Abstract
Purpose
Early diagnosis of systemic inflammatory response syndrome is fundamentally important for an effective and a goal-directed therapy. Various inflammation biomarkers have been used in clinical and experimental practice. However, a definitive diagnostic tool for an early detection of systemic inflammation remains to be identified. Acetylcholine (Ach) has been shown to play an important role in the inflammatory response. Serum cholinesterase (butyrylcholinesterase [BChE]) is the major Ach hydrolyzing enzyme in blood. The role of this enzyme during inflammation has not yet been fully understood. This study tests whether a reduction in the BChE activity could indicate the onset of the systemic inflammatory response upon traumatic injury.
Patients and methods
This observational study measured BChE activity in patients with traumatic injury admitted to the emergency room by using point-of-care-test system (POCT). In addition, the levels of routine inflammation biomarkers during the initial treatment period were measured. Injury Severity Score was used to assess the trauma severity.
Results
Altered BChE activity was correlated with trauma severity, resulting in systemic inflammation. Reduction in the BChE activity was detected significantly earlier compared to those of routinely measured inflammatory biomarkers.
Conclusion
This study suggests that the BChE activity reduction might serve as an early indicator of acute systemic inflammation. Furthermore, BChE activity, measured using a POCT system, might play an important role in the early diagnosis of the trauma-induced systemic inflammation.
Keywords: trauma, injury, early diagnostics, cholinergic, pseudocholinesterase, SIRS
Introduction
Systemic inflammation is a generalized response to noxious stimuli mediated by an activation of the innate immune system.1 An effective immunologic reaction relies on a balanced interplay of pro- and anti-inflammatory mechanisms.2,3 Cholinergic activation can modulate the immune response to limit pro-inflammatory processes to a nontoxic range. Vagus nerve stimulation releases acetylcholine (Ach), which on one hand inhibits macrophage activation and release of pro-inflammatory cytokines and simultaneously promotes the synthesis and secretion of anti-inflammatory cytokines, thereby attenuating the development of endotoxic shock in rats.4
Serum cholinesterase termed “butyrylcholinesterase” (BChE) is an enzyme that hydrolyses Ach. BChE is a nonspecific choline esterase, which is abundant in blood, liver, and brain.5 Altered serum BChE activity might indicate a disrupted Ach hydrolysis, which would, in turn, indirectly signal an imbalance between the pro- and anti-inflammatory systemic responses mediated by nonneuronal cholinergic activity.4,6 Previously, it has been shown that patients diagnosed with severe systemic inflammation show a marked reduction in the activity of serum cholinesterase.7 However, due to the high variability in the onset and etiology of the observed conditions, it was difficult to determine whether the change in the enzyme activity correlated with the emergence of the disease. Ba et al reported a dynamic change of serum cholinesterase activity following a severe traumatic injury.8 Patients with severe polytrauma showed a continuous decrease of serum cholinesterase during an observation period of 7 days. The authors suggested that the observed decrease in serum cholinesterase may be regarded as a supplemental trauma severity indicator and as a prognostic patient outcome tool.
Fast and accurate diagnosis of a systemic inflammation plays a pivotal role in intensive care therapy.9 Indeed, early goal-directed therapy that has been provided in the first 6 h following disease presentation in the emergency unit has been shown to reduce mortality by 16% over standard care.10 Various inflammation biomarkers have been proposed; however, none has proven sufficient for a prompt, specific, and accurate diagnosis of systemic inflammation.11 A combination of laboratory tests, clinical signs, and patient history record should be used in a process of diagnosing systemic inflammation.
To better understand the pattern of the BChE activity during the onset of systemic inflammation and to investigate the role of BChE in the inflammatory mechanisms, the activity of this enzyme was measured in patients within the initial 5 h following traumatic injury. Trauma is a sudden and initially pathogen-free inflammatory challenge that causes a prompt immune response. Previous studies have shown that the magnitude and duration of the immune reaction correlate well with the extent of the trauma.12,13 The role of cholinergic activity during inflammation has been suggested;4 however, rapid activity changes of the serum cholinesterase during trauma-induced inflammatory responses have not yet been investigated.
The present study shows that the activity of serum cholinesterase decreases following a traumatic injury. The observed enzyme activity change correlates with the trauma onset, suggesting that the cholinergic activity plays an important role in the acute response to an inflammatory challenge. By using a point-of-care-testing system (POCT system), measuring the dynamics of the BChE activity might prove useful for early diagnostics of the emerging systemic inflammation.
Materials and methods
Study design
This observational clinical study was approved by the Ethics Committee of the Medical Faculty of Heidelberg (Trial-Code No. S-196/2014) and was conducted in the emergency room and surgical intensive care unit of the University Hospital of Heidelberg, Germany. All patients or their legal designees gave written informed consent to the work.
