There are a number of challenges in assessing the long-term effects of exposure to THC. Firstly, it is plausible that discrepant findings may be attributable to the duration of THC treatment. Animal experiments are typically conducted following administration of THC for no longer than three weeks65,81,87 whilst human studies are conducted in participants who have taken cannabis over years (the duration of regular cannabis use is typically 3+ years76). Furthermore, it remains unclear to what degree homeostatic mechanisms are able to compensate for these alterations over time. Additionally, in humans there are a number of psychosocial and genetic risk factors that may predispose an individual to develop a cannabis use disorder. Given animal evidence of interactions between early life psychological deprivation and THC-induced effects on dopamine77, it is possible that the human data in cannabis-dependent participants is confounded by a range of environmental factors including parental loss, amongst other psychosocial factors105. Other environmental and physiological factors have been found to influence the dopaminergic effects of THC. Of particular potential human significance is sleep deprivation, which decreases dopamine turnover in response to THC106; and stress, which increases THC-induced dopamine synthesis107. Likewise, cannabis contains a multitude of other compounds, called phytocannabinoids, which likely exert differential and complex actions on the dopamine system which could have resulted in the varied response in human cannabis users vs. the more consistent animal data using THC only. Similarly, due to co-morbidity of addictions, it is particularly challenging for human imaging researchers to recruit participants with cannabis dependence who do not use other psychoactive compounds, including nicotine and alcohol, in more naturalistic studies.
Finally, whether THC is delivered in a contingent or non-contingent way is likely to be important given evidence that anticipation leads to dopamine release. In general, the animal studies include a variety of contingencies around the dosing of THC. In contrast, in the human acute studies THC was given to participants with a prior exposure to the drug but was not delivered in the habitual manner in which the drug is consumed, which may have reduced the degree of dopamine release.
Dopamine and the behavioural effects of THC
THC is associated with a number of behavioural effects which likely involve alterations in dopaminergic function. The first evidence of this came from early animal studies, which reported similarities between the behavioural effects of THC, including catalepsy and hypothermia108, and dopamine antagonists109. Likewise, THC antagonises the locomotor and hyperthermic effects of amphetamine, which causes dopamine release110. These seemingly paradoxical effects may be due to the high doses used in the early research together with the partial agonist effects of THC and/or non-specific receptor-independent effects.
THC promotes increased food intake in animals111 and humans112, colloquially referred to amongst users as “the munchies”. Since appetite is modulated by the dopamine system113, studies have investigated dopaminergic involvement in THC-induced feeding, finding that the dopamine D1 receptor antagonist SCH23390 attenuated THC-induced feeding at a dose that did not affect feeding on its own114. Recent research has identified CB1R mediated changes in hypothalamic pro-opiomelanocortin (POMC) neurons115 as potentially underlying this process via mitochondrial uncoupling protein 2 (UCP2), which is involved in ghrelin-mediated dopaminergic function116.
Heavy cannabis use is associated with impaired educational and occupational outcomes117. Factors that may underlie this include cognitive impairment, including involving executive dysfunction118, working memory impairments119 and amotivation120, defined as reduced motivation for goal-directed behaviour121. These functions are susceptible to mesocortical dopaminergic manipulation122 including prefrontal D1 receptor blockade123, for example. Whilst a preclinical study reported that D2 receptor antagonism blocks THC-induced working memory deficits124, this was not replicated in humans125. Nonetheless, there is recent evidence that THC-induced working memory deficits are moderated by catechol-O-methyltransferase (COMT), a key enzyme in the dopamine metabolic pathway126, which also modulates the effects of THC in adolescence on dopaminergic cells size87. Heavy chronic cannabis use produces apathetic behaviours in rhesus monkeys127 and a study in humans76 found that reduced dopamine synthesis capacity observed in heavy cannabis users was inversely related to amotivation. An overlapping feature of the amotivational syndrome associated with cannabis use disorders is negative emotionality128, such as reduced reward sensitivity and negative emotionality was also found to be inversely related to methylphenidate-induced dopamine ventral striatal dopamine release70.
The dopamine system is involved in risk for psychosis129. An early case report described increased striatal dopamine following cannabis intoxication associated with the exacerbation of psychotic symptoms in a patient with schizophrenia130. Furthermore, cannabis users with a diagnosis of schizophrenia and those at clinical high risk for schizophrenia displayed blunted striatal stress-induced dopamine release131. Although dopamine release was blunted in cannabis users with schizophrenia, it was nevertheless directly related to the induction of psychotic symptoms132. Supersensivity of post-synaptic D2 receptors65 could explain this apparent paradox, or it could be due to impaired endocannabinoid regulation of dopamine signal transduction. Supporting the latter explanation, patients with schizophrenia using high levels of cannabis show reduced anandamide levels133, and cannabidiol, a compound that elevates anandamide levels, has been shown to reduce psychotic symptoms134. Alternative post-synaptic mechanisms include CB1R-D2R heterodimerisation135 and downstream intracellular mechanisms including the neuregulin 1-erb-b2 receptor tyrosine kinase-phosphoinositide 3 kinase-protein kinase B (NRG1-ERBB4-PI3K-AKT1) pathway136.
Outlook
There is now a substantial body of evidence in animals showing that THC exerts effects on the dopamine system. The key challenges for the field must be to understand the complexity of these effects, how these translate to humans and relate to the potential negative effects of the drug in humans (Box 2). Animal studies demonstrate that acute administration of THC causes region-specific increases in dopamine release and nerve activity and we must understand the functional significance of this. The available preclinical evidence suggests that chronic THC administration causes long-term changes on the dopamine system, but a general limitation of these models is that they do not reflect typical patterns of human use. Thus future studies should be of longer duration to reflect human use. This should also include co-administration with other drugs such as nicotine and alcohol that are commonly used with cannabis. Related to this is a need to understand how the dopaminergic effects of THC are moderated by the other phytocannabinoids. Human PET studies have demonstrated blunted dopamine synthesis and dopamine release in cannabis users relative to non-users, yet we still need to understand the precise mechanisms through which this occurs.
Box 2: Strategy for future work.
Preclinical and Clinical Research
Determine the relationships between cannabis-induced alterations in the dopamine system and behavioural phenomena in humans and animal models.
Use translational techniques that can be applied in human and animal studies alike and employ study designs that better reflect patterns of human use, including modelling contingency in acute THC challenge studies
Consensus needed on dose equivalence across species.
Determine if THC-induced dopaminergic changes during key developmental phases persists into later life and if this is linked to behavioural changes
Investigate how gene variants that modulate the endocannabinoid and dopamine systems influence the sensitivity to the rewarding effects of THC and the vulnerability to addiction, amotivation and psychosis following chronic exposures
Investigate the effects of sex in THC response. This will be useful to understand the different susceptibility to cannabis effects that have been reported between males and females74.
Preclinical Research
Determine the effects of long-term THC exposures on the dopamine system alongside co-administration with nicotine to reflect typical patterns of human use.
Determine the mechanisms underlying the complex dose-response effects of THC on dopaminergic function.
Elucidate the mechanisms for regional differences in dopaminergic effects and the functional significance of this on behaviour.
Determine if the long-term effects of THC are reversible with abstinence.
Clinical Research
Determine if the blunted dopamine release and synthesis seen in chronic users is a pre-existing vulnerability factor or a direct result of repeated THC exposure.
Determine the dopaminergic changes over the course of repeated THC exposures and dose-response effects.
Consensus needed in the human literature on how to report previous exposure to cannabis use.
Determine if there are regional differences in dopamine release to THC in humans.
Determine the dose and the context dependency for THC effects on the dopamine system.
A key outstanding question remains “are the effects of THC on the dopamine system reversible, and, if not, at which point do these changes become irreversible?” Likewise, further molecular imaging studies in humans are needed to determine the dose-response and timing of the acute effects of THC on dopamine release, and to determine if there are regional differences in dopamine release, particularly between cortical and sub-cortical regions.
Ultimately, a key challenge in interpreting the animal and clinical work is the use of different techniques, some of which cannot be conducted in humans. Likewise, we must reach consensus on THC dose equivalence across species. It is therefore difficult to know what the implications of some preclinical work are for human research and how to back-translate human findings into preclinical models. We must move out of silos and use translational techniques, such as PET and MR imaging approaches that can be conducted in humans and preclinical models that capture human use patterns in combination with traditional preclinical techniques. We must also understand how the psychoactive metabolites of THC, and the endocannabinoids modulate THC-induced changes in dopaminergic function.
Given changing patterns in cannabis use across the world, particularly in young people, and the consumption of cannabis with higher THC content, there is clearly a pressing need to understand how THC alters dopaminergic function. This is especially important given the emerging evidence that dopaminergic alterations are linked to a number of the adverse cognitive and behavioural consequences of THC, and the lack of current effective biological interventions for many of the psychiatric sequelae of cannabis use. Dopaminergic dysfunction may thus represent an area for future treatment targets.
The evidence that gestational exposure to THC is associated with dysregulated dopamine synthesis in later life has major potential public health implications given the prevalence of cannabis use in women of child-bearing age and that the liberalisation of cannabis laws around the world may be associated with increased use of the drug amongst gravid and nursing mothers. However, questions about the developmental effects of THC remain. In particular, how long do the dopaminergic effects persist and what are their behavioural consequences? A related critical question is what are the effects of THC exposure during adolescence on the dopamine system?
In summary, the available evidence indicates that THC exposure produces complex, diverse and potentially long-term effects on the dopamine system including increased nerve firing and dopamine release in response to acute THC and dopaminergic blunting associated with long-term use. Future research should focus on probing the relationships between cannabis-induced alterations in the dopamine system and behavioural effects in humans and animal models.
Box 1: Glossary of methods used to assess dopaminergic activity after THC exposure.
High performance liquid chromatography (HPLC): A technique to separate, identify and quantify a particular chemical from a mixture.
In vivo electrophysiology: A method of studying single neuron responses by using microelectrodes to record from cells, including in the living animal brain.
Microdialysis: A technique used for continuous measurement of neurochemicals and metabolites by inserting a probe into a specific area of brain.
Positron Emission Tomography (PET): An imaging technique that uses a positron-emitting radiotracer that binds to specific proteins including receptors, enzymes and transporters. The gamma rays produced by the radionuclide allow quantification of proteins in living tissue non-invasively with very high chemical specificity.
Single photon emission (computed) tomography (SPECT): An imaging technique similar to positron emission tomography, but with lower spatial resolution.
Synaptosome: A homogenized mixture of isolated synaptic terminals.
Acknowledgements
We would like to thank Dr Veera Manikandan for assistance with illustrations.
Footnotes
Competing financial interests statement.
Dr Bloomfield conducts research funded by the Medical Research Council (UK), the National Institute of Health Research (UK) and the British Medical Association. Dr Ashok conducts research funded by the Medical Research Council (UK) and Kings College London. Dr Volkow is Director of the National Institute on Drug Abuse (USA). Professor Howes conducts research funded by the Medical Research Council (UK), the National Institute of Health Research (UK) and the Maudsley Charity. Professor Howes has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Astra-Zeneca, BMS, Eli Lilly, Jansenn, Lundbeck, Lyden-Delta, Servier, and Roche. Neither Professor Howes nor his family have been employed by or have holdings/a financial stake in any biomedical company.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; 2014. Vol. HHS Publication No. (SMA) 14-4863. [Google Scholar]
- 2.EMCDDA. European Drug Report 2015: Trends and Developments. European Monitoring Centre for Drugs and Drug Addiction; Lisbon: 2015. [Google Scholar]
- 3.Volkow ND, et al. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- 4.Di Forti M, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophrenia bulletin. 2014;40:1509–1517. doi: 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fergusson DM, Boden JM, Horwood LJ. Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction (Abingdon, England) 2006;101:556–569. doi: 10.1111/j.1360-0443.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- 6.Horwood LJ, et al. Cannabis and depression: an integrative data analysis of four Australasian cohorts. Drug and alcohol dependence. 2012;126:369–378. doi: 10.1016/j.drugalcdep.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Silins E, et al. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1:286–293. doi: 10.1016/S2215-0366(14)70307-4. [DOI] [PubMed] [Google Scholar]
- 8.Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychology review. 2013;23:117–137. doi: 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherek DR, Lane SD, Dougherty DM. Possible amotivational effects following marijuana smoking under laboratory conditions. Experimental and clinical psychopharmacology. 2002;10:26–38. doi: 10.1037//1064-1297.10.1.26. [DOI] [PubMed] [Google Scholar]
- 10.Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology. 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- 11.Felder CC, Veluz JS, Williams HL, Briley EM, Matsuda LA. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol Pharmacol. 1992;42:838–845. [PubMed] [Google Scholar]
- 12.Mehmedic Z, et al. Potency trends of Delta9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. Journal of forensic sciences. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 13.Spaderna M, Addy PH, D'Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology. 2013;228:525–540. doi: 10.1007/s00213-013-3188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin DM, Fossler MJ. Edible Cannabis Products: It Is Time for FDA Oversight. Journal of clinical pharmacology. 2016 doi: 10.1002/jcph.778. [DOI] [PubMed] [Google Scholar]
- 15.Varlet V, et al. Drug vaping applied to cannabis: Is “Cannavaping” a therapeutic alternative to marijuana? Scientific Reports. 2016;6:25599. doi: 10.1038/srep25599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallee M, et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huestis MA, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Archives of general psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [The psychoactive active effects of cannabis are mediated via the CB1 receptor.] [DOI] [PubMed] [Google Scholar]
- 18.Elphick MR, Egertova M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2001;356:381–408. doi: 10.1098/rstb.2000.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. British journal of pharmacology. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kathmann M, Flau K, Redmer A, Trankle C, Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn-Schmiedeberg's archives of pharmacology. 2006;372:354–361. doi: 10.1007/s00210-006-0033-x. [DOI] [PubMed] [Google Scholar]
- 21.Chartoff EH, Connery HS. It's MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol. 2014;5:116. doi: 10.3389/fphar.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollister LE, Gillespie HK. Action of delta-9-tetrahydrocannabinol. An approach to the active metabolite hypothesis. Clin Pharmacol Ther. 1975;18:714–719. doi: 10.1002/cpt1975186714. [DOI] [PubMed] [Google Scholar]
- 23.Garriott JC, King LJ, Forney RB, Hughes FW. Effects of some tetrahydrocannabinols on hexobarbital sleeping time and amphetamine induced hyperactivity in mice. Life sciences. 1967;6:2119–2128. doi: 10.1016/0024-3205(67)90232-9. [DOI] [PubMed] [Google Scholar]
- 24.Howes J, Osgood P. The effect of delta9-tetrahydrocannabinol on the uptake and release of 14C-dopamine from crude striatal synaptosoma; preparations. Neuropharmacology. 1974;13:1109–1114. doi: 10.1016/0028-3908(74)90060-4. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Ruiz J, Hernandez M, Ramos JA. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e72–91. doi: 10.1111/j.1755-5949.2010.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herkenham M, Lynn AB, Decosta BR, Richfield EK. Neuronal Localization of Cannabinoid Receptors in the Basal Ganglia of the Rat. Brain research. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- 27.Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. Journal of neurochemistry. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- 28.De Luca MA, et al. Endocannabinoid 2-Arachidonoylglycerol Self-Administration by Sprague-Dawley Rats and Stimulation of in vivo Dopamine Transmission in the Nucleus Accumbens Shell. Front Psychiatry. 2014;5:140. doi: 10.3389/fpsyt.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:1164–1176. doi: 10.1038/npp.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marinelli S, et al. N-arachidonoyl-dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:298–308. doi: 10.1038/sj.npp.1301118. [DOI] [PubMed] [Google Scholar]
- 31.Bloom AS, Dewey WL. A comparison of some pharmacological actions of morphine and delta9-tetrahydrocannabinol in the mouse. Psychopharmacology. 1978;57:243–248. doi: 10.1007/BF00426745. [DOI] [PubMed] [Google Scholar]
- 32.Hershkowitz M, Szechtman H. Pretreatment with delta 1-tetrahydrocannabinol and psychoactive drugs: effects on uptake of biogenic amines and on behavior. European journal of pharmacology. 1979;59:267–276. doi: 10.1016/0014-2999(79)90290-5. [DOI] [PubMed] [Google Scholar]
- 33.Poddar MK, Dewey WL. Effects of cannabinoids on catecholamine uptake and release in hypothalamic and striatal synaptosomes. The Journal of pharmacology and experimental therapeutics. 1980;214:63–67. [PubMed] [Google Scholar]
- 34.Aulakh CS, Bhattacharyya AK, Hossain MA, Pradhan SN. Behavioral and neurochemical effects of repeated administration of delta 9-tetrahydrocannabinol in rats. Neuropharmacology. 1980;19:97–102. doi: 10.1016/0028-3908(80)90171-9. [DOI] [PubMed] [Google Scholar]
- 35.Maitre L, Staehelin M, Bein HJ. Effect of an extract of cannabis and of some cannabinols on catecholamine metabolism in rat brain and heart. Agents and actions. 1970;1:136–143. doi: 10.1007/BF01982400. [DOI] [PubMed] [Google Scholar]
- 36.Bloom AS, Johnson KM, Dewey WL. The effects of cannabinoids on body temperature and brain catecholamine synthesis. Research communications in chemical pathology and pharmacology. 1978;20:51–57. [PubMed] [Google Scholar]
- 37.Romero J, Demiguel R, Garciapalomero E, Fernandezruiz JJ, Ramos JA. Time-Course of the Effects of Anandamide, the Putative Endogenous Cannabinoid Receptor-Ligand, on Extrapyramidal Function. Brain research. 1995;694:223–232. doi: 10.1016/0006-8993(95)00835-e. [DOI] [PubMed] [Google Scholar]
- 38.Bosier B, et al. Differential modulations of striatal tyrosine hydroxylase and dopamine metabolism by cannabinoid agonists as evidence for functional selectivity in vivo. Neuropharmacology. 2012;62:2328–2336. doi: 10.1016/j.neuropharm.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Navarro M, et al. An acute dose of delta9-tetrahydrocannabinol affects behavioral and neurochemical indices of mesolimbic dopaminergic activity. Behavioural brain research. 1993;57:37–46. doi: 10.1016/0166-4328%2893%2990059-Y. [DOI] [PubMed] [Google Scholar]
- 40.Heien ML, et al. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pistis M, et al. DELTA9-Tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: An in vivo microdialysis study. Brain research. 2002;948:155–158. doi: 10.1016/S0006-8993%2802%2903055-X. [DOI] [PubMed] [Google Scholar]
- 42.Ng Cheong Ton JM, et al. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain research. 1988;451:59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- 43.Chen JP, et al. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- 44.Castaneda E, Moss D, Oddie S, Whishaw I. THC does not affect striatal dopamine release: Microdialysis in freely moving rats. Pharmacol Biochem Behav. 1991;40:587–591. doi: 10.1016/0091-3057%2891%2990367-B. [DOI] [PubMed] [Google Scholar]
- 45.Nahas GG. In: Medical Aspects of Drug Abuse. Richter RW, editor. Harper & Row; 1975. pp. 16–36. [Google Scholar]
- 46.Chen J, Paredes W, Lowinson J, Gardner E. Strain-specific facilitation of dopamine efflux by DELTA9-tetrahydrocannabinol in the nucleus accumbens of rat: An in vivo microdialysis study. Neuroscience letters. 1991;129:136–140. doi: 10.1016/0304-3940%2891%2990739-G. [DOI] [PubMed] [Google Scholar]
- 47.Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harbor perspectives in medicine. 2012;2 doi: 10.1101/cshperspect.a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.French E. DELTA9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neuroscience letters. 1997;226:159–162. doi: 10.1016/S0304-3940%2897%2900278-4. [DOI] [PubMed] [Google Scholar]
- 49.Ali SF, et al. Chronic marijuana smoke exposure in the rhesus monkey. IV: Neurochemical effects and comparison to acute and chronic exposure to delta-9-tetrahydrocannabinol (THC) in rats. Pharmacology, biochemistry, and behavior. 1991;40:677–682. doi: 10.1016/0091-3057(91)90381-b. [DOI] [PubMed] [Google Scholar]
- 50.Navarro M, et al. Motor disturbances induced by an acute dose of DELTA9-tetrahydrocannabinol: Possible involvement of nigrostriatal dopaminergic alterations. Pharmacol Biochem Behav. 1993;45:291–298. doi: 10.1016/0091-3057%2893%2990241-K. [DOI] [PubMed] [Google Scholar]
- 51.Defonseca FR, et al. Acute Effects of Delta-9-Tetrahydrocannabinol on Dopaminergic Activity in Several Rat-Brain Areas. Pharmacol Biochem Behav. 1992;42:269–275. doi: 10.1016/0091-3057(92)90526-l. [DOI] [PubMed] [Google Scholar]
- 52.Volkow ND, et al. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res. 1996;67:29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- 53.Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 54.Borgwardt SJ, et al. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biological psychiatry. 2008;64:966–973. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Bhattacharyya S, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Hell HH, et al. Involvement of the endocannabinoid system in reward processing in the human brain. Psychopharmacology. 2012;219:981–990. doi: 10.1007/s00213-011-2428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bossong MG, et al. Further human evidence for striatal dopamine release induced by administration of 9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology. 2015 doi: 10.1007/s00213-015-3915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stokes PR, et al. Significant decreases in frontal and temporal [11C]-raclopride binding after THC challenge. NeuroImage. 2010;52:1521–1527. doi: 10.1016/j.neuroimage.2010.04.274. [DOI] [PubMed] [Google Scholar]
- 59.Barkus E, et al. Does intravenous Delta9-tetrahydrocannabinol increase dopamine release? A SPET study. Journal of psychopharmacology. 2011;25:1462–1468. doi: 10.1177/0269881110382465. [DOI] [PubMed] [Google Scholar]
- 60.Volkow ND, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. The Journal of pharmacology and experimental therapeutics. 1999;291:409–415. [PubMed] [Google Scholar]
- 61.Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD. The test-retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. NeuroImage. 2010;50:524–531. doi: 10.1016/j.neuroimage.2009.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jentsch J, Verrico C, Le D, Roth R. Repeated exposure to DELTA9-tetrahydrocannabinol reduces prefrontal cortical dopamine metabolism in the rat. Neuroscience letters. 1998;246:169–172. doi: 10.1016/S0304-3940%2898%2900254-7. [DOI] [PubMed] [Google Scholar]
- 63.Avraham Y, et al. Very low doses of delta 8-THC increase food consumption and alter neurotransmitter levels following weight loss. Pharmacology, biochemistry, and behavior. 2004;77:675–684. doi: 10.1016/j.pbb.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 64.Jentsch J, Andrusiak E, Tran A, Bowers, Roth R. delta9-Tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: Blockade of dopaminergic effects with HA966. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1997;16:426–432. doi: 10.1016/S0893-133X%2897%2900018-3. [DOI] [PubMed] [Google Scholar]
- 65.Ginovart N, et al. Chronic Delta(9)-tetrahydrocannabinol exposure induces a sensitization of dopamine D(2)/(3) receptors in the mesoaccumbens and nigrostriatal systems. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2355–2367. doi: 10.1038/npp.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [THC increased extracellular dopamine concentrations nucleus accumbens shell] [DOI] [PubMed] [Google Scholar]
- 67.Cadoni C, Valentini V, Di Chiara G. Behavioral sensitization to delta 9-tetrahydrocannabinol and cross-sensitization with morphine: differential changes in accumbal shell and core dopamine transmission. Journal of neurochemistry. 2008;106:1586–1593. doi: 10.1111/j.1471-4159.2008.05503.x. [DOI] [PubMed] [Google Scholar]
- 68.Cadoni C, Simola N, Espa E, Fenu S, Di Chiara G. Strain dependence of adolescent Cannabis influence on heroin reward and mesolimbic dopamine transmission in adult Lewis and Fischer 344 rats. Addiction biology. 2015;20:132–142. doi: 10.1111/adb.12085. [DOI] [PubMed] [Google Scholar]
- 69.Bloomfield MAP, et al. Dopaminergic Function in Cannabis Users and Its Relationship to Cannabis-Induced Psychotic Symptoms. Biological psychiatry. 2014;75:470–478. doi: 10.1016/j.biopsych.2013.05.027. [Dopamine synthesis capacity is reduced in human long term cannabis users.] [DOI] [PubMed] [Google Scholar]
- 70.Volkow ND, et al. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3149–3156. doi: 10.1073/pnas.1411228111. [Dopamine release is blunted in chronic human cannabis users.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van de Giessen E, et al. Deficits in striatal dopamine release in cannabis dependence. Molecular psychiatry. 2016 doi: 10.1038/mp.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Urban NBL, et al. Dopamine Release in Chronic Cannabis Users: A [C-11]Raclopride Positron Emission Tomography Study. Biological psychiatry. 2012;71:677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizrahi R, et al. Dopamine response to psychosocial stress in chronic cannabis users: a PET study with [11C]-+-PHNO. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:673–682. doi: 10.1038/npp.2012.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wiers CE, et al. Cannabis Abusers Show Hypofrontality and Blunted Brain Responses to a Stimulant Challenge in Females but not in Males. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leroy C, et al. Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: a high-resolution PET study. Addiction biology. 2012;17:981–990. doi: 10.1111/j.1369-1600.2011.00356.x. [DOI] [PubMed] [Google Scholar]
- 76.Bloomfield MA, Morgan CJ, Kapur S, Curran HV, Howes OD. The link between dopamine function and apathy in cannabis users: an [18F]-DOPA PET imaging study. Psychopharmacology (Berl) 2014;231:2251–2259. doi: 10.1007/s00213-014-3523-4. [DOI] [PubMed] [Google Scholar]
- 77.Zamberletti E, et al. Gender-dependent behavioral and biochemical effects of adolescent delta-9-tetrahydrocannabinol in adult maternally deprived rats. Neuroscience. 2012;204:245–257. doi: 10.1016/j.neuroscience.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 78.Cortright JJ, Lorrain DS, Beeler JA, Tang WJ, Vezina P. Previous exposure to delta9-tetrahydrocannibinol enhances locomotor responding to but not self-administration of amphetamine. The Journal of pharmacology and experimental therapeutics. 2011;337:724–733. doi: 10.1124/jpet.111.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gifford A, Gardner E, Ashby The effect of intravenous administration of delta-9-tetrahydrocannabinol on the activity of A10 dopamine neurons recorded in vivo in anesthetized rats. Neuropsychobiology. 1997;36:96–99. doi: 10.1159/000119369. [DOI] [PubMed] [Google Scholar]
- 80.French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- 81.Wu X, French E. Effects of chronic DELTA9-tetrahydrocannabinol on rat midbrain dopamine neurons: An electrophysiological assessment. Neuropharmacology. 2000;39:391–398. doi: 10.1016/S0028-3908%2899%2900140-9. [DOI] [PubMed] [Google Scholar]
- 82.Diana M, Melis M, Muntoni AL, Gessa GL. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10269–10273. doi: 10.1073/pnas.95.17.10269. [Chronic THC exposure is associated with reduced meso-accumbens dopamine neuron activity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Albrecht DS, et al. Striatal D(2)/D(3) receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug and alcohol dependence. 2013;128:52–57. doi: 10.1016/j.drugalcdep.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sevy S, et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology. 2008;197:549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stokes PRA, et al. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. Journal of psychopharmacology. 2012;26 doi: 10.1177/026988111141409021890594. [DOI] [PubMed] [Google Scholar]
- 86.Spiga S, Lintas A, Migliore M, Diana M. Altered architecture and functional consequences of the mesolimbic dopamine system in cannabis dependence. Addiction biology. 2010;15:266–276. doi: 10.1111/j.1369-1600.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 87.Behan A, et al. Chronic adolescent exposure to delta-9-tetrahydrocannabinol in COMT mutant mice: Impact on indices of dopaminergic, endocannabinoid and GABAergic pathways. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1773–1783. doi: 10.1038/npp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60:429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- 89.Renard J, Krebs MO, Le Pen G, Jay TM. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Frontiers in neuroscience. 2014;8:361. doi: 10.3389/fnins.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berghuis P, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 91.Bonnin A, de Miguel R, Hernandez ML, Ramos JA, Fernandez-Ruiz JJ. The prenatal exposure to delta 9-tetrahydrocannabinol affects the gene expression and the activity of tyrosine hydroxylase during early brain development. Life sciences. 1995;56:2177–2184. doi: 10.1016/0024-3205(95)00205-k. [DOI] [PubMed] [Google Scholar]
- 92.Walters DE, Carr LA. Perinatal exposure to cannabinoids alters neurochemical development in rat brain. Pharmacology, biochemistry, and behavior. 1988;29:213–216. doi: 10.1016/0091-3057(88)90300-0. [DOI] [PubMed] [Google Scholar]
- 93.DiNieri JA, et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biological psychiatry. 2011;70:763–769. doi: 10.1016/j.biopsych.2011.06.027. [In humans prenatal cannabis exposure decreases dopamine receptor D2 messenger RNA expression in the ventral striatum of offspring] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia-Gil L, et al. Perinatal DELTA9-tetrahydrocannabinol exposure alters the responsiveness of hypothalamic dopaminergic neurons to dopamine-acting drugs in adult rats. Neurotoxicology and teratology. 1997;19:477–487. doi: 10.1016/S0892-0362%2897%2900048-2. [DOI] [PubMed] [Google Scholar]
- 95.Mokler DJ, Robinson SE, Johnson JH, Hong JS, Rosecrans JA. Neonatal administration of delta-9-tetrahydrocannabinol (THC) alters the neurochemical response to stress in the adult Fischer-344 rat. Neurotoxicology and teratology. 1987;9:321–327. doi: 10.1016/0892-0362(87)90023-7. [DOI] [PubMed] [Google Scholar]
- 96.Scherma M, et al. Adolescent Delta(9)-Tetrahydrocannabinol Exposure Alters WIN55,212-2 Self-Administration in Adult Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:1416–1426. doi: 10.1038/npp.2015.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bossong MG, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. [Combined analysis of two previous PET studies showing acute THC causes dopamine release in humans.] [DOI] [PubMed] [Google Scholar]
- 98.Stokes P, Mehta M, Curran H, Breen G, Grasby P. Can recreational doses of THC produce significant dopamine release in the human striatum? NeuroImage. 2009;48:186–190. doi: 10.1016/j.neuroimage.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 99.Mawlawi O, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 100.Do we need an ethics of self-organizing tissue? Nat Meth. 2015;12:895–895. doi: 10.1038/nmeth.3618. [DOI] [PubMed] [Google Scholar]
- 101.Lindgren JE, Ohlsson A, Agurell S, Hollister L, Gillespie H. Clinical effects and plasma levels of delta 9-tetrahydrocannabinol (delta 9-THC) in heavy and light users of cannabis. Psychopharmacology. 1981;74:208–212. doi: 10.1007/BF00427095. [DOI] [PubMed] [Google Scholar]
- 102.Hardwick S, K L. Home Office Cannabis Potency Study. Home Office; 2008. [Google Scholar]
- 103.Hunault CC, et al. Disposition of smoked cannabis with high Delta(9)-tetrahydrocannabinol content: a kinetic model. Toxicology and applied pharmacology. 2010;246:148–153. doi: 10.1016/j.taap.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 104.Banerjee SP, Snyder SH, Mechoulam R. Cannabinoids: influence on neurotransmitter uptake in rat brain synaptosomes. The Journal of pharmacology and experimental therapeutics. 1975;194:74–81. [PubMed] [Google Scholar]
- 105.von Sydow K, Lieb R, Pfister H, Hofler M, Wittchen HU. What predicts incident use of cannabis and progression to abuse and dependence? A 4-year prospective examination of risk factors in a community sample of adolescents and young adults. Drug and alcohol dependence. 2002;68:49–64. doi: 10.1016/s0376-8716(02)00102-3. [DOI] [PubMed] [Google Scholar]
- 106.Carlini EA, Lindsey CJ, Tufik S. Cannabis, catecholamines, rapid eye movement sleep and aggressive behaviour. British journal of pharmacology. 1977;61:371–379. doi: 10.1111/j.1476-5381.1977.tb08429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.MacLean KI, Littleton JM. Environmental stress as a factor in the response of rat brain catecholamine metabolism to delta8-tetrahydrocannabinol. European journal of pharmacology. 1977;41:171–182. doi: 10.1016/0014-2999(77)90206-0. [DOI] [PubMed] [Google Scholar]
- 108.Lomax P. Acute tolerance to the hypothermic effect of marihuana in the rat. Research communications in chemical pathology and pharmacology. 1971;2:159–167. [PubMed] [Google Scholar]
- 109.Anden NE. Dopamine turnover in the corpus striatum and the lumbic system after treatment with neuroleptic and anti-acetylcholine drugs. The Journal of pharmacy and pharmacology. 1972;24:905–906. doi: 10.1111/j.2042-7158.1972.tb08912.x. [DOI] [PubMed] [Google Scholar]
- 110.Hattendorf C, Hattendorf M, Coper H, Fernandes M. Interaction between delta(9)-tetrahydrocannabinol and d-amphetamine. Psychopharmacology. 1977;54:177–182. doi: 10.1007/BF00426776. [DOI] [PubMed] [Google Scholar]
- 111.Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral delta9-THC. Physiol Behav. 1998;65:343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 112.Foltin RW, Brady JV, Fischman MW. Behavioral analysis of marijuana effects on food intake in humans. Pharmacology, biochemistry, and behavior. 1986;25:577–582. doi: 10.1016/0091-3057(86)90144-9. [DOI] [PubMed] [Google Scholar]
- 113.Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 114.Verty A, McGregor I, Mallet P. The dopamine receptor antagonist SCH 23390 attenuates feeding induced by DELTA9-tetrahydrocannabinol. Brain research. 2004;1020:188–195. doi: 10.1016/j.brainres.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 115.Koch M, et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andrews ZB, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14057–14065. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fergusson DM, Horwood LJ, Beautrais AL. Cannabis and educational achievement. Addiction. 2003;98:1681–1692. doi: 10.1111/j.1360-0443.2003.00573.x. [Longidutinal birth cohort study indicating that adolescent and early adult cannabis use is associated with reduced educational acheivement.] [DOI] [PubMed] [Google Scholar]
- 118.Hooker WD, Jones RT. Increased susceptibility to memory intrusions and the Stroop interference effect during acute marijuana intoxication. Psychopharmacology. 1987;91:20–24. doi: 10.1007/BF00690920. [DOI] [PubMed] [Google Scholar]
- 119.Ranganathan M, D'Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology. 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- 120.McGlothlin WH, W L. The marihuana problem: an overview. Am J Psychiatry. 1968;125:126–134. [PubMed] [Google Scholar]
- 121.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- 122.Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 124.Nava F, Carta G, Gessa G. Permissive role of dopamine D2 receptors in the hypothermia induced by DELTA9-tetrahydrocannabinol in rats. Pharmacol Biochem Behav. 2000;66:183–187. doi: 10.1016/S0091-3057%2800%2900231-8. [DOI] [PubMed] [Google Scholar]
- 125.D'Souza DC, et al. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology. 2008;198:587–603. doi: 10.1007/s00213-007-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tunbridge EM, et al. Genetic moderation of the effects of cannabis: catechol-O-methyltransferase (COMT) affects the impact of Delta9-tetrahydrocannabinol (THC) on working memory performance but not on the occurrence of psychotic experiences. Journal of psychopharmacology. 2015;29:1146–1151. doi: 10.1177/0269881115609073. [DOI] [PubMed] [Google Scholar]
- 127.Paule MG, et al. Chronic marijuana smoke exposure in the rhesus monkey. II: Effects on progressive ratio and conditioned position responding. The Journal of pharmacology and experimental therapeutics. 1992;260:210–222. [PubMed] [Google Scholar]
- 128.Campbell I. The amotivational syndrome and cannabis use with emphasis on the Canadian scene. Annals of the New York Academy of Sciences. 1976;282:33–36. doi: 10.1111/j.1749-6632.1976.tb49882.x. [DOI] [PubMed] [Google Scholar]
- 129.Howes OD, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Archives of general psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Voruganti LN, Slomka P, Zabel P, Mattar A, Awad AG. Cannabis induced dopamine release: an in-vivo SPECT study. Psychiatry Res. 2001;107:173–177. doi: 10.1016/s0925-4927(01)00104-4. [DOI] [PubMed] [Google Scholar]
- 131.Mizrahi R, et al. Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:1479–1489. doi: 10.1038/npp.2013.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thompson JL, et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Molecular psychiatry. 2013;18:909–915. doi: 10.1038/mp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leweke FM, et al. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: Impact of cannabis use. Schizophrenia research. 2007;94:29–36. doi: 10.1016/j.schres.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 134.Leweke FM, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- 136.Di Forti M, et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biological psychiatry. 2012;72:811–816. doi: 10.1016/j.biopsych.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 137.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nature reviews Neuroscience. 2015;16:579–594. doi: 10.1038/nrn4004. [Impaired eCB signalling dysregulates synaptic plasticity, increases stress responsivity, negative emotional states and cravings that propel addiction.] [DOI] [PMC free article] [PubMed] [Google Scholar]