Abstract
Background
Cingulate gyrus epilepsy is controversial because it may overlap with other frontal lobe epilepsy syndromes. Reported cases are rare in the pre–magnetic resonance imaging literature but are more common thereafter. Information about peri-ictal and ictal behaviors is scarce.
Objectives
To characterize epilepsy originating from the cingulate gyrus and to report surgical outcomes.
Design
Case studies.
Setting
Academic research.
Patients
We report 3 surgically treated cases of cingulate gyrus epilepsy, with seizure-free or almost seizure-free outcomes. The cases were identified from a database of 4201 consecutive epilepsy monitoring unit admissions since October 1998 through September 2008. All 3 cases involved cingulate lesions.
Main Outcome Measures
Neuroimaging, video electroencephalographic, pathologic, and surgical outcome data were reviewed.
Results
All 3 patients had lesional left anterocingulate seizures confirmed by magnetic resonance imaging and experienced cessation of seizures after lesionectomy. Two patients had auras (fear and laughter) previously associated with cingulate gyrus epilepsy. All patients had clinical features consistent with frontal lobe epilepsy, including hyperkinetic behavior and ictal vocalization. Two patients had behavioral changes with aggression, personality disorder, and poor judgment; some behavioral episodes lasted for days and were socially devastating. One patient, a commercial pilot, showed behavior as a passenger that resulted in a diversionary landing. The other patient demonstrated behavior that led to his arrest, and he was almost arrested again in the hospital for threatening security officers. Aberrant behaviors in all 3 patients completely resolved after lesionectomy.
Conclusions
Lesional cingulate gyrus epilepsy is uncommon. Our 3 confirmed cases included 2 patients with unique and severe behavioral changes that resolved with lesionectomy.
Cingulate gyrus epilepsy is controversial because it may have distinct clinical features or it may overlap with other frontal lobe epilepsy syndromes. The term anterior cingulate epilepsy was first used in 1957 by Andy and Chinn1 to describe an experimental epilepsy model in cats. In 1970, Mazars2 published “Criteria for Identifying Cingulate Epilepsies” and reported 36 cases. This study predated magnetic resonance (MR) imaging and computed tomography (CT), and localization by intracerebral depth electrodes was problematic by today’s standards. In 1989, the International League Against Epilepsy3 included cingulate epilepsy as a type of frontal lobe epilepsy in the proposed classification of epilepsy and epileptic syndromes. The commission first described general features that strongly suggest an epilepsy diagnosis of frontal origin (short seizures, minimal or no postictal confusion, rapid progression to secondary generalized seizures, prominent motor manifestations, complex gestural automatisms, and frequent falling when the discharge is bilateral), and then briefly described seizure subtypes. Cingulate gyrus epilepsies were described as consisting of complex partial seizures with complex motor gestural automatisms at onset, autonomic signs, and changes in mood and affect. Approximately 3 years later, Chauvel et al4 published a review of patients with anterocingulate epilepsy. They attempted to describe a syndrome based on 66 seizures in 16 patients, who were not discussed or described individually; it was unclear how the epileptic focus was identified. They described intense manifestations exceeding those produced by electrical stimulation, with intense fright, facial expression of fear, and incomplete loss of consciousness. In 2000, Williamson et al5 debated the existence of such an entity, claiming that it is hard to distinguish clinically between anterocingulate epilepsy and other frontal lobe syndromes.
METHODS
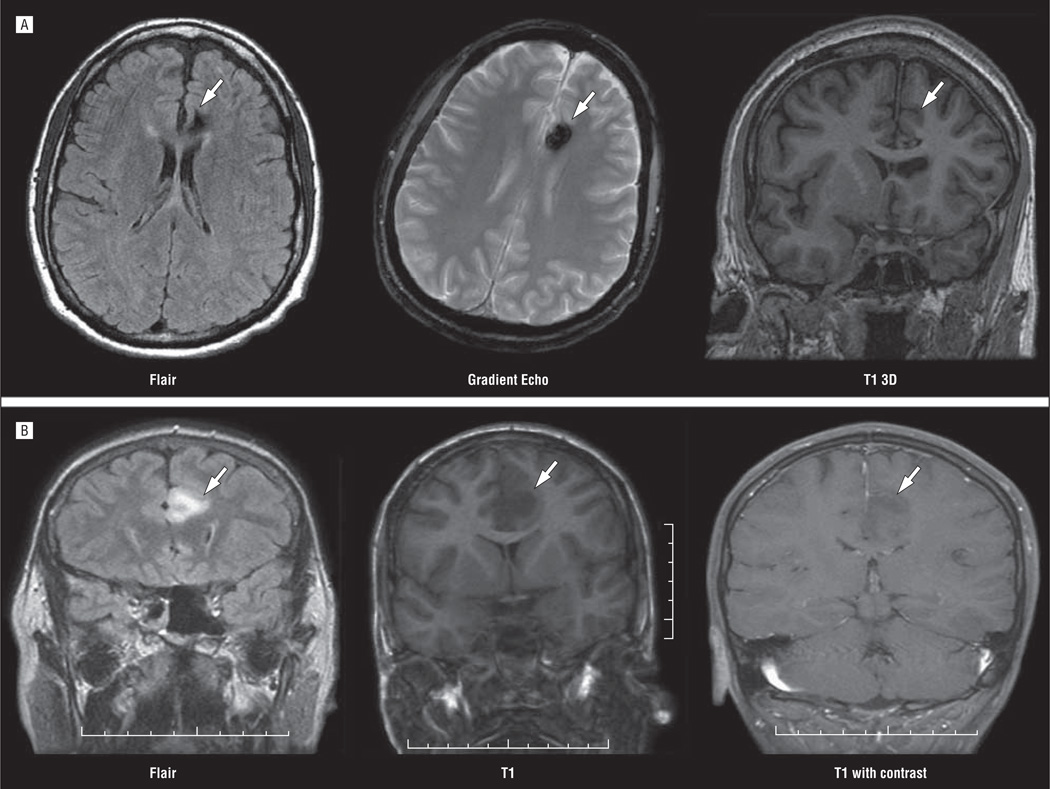
We report 3 surgically treated cases of lesional cingulate gyrus epilepsy, with early seizure-free outcomes. Cases were identified from a database of 4201 consecutive epilepsy monitoring unit admissions from October 1998 through September 2008, at Parkland Memorial Hospital and the University of Texas Southwestern Epilepsy Center. Cases with a mesial frontal focus were then limited to 3 patients having seizures originating from the cingulate gyrus. The epileptogenic zone was confirmed by MR imaging (Figure), video electroencephalogram (EEG), intraoperative electrocorticography, single-photon emission CT images, neuropsychological evaluation, and good postsurgical reduction in seizure frequency. We reviewed the history, clinical manifestations, radiological findings, neurophysiological data, and surgical outcomes. Written consent to approve research on cases is routinely obtained from all patients before proceeding with EEG monitoring.
Figure.
Magnetic resonance images showing old left anterocingulate hemorrhage (arrows) in case 1 (A) and left cingulate glioma (arrows) in case 3 (B).
The EEG for case 2 (Table 1) was recorded using a commercially available system (BMSI; Nicolet, Milwaukee, Wisconsin). The EEGs for the other 2 cases were recorded using a digital video system (Stellate; Natus Medical Incorporated, San Carlos, California) with a 250-Hz sampling rate and the 10-10 modified combinatorial nomenclature system of electrode placement with the addition of ECG, FT9–10, TP9–10, AF7–8, FPz, Oz, Cz, and Fz. Ictal recordings and behavioral events were identified by the patient, family, or epilepsy monitoring unit staff or by computer. Informative ictal and interictal digital EEG samples were analyzed by montage reformatting using referential, bipolar, mean reference, and Laplacian montages with digital high-pass and low-pass filtering or 60-Hz notch filtering as needed for optimal waveform display.
Table 1.
Clinical Data, Electrophysiological Results and Neuroimaging, and Postsurgical Outcomes in Our Series
| Case No./Sex/Age at Onset, y | |||
|---|---|---|---|
| Variable | 1/M/38 | 2/M/37 | 3/F/31 |
| Risk factor | Head trauma | Head trauma | Brain