Subjects
Patients were recruited upon arrival to the emergency room, following an acute traumatic injury. The criteria for the admission of injured patients to the emergency room are based on the S3-guideline for the early phase of care of the severely injured issued by the German Society for Trauma Surgery (DGU).14 Relevant patient data (demographic data, preinjury illness, medications, etc.) were collected. The trauma severity was calculated using the Injury Severity Score (ISS).15 In order to assess the BChE enzyme activity in the healthy population and validate criteria for the control group within the study population, blood samples from 10 healthy volunteers were collected for BChE analysis.
Measurements
Patients admitted to the emergency department with an ISS≤3 (n = 12 patients) were considered mildly injured or not injured and served as a control group in this study. Trauma patients with an ISS>3 had at least one body region with a moderate injury and were considered injured in this study (n = 38 patients). All patients received prompt and goal-directed treatment following admission to the hospital. Patients included in the study were in the intensive care unit (ICU) under observation and treatment for at least 5 h. Upon arrival to the emergency room, blood samples were taken for laboratory analysis.
Blood samples for BChE analysis were collected from all patients hourly for up to 5 h and kept at +4°C until measured. In order to test the blood sample stability, an independent, separate set of measurements were made, where 6 blood samples from 6 volunteers were kept at +4°C for 9 days. BChE measurements were performed daily for 9 days in order to monitor possible result variations (Figure S1). The mean coefficient of variation for all samples over 9 days was 7.01%.
After routine blood gas analysis collection (pH, pCO2, pO2, base excess, K+, Na+, Ca2+, Cl−, CO-Hb, Met-Hb, hematocrit, glucose, lactate, bicarbonate, and cSO2), taken hourly in the first 5 h after trauma, as a part of the standardized ICU diagnostic and therapeutic procedure (RAPIDPoint 500; Siemens AG, Germany), 10 µl of blood was taken for the analysis of BChE activity (1 mL Blood Gas Monovette; Sarstedt, Germany). ChE Check (Securetec Detektions-Systeme AG, Neubiberg, Germany; In-Vitro-Diagnostics Guideline 98/79/EG; DIN EN ISO 18113-2 and -3), a POCT device, was used to determine the BChE activity according to the manufacturers’ instructions. This is an enzymatic assay providing rapid and precise determination of BChE activity in human whole blood without any pretreatment of the samples. BChE activity is assayed by measuring the production of thiocholine indirectly from the hydrolysis of the specific substrate s-butyrylthiocholine iodide. Thiocholine reacts with 5,5′-dithio-bis-2-nitrobenzoic acid (DTNB, Ellman’s reagent) as a chromogenic reagent, producing the yellow 5-thio-2-nitrobenzoate anion (TNB, Ellman’s anion). The production of TNB was monitored at 470 nm.16 Enzyme activity is expressed as U/L. Standard laboratory blood analysis for routine parameters (Na+, K+, Ca2+, Cl−, creatinine, glomerular filtration rate, urea, glucose, creatinekinase [CK], CK-MB, troponin-T, lactate dehydrogenase, aspartate-aminotransferase [AST], alanine-aminotransferase [ALT], gamma-glutamyl transferase [GGT], bilirubin, pancreas-amylase, lipase, C-reactive protein [CRP], white blood cell count [WBCC], erythrocyte count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin (MCH), MCH concentration, red cell distribution width, platelets, hypochrome erythrocytes, international normalized ratio (INR), activated partial thromboplastin time) was taken at the admission and after 5, 24, and 72 h (S-Monovette, Sarstedt, Germany). All routine laboratory tests were performed in the central laboratory of the clinic according to the internal standards and manufacturers’ instructions.
The relative BChE activity change over the first 5 h following hospital admission was defined as the primary outcome. The pattern of BChE activity change was calculated as follows: relative activity change for the given time point was calculated as the ratio between the measured absolute enzyme activity for the given time point divided by the enzyme activity at admission. Ratios obtained over 5 h were added together. A sum ≤5 was considered as a negative activity change and a sum >5 a positive activity change. Secondary outcomes are correlation of the enzyme activity change with the ISS, CRP, WBCC, and liver function tests.
Statistical analysis
Cohen’s d test (d = 0.57; power 80%, significance level alpha =5%) was used to determine the sample size. The resulting data were entered into an electronic database (Microsoft Excel 2010, Microsoft Corp., Redmond, WA, USA) and evaluated using GraphPad Prism version 6.0h for Mac (GraphPad Software, La Jolla, CA, USA, www.graphpad.com). Data were tested for Gaussian distribution using D’Agostino and Pearson omnibus normality test and presented as median with inter-quartile range (IQR). Statistical significance was tested using Friedman, Mann–Whitney U, and Kruskal–Wallis test. Post hoc analysis was performed by using Dunn’s multiple comparison test including a correction for multiple comparisons. Correlation analysis was performed using Spearman’s rank correlation test. P<0.05 was considered statistically significant.
Results
The study included 50 patients who were admitted to the emergency room following a traumatic injury and 10 volunteers whose blood samples were collected once. The time point of the arrival to the emergency room marked the initial measurement of our analysis. Patient demographic information and clinical data are listed in Table 1. Patients with an ISS≤3 served as a control and patients with an ISS>3 during admission were considered injured in this study (see “Materials and methods” section).
Table 1.
Patient demographic and clinical data obtained upon arrival to the emergency department
| Number of patients | 50 |
| Age (years) | 49 (28–59)* |
| Gender (male/female) | 30/20 |
| Traumatic injury | |
| ISS | 6 (4–13)* |
| ISS≤3 | 12 |
| ISS>16 | 9 |
| ISS>25 | 2 |
| Duration of prehospital emergency care (minutes) | 60 (46–68)* |
| ISS body regions | |
| Head or neck | 28 |
| Face | 8 |
| Chest | 27 |
| Abdomen or pelvic contents | 11 |
| Extremities or pelvic girdle | 26 |
| External | 18 |
| Clinical data | |
| Preinjury Illness | 12 |
| Cardiovascular | 9 |
| Neurologic | 1 |
| Metabolic | 2 |
| Patient outcome | |
| Hospital stay duration of trauma patients (days) | 6 (1–12)* |
| Survivors | 48 |
Note:
Median (interquartile range).
Abbreviation: ISS, Injury Severity Score.
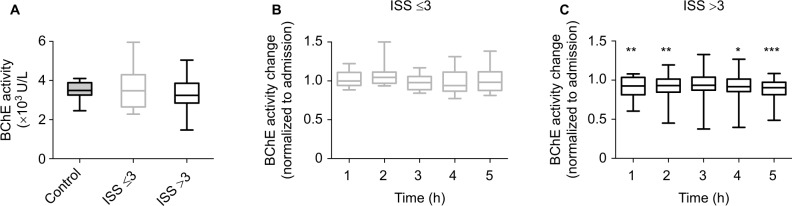
By using a POCT, the enzyme activity of BChE was measured every hour for the duration of 5 h. In addition, BChE enzyme activity was measured once from blood samples collected from 10 healthy volunteers. The BChE enzyme activity measured at the admission to the emergency room did not differ between the groups (Figure 1A and Table S1). The initial BChE activity measured from all the patients at hospital admission was comparable to that observed in the healthy volunteers (Table S1).
Figure 1.
BChE activity reduction in patients with traumatic injury.
Notes: (A) Initial BChE activity measured at the hospital admission did not differ between healthy volunteers (control, filled gray), patients with an ISS≤3 (open gray), and those with ISS>3 (black, Kruskal–Wallis test followed by Dunn’s multiple comparisons test). Blood samples from patients without severe injury (ISS≤3, gray, B) and patients with traumatic injury (ISS>3, black, C) were tested for BChE activity changes over 5 h following hospital admission. Values represent relative change of BChE activity, normalized to the initial enzyme activity recorded on admission (A). Box plots in panels (A), (B), and (C) represent medians with 25% and 75% percentiles; error bars are minimum and maximum values. *P<0.05; **P<0.01; ***P<0.001. Observed BChE activity change has been tested using Friedman test for repeated measures followed by Dunn’s multiple comparisons test.
Abbreviations: BChE, butyrylcholinesterase; ISS, Injury Severity Score.
Compared to their initial BChE activity, control group did not show any difference in the relative BChE activity change throughout the observation time of 5 h (Figure 1B and Table S1). Relative change of BChE activity measured from patients with the traumatic injury was markedly reduced from 1 h after being admitted. Surprisingly, there was no difference in the relative change of BChE activity after 3 h of admission. However, the relative change of BChE activity remained reduced in these patients when measured 4 and 5 h after hospital admission (Figure 1C and Table S1).
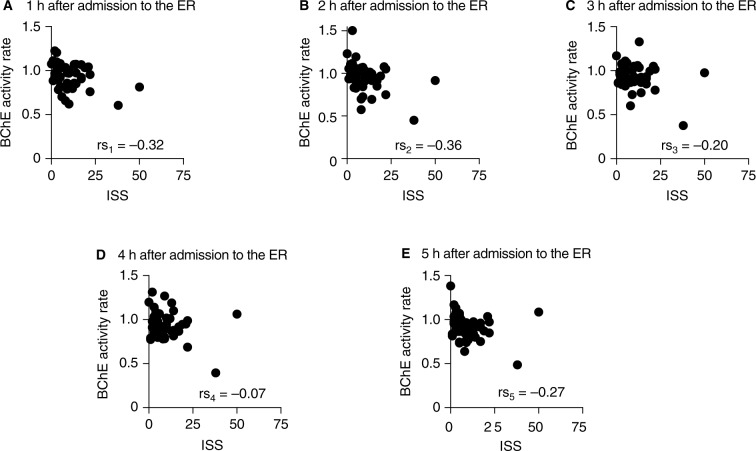
Next, the study questioned whether the BChE enzyme activity correlates with the severity of the trauma. The ISSs did not correlate with the BChE activity at admission to the emergency unit or at any of the individual time points during the 5 h following admission (Figure S2A–F). However, the BChE activity change relative to the initial time point (ie, at admission) did correlate with the ISS at 1 and 2 h after the time of admission (Figure 2A and B). No correlation was observed 3, 4, and 5 h after the admission (Figure 2C and E). A similar correlation between BChE activity and the trauma severity was also seen when using the New Injury Severity Score (NISS) (Figures S3A–F and S4A–E).
Figure 2.
Correlation between the relative change of the BChE activity and the ISS of patients admitted to the emergency unit after traumatic injury.
Notes: BChE activity change (normalized to admission) correlated with the ISS when measured 1 and 2 h after admission to the ER (A and B, respectively). The correlation was lost when measured 3–5 h after admission (C–E). rs1–5 is the Spearman’s rank correlation coefficient.
Abbreviations: BChE, butyrylcholinesterase; ER, emergency room; ISS, Injury Severity Score.
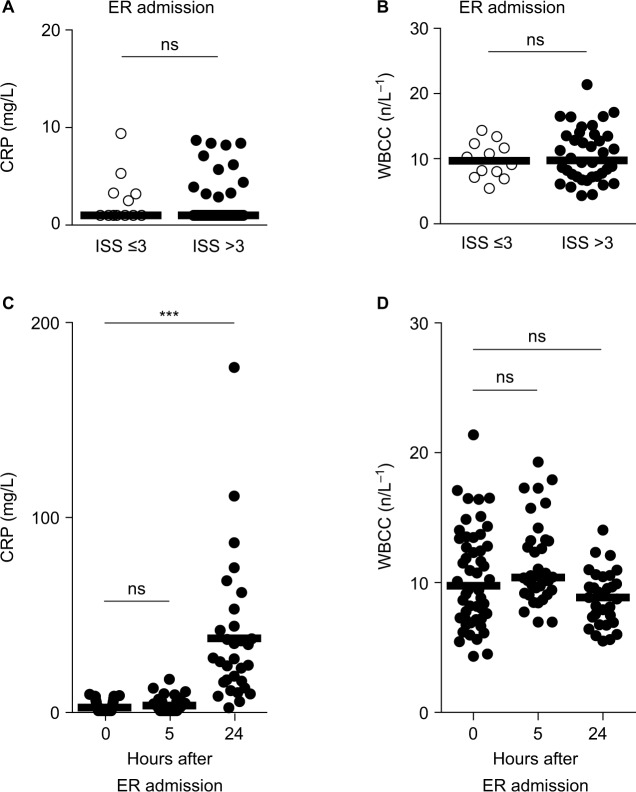
The initial blood analysis at the arrival to the emergency unit included measuring inflammation biomarkers (CRP and WBCC). Hospital records and inflammation biomarkers measured on admission did not indicate any chronic inflammatory diseases or ongoing inflammation in any of the patients. In addition the initial laboratory testing for CRP and WBCC concentrations of all patients included in the study showed CRP and WBCC concentrations within the normal range (Figure 3A and B). Thus, this study presumes that there was no chronic or recent inflammation in these patients and the traumatic injury could be considered an acute inflammatory insult in these patients.
Figure 3.
CRP activity changes 24 h after traumatic injury.
Notes: Blood samples from patients admitted to the ER were taken for laboratory tests at the admission, as well as 5 and 24 h later. (A and B) CRP activity and WBCC did not differ between patients with traumatic injury (ISS >3, closed circles) and their controls (ISS≤3, open circles) when measured at the admission to the ER (Mann–Whitney U-test). In trauma patients, CRP activity (C) and WBCC (D) did not change when measured 5 h after admission. CRP but not WBCC was greatly increased 24 h after admission (C and D, right scatter plot). ***P<0.001; Kruskal–Wallis test followed by Dunn’s multiple comparisons test.
Abbreviations: CRP, C-reactive protein; ER, emergency room; ISS, Injury Severity Score; WBCC, white blood cell count; ns, not significant.
The CRP and WBCC concentrations were within the laboratory reference range in all patients in both the patient groups upon admission to the ICU (Figure 3A and B and Table S2) and remained unchanged 5 h after admission to the hospital (Figure 3C and D and Table S2). Elevated CRP concentration was observed 24 h after hospital admission in the group of trauma injured patients (Figure 3C and Table S2). CRP concentration, measured at this time point, correlated with ISS (Spearman r = 0.56). CRP concentration remained elevated when measured 72 h later in the injured patients (Table S2). The analysis of the WBCC performed 24 h following trauma revealed no significant change in the WBCC (Figure 3D and Table S2). Blood analysis was not performed at the 24 h time point for patients with mild or no injuries (ISS≤3) because they were discharged from the hospital after 6 h.
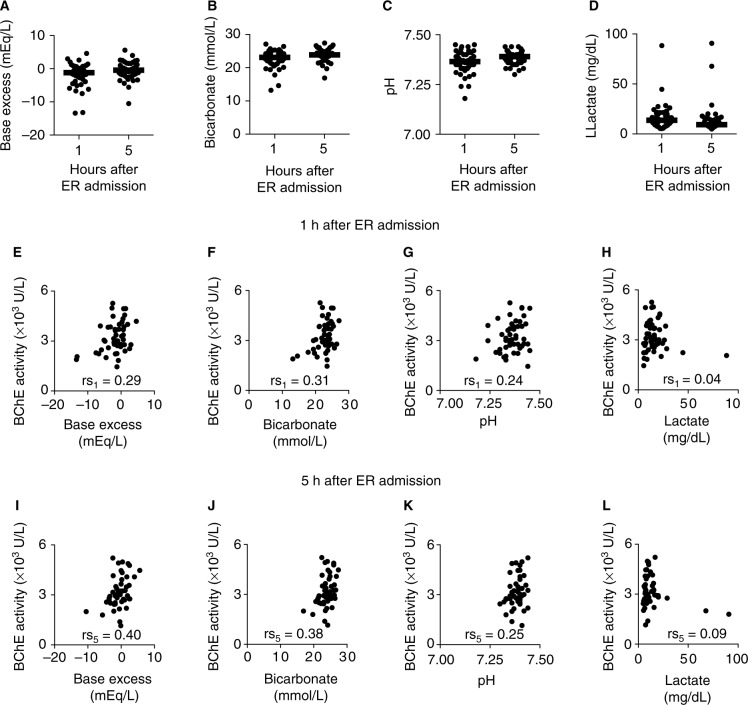
Altered base excess, bicarbonate concentration, pH, and lactate concentration in blood, measured upon traumatic injury, have been associated with poor patient outcome. To test whether BChE activity correlates with the activity changes of these parameters, measurements were taken 1 and 5 h after being admitted in the hospital. The analysis revealed that mean levels of base excess, bicarbonate, pH, and lactate measured after first and fifth hour following hospital admission remained in the normal laboratory range (Figure 4A–D and Table S3). Next, whether these parameters correlate with the trauma severity of the patients was tested. The analysis revealed no correlation between the ISSs and the base excess or bicarbonate concentration at 1 or 5 h after admission (Figure S5A–D). Correlation between ISS and pH as well as lactate concentration was observed 1 h (Figure S5E and G) but not 5 h after hospital admission (Figure S5F and H). Moreover, whether levels of the metabolic state indicators correlate with BChE enzyme activity during the initial post-traumatic time period was tested. The analysis revealed that the BChE enzyme activity correlated with base excess and bicarbonate concentration throughout the first 5 h after ICU admission (Figure 4E, F, I and J and Table S3). No correlation was observed between BChE activity and pH or lactate concentration throughout the first 5 h in either group (Figure 4G, H, K, and L and Table S3).
Figure 4.
BChE activity correlates with the indicators of the metabolic state change following traumatic injury.
Notes: Scatter plots show base excess (A), bicarbonate concentration (B), pH (C), and lactate concentration (D) measured in blood of trauma patients 1 and 5 h following hospital admission. Base excess (E and I) and bicarbonate (F and J) concentration correlated with the BChE activity throughout the first 5 h after hospital admission. No correlation was observed between BChE activity and pH (G and K) or serum lactate concentration (H and L) during the first 5 h of hospital stay. rs1, rs5: Spearman’s rank correlation coefficient.
Abbreviations: BChE, butyrylcholinesterase; ER, emergency room.
These data suggest that the relative change in BChE activity within the first 2 h after hospital admission might indicate a systemic inflammation. Base excess and bicarbonate concentration, conventional early indicators of poor outcome after traumatic injury, correlated with the BChE activity changes when measured within the first 5 h after hospital admission.
BChE is an enzyme synthesized in liver. Disrupted liver synthesis might affect BChE activity. By using a combination of standard laboratory liver function tests, namely AST, ALT, GGT, alkaline phosphatase (AP), bilirubin, and INR, whether the trauma patients suffered from liver dysfunction was assessed. Based on ISS, the patients were divided into abdominal-trauma and no-abdominal-trauma groups. Patients with abdominal trauma did show a mild elevation of AST and ALT compared to those without abdominal trauma. However, median values stayed within the normal range, suggesting no indication of an acute liver failure within the two groups, when measured at the hospital admission (Figure S6A–C and Table S4). To further assess the interdependence of BChE activity and the liver function following abdominal trauma, the tests were repeated 5 h after injury. An elevation of AST, ALT, and GGT in the group of patients with abdominal trauma could be observed (Figure S6E–G and Table S4). The observed elevated levels of AST, ALT, and GGT were within the normal range. There was no difference between two groups with regard to AP, bilirubin, or INR function tests. Next, the BChE activities among the two groups measured at the admission to the emergency unit and 5 h after admission were compared. The BChE remained unaltered between the two groups (Figure S6D and H and Table S4).
Taken together, these data suggest that the change in BChE activity may serve as an early indicator of acute systemic inflammation in trauma patients. Measuring enzyme activity by means of a POCT system, in combination with conventional methods, enables a quick diagnosis in an emergency care environment.
Discussion
This study shows that the BChE activity decreases 1 h after hospital admission in patients with traumatic injury categorized with ISS>3. Furthermore, individuals with ISS≤3 showed no alteration in the BChE activity during the observation period of 5 h. In contrast, the conventional laboratory tests for inflammation (eg, CRP and WBCC) measured from injured patients did not deviate from the normal range within 5 h of admission. Our results suggest that using a POCT system to measure BChE activity in the initial phase of trauma care might help to identify patients at risk of an emerging inflammatory response. Early detection of reduced BChE levels and intervention during the primary therapeutic and diagnostic procedures in the emergency department and intensive care unit should improve patient outcome. Implementation of greater vigilance, escalation of monitoring strategies, or intensifying the supporting therapy represent a few treatment suggestions for patients showing reduced BChE activity during their hospital stay.
Ba et al8 showed that serum cholinesterase activity in patients with severe trauma (ISS>25) is strongly decreased. This change, observed within 7 days following trauma correlated with the CRP and injury severity scores.8 Our results support these findings. Moreover, a change in the BChE activity as early as 1 h after hospital admission was observed. In addition, this data analysis detected a reduction in the BChE activity even in patients with notably lower trauma scores (starting from ISS>3) than those described by Ba et al. Thus, monitoring BChE activity changes within the first 2 h after hospital admission may be sensitive indicator for an early identification of systemic inflammation.
Ofek et al validated the importance of Ach in the acute phase of the immune response and suggested that cholinergic modulation of the immune system might affect individual differences in patient outcome.6 The authors determined an individual cholinergic status by measuring plasma levels of acetylcholinesterase (AChE), BChE, and paraoxonase (PON1) activity 1.5 h following endotoxic challenge. Based on the ability to recruit the immune system in response to endotoxic challenge, the authors described two types of inflammation responders: individuals with an increased activity of enzymes (enhancers) and individuals showing decreased enzyme activity following endotoxic challenge (suppressors). Data from the present study supported this finding by identifying two distinct patterns of the BChE activity change in trauma patients during the first 2 h following hospital admission (see “Materials and methods” section), those with a net decrease in BChE activity (79% of patients), suggesting an anti-inflammatory response and those with net increase in BChE activity (21% of patients), indicating a pro-inflammatory reaction (Figure S7 and Table S1).
A bidirectional enzyme activity change, apparent 1–2 hours after admission, compares favorably with the results described by Ofek et al. Data from the present study confirm the existence of individual variabilities in the early cholinergic response to inflammation (cholinergic status) and demonstrates an early onset of this response.
CRP is an early marker of inflammation and infection. It has extensively been utilized in the clinical environment for diagnosing and monitoring the disease activity. CRP is normally present in trace levels in blood but increases during the first 24 h in response to an inflammatory or infectious process.17–19 Data from the present study suggest that an increase in CRP levels is not apparent within the first 5 h following hospital admission.
Previous studies report a peak response for WBCC after sterile inflammation to be 24–48 h. Moreover, WBCC appears not to reflect the magnitude of the injury. The results of the present study are in accordance with a previous report20 as no change in the WBCC in the first 5 h after hospital admission was observed.
Compared to the conventional laboratory tests that are used to diagnose inflammation (CRP and WBCC), BChE activity change can be detected much earlier, indicating an emerging systemic inflammation. A reduction in BChE activity within the first 2 h after admission to ICU might signal an ongoing inflammation which is first detected by an increased blood CRP level 24 h later. POCT system, available at the bedside, enables early identification of high-risk patients, thereby allowing for quicker diagnosis and therapy of systemic inflammation.
Base excess, blood bicarbonate, albumin, and lactate concentration have long been recognized as independent predictors of mortality in trauma patients.21–23 These parameters did not correlate with the severity of injury in this study most likely because of the relatively low severity of injury in the patient data set (19% showed ISS>16), as well as the brief time period between trauma and hospital admission, providing prompt commencement of therapy. The base excess and blood bicarbonate correlated with the BChE activity when measured in trauma patients 1 h, as well as 5 h after hospital admission, suggesting that BChE activity might either interact with the acid–base balance in serum or that the change in the BChE activity might mirror the severity of trauma-induced inflammation. This analysis revealed that BChE activity does not correlate with the pH measured from trauma patients, supporting the latter assumption.
Albumin concentration has been associated with trauma severity.24 Reduced serum albumin concentration correlates with the increased mortality of critically ill patients.25,26 Previously, it has been shown that the BChE activity correlates with the albumin concentration in critically ill patients with severe systemic inflammation.7 However, in the present study, no correlation was observed between albumin concentration and the BChE activity in trauma patients.
BChE is synthesized in liver, and its activity strongly depends on liver function.27 Indeed, it was observed that liver function tests obtained from patients with abdominal trauma varied in comparison with their controls. Patients with abdominal trauma demonstrated isolated higher transaminase levels compared to those without abdominal trauma. However, these results were still in the reference laboratory range. No difference in the relative change of BChE activity between the two groups was observed. These data suggest that the BChE activity remains unaffected upon mild liver dysfunction during the initial period following a traumatic injury.
Several limitations should be considered when interpreting the findings. First, this was a single center observational pilot trial with a relatively small study group, which potentially reduced the power to detect some differences (eg, lactate concentration and WBCC). It would be necessary to repeat the study in a larger patient group to further investigate the correlation between BChE activity change and the secondary outcome parameters during acute traumatic injury.
Second, patients included in this study, presented with rather low injury scores (median ISS = 6). This finding, being unanticipated, could have obscured the effect of BChE changes on the study outcomes; however, a rapid and significant change in BChE activity, observed in mild traumatic injury but not in the control, renders this assay quick and very sensitive in detecting inflammatory response changes. Indeed, 68% of patients with reduced BChE activity observed within first 5 h following traumatic injury stayed in hospital longer than 2 days, suggesting that early change in BChE activity might be predictive of subsequent systemic complications.
Third, any further inflammatory biomarker analysis (eg, IL-6, TNF-alpha, and procalcitonine) which would require additional patient blood samples and ethical clearance was not included. Next, there are concomitant factors that might affect the activity of BChE in trauma patients. One possible scenario might be that a trauma patient is found to have positive toxicology screenings upon admission. Substance abuse is frequently associated with the events resulting in a trauma and can often play an important role in the subsequent medical treatment of these patients.28 The patients were tested for ethanol in the blood. Only 6% of patients included in this study were positive, showing no significant difference in BChE activity compared to their controls. Thus, it is presumed that elevated serum ethanol concentration did not bias the analysis and interpretation of BChE activity.
The initial time point for the data analysis in this study is the arrival of the patient to the hospital. It was estimated that the average time from injury until the admission to the emergency unit was 60 min. However, due to the high inter-case variability and lack of accurate prehospital information, this time interval was not included in the data analysis and result interpretation. However, time period from traumatic injury until hospital admission should be considered while interpreting the data.
Finally, several conditions are known to affect BChE activity in the blood. An elevated BChE has been described in patients with a nephrotic syndrome.29 A possible reason for an elevated BChE concentration is a compensatory elevation of albumin synthesis in hepatocytes as a response to a massive protein loss during nephrotic disease. In contrast, a number of conditions may cause decreased BChE activity. Liver disease may cause decreased BChE activity. Further laboratory tests (eg, transaminases, bilirubin, and coagulation) and clinical signs could help distinguish between inflammation and liver disease. Reduced level of BChE has been observed in the last trimester of pregnancy.30 Decreased cholinergic status has been reported in burn patients31 and is associated with an increased risk for MACE32 and presence of carcinoma.33 Thus, only a combined diagnostic approach, comprising various laboratory tests and clinical examinations would identify the most effective treatment decision in the therapy of acute inflammation. Therefore, monitoring BChE activity level, along with a relative change of BChE activity during the initial inflammatory process may prove to be of substantial importance.
Conclusion
This study suggests that measuring the relative change of BChE activity by using POCT system might prove helpful for a rapid indication/prediction of an emerging inflammation in patients with trauma. The reduction in the enzyme activity is apparent 1–2 h after hospital admission, which makes this assay one of the earliest indicators of inflammation described so far. Measuring BChE activity by using a POCT system at the bed side minimizes time delays often associated with conventional laboratory testing. This assay might prove beneficial in early recognition of emerging inflammation permitting early commencement of anti-inflammatory therapy.
Acknowledgments
The authors would like to thank Dr C Peter Bengtson for critically reading the manuscript. This study was carried out with financial resources of the Department of Anesthesiology, Heidelberg University Hospital, Germany.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rock K, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–243. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 4.Borovikova L, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 5.Kaplay SS. Acetylcholinesterase and butyrylcholinesterase of developing human brain. Biol Neonate. 1976;28:65–73. doi: 10.1159/000240805. [DOI] [PubMed] [Google Scholar]
- 6.Ofek K, Krabbe K, Evron T, et al. Cholinergic status modulations in human volunteers under acute inflammation. J Mol Med. 2007;85:1239–1251. doi: 10.1007/s00109-007-0226-x. [DOI] [PubMed] [Google Scholar]
- 7.Zivkovic AR, Schmidt K, Sigl A, Decker SO, Brenner T, Hofer S. Reduced serum butyrylcholinesterase activity indicates severe systemic inflammation in critically ill patients. Mediators Inflamm. 2015;2015:274607. doi: 10.1155/2015/274607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ba L, Wu D, Qian A, Zhang M, Xiong B. Dynamic changes of serum cholinesterase activity after severe trauma. J Zhejiang Univ Sci B. 2014;15:1023–1031. doi: 10.1631/jzus.B1400129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 10.Rivers EP, Katranji M, Jaehne KA, et al. Early interventions in severe sepsis and septic shock: a review of the evidence one decade later. Minerva Anesthesiol. 2012;78:712–724. [PubMed] [Google Scholar]
- 11.Pierrakos C, Vincent J-L. Sepsis biomarkers: a review. Crit Care. 2010;14:1–18. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shih HC, Su CH, Lee CH. Alternations of surface antigens on leukocytes after severe injury: correlation with infectious complications. Intensive Care Med. 1998;24:152–156. doi: 10.1007/s001340050537. [DOI] [PubMed] [Google Scholar]
- 13.Choileain N, Redmond P. Cell response to surgery. Arch Surg. 2006;141:1132–1140. doi: 10.1001/archsurg.141.11.1132. [DOI] [PubMed] [Google Scholar]
- 14.Ruchholtz S, Bail H, Bardenheuer M, et al. Die S3-Leitlinie Polytrauma. Notfallmedizin. 2011;6:e33–e64. [Google Scholar]
- 15.Baker SP, O’Neill B, Haddon W, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 16.Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chimica Acta Int J Clin Chem. 1999;288:73–90. doi: 10.1016/s0009-8981(99)00144-8. [DOI] [PubMed] [Google Scholar]
- 17.Kolb-Bachofen A review on the biological properties of C-reactive protein. Immunobiology. 1991;183:133–145. doi: 10.1016/S0171-2985(11)80193-2. [DOI] [PubMed] [Google Scholar]
- 18.Gewurz H, Mold C, Siegel J, Fiedel B. C-reactive protein and the acute phase response. Adv Intern Med. 1982;27:345–372. [PubMed] [Google Scholar]
- 19.Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992;37:313–336. [PubMed] [Google Scholar]
- 20.Hoffmann C, Hoffmann P, Zimmermann M. Diagnostic testing for a high-grade inflammation: parameter dynamics and novel markers. Clin Chem Lab Med. 2015;53:541–570. doi: 10.1515/cclm-2014-0482. [DOI] [PubMed] [Google Scholar]
- 21.Tremblay LN, Feliciano DV, Rozycki GS. Assessment of initial base deficit as a predictor of outcome: mechanism of injury does make a difference. Am Surg. 2002;68:689–693. [PubMed] [Google Scholar]
- 22.Guyette F, Suffoletto B, Castillo J-L, Quintero J, Callaway C, Puyana J-C. Prehospital serum lactate as a predictor of outcomes in trauma patients: a retrospective observational study. J Trauma Acute Care Surg. 2011;70:782–786. doi: 10.1097/TA.0b013e318210f5c9. [DOI] [PubMed] [Google Scholar]
- 23.Laulund A, Lauritzen J, Duus B, Mosfeldt M, Jørgensen H. Routine blood tests as predictors of mortality in hip fracture patients. Injury. 2012;43:1014–1020. doi: 10.1016/j.injury.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Garwe T, Albrecht R, Stoner J, Mitchell S, Motghare P. Hypoalbuminemia at admission is associated with increased incidence of in-hospital complications in geriatric trauma patients. Am J Surg. 2016;212:109–115. doi: 10.1016/j.amjsurg.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleck A, Hawker F, Wallace PI, Raines G, Trotter J. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1:781–784. doi: 10.1016/s0140-6736(85)91447-3. [DOI] [PubMed] [Google Scholar]
- 26.McCluskey A, Thomas AN, Bowles BJ, Kishen R. The prognostic value of serial measurements of serum albumin concentration in patients admitted to an intensive care unit. Anaesthesia. 1996;51:724–727. doi: 10.1111/j.1365-2044.1996.tb07883.x. [DOI] [PubMed] [Google Scholar]
- 27.Jokanović M, Maksimović M. Abnormal cholinesterase activity: understanding and interpretation. Eur J Clin Chem Clin Biochem. 1997;35:11–16. [PubMed] [Google Scholar]
- 28.Gustafson M, Hollosi S, Chumbe J, Samanta D, Modak A, Bethea A. The Effect of ethanol on lactate and base deficit as predictors of morbidity and mortality in trauma. Am J Emerg Med. 2015;33:607–613. doi: 10.1016/j.ajem.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farber M. Serum cholinesterase in patients with proteinuria. Acta Med Scand. 1943;115:475. [Google Scholar]
- 30.Shnider SM. Serum cholinesterase activity during pregnancy, labor and the puerperium. Anesthesiology. 1965;26:335–339. doi: 10.1097/00000542-196505000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Viby-Mogensen J, Hanel HK, Hansen E, Sorensen B, Graae J. Serum cholinesterase activity in burned patients. I: biochemical findings. Acta Anaesthesiol Scand. 1975;19:159–168. doi: 10.1111/j.1399-6576.1975.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 32.Arbel Y, Shenhar-Tsarfaty S, Waiskopf N, et al. Decline in serum cholinesterase activities predicts 2-year major adverse cardiac events. Mol Med. 2014;20:38–45. doi: 10.2119/molmed.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaniaris P, Fassoulaki A, Liarmakopoulou K, Dermitzakis E. Serum cholinesterase levels in patients with cancer. Anesth Analg. 1979;58:82–84. [PubMed] [Google Scholar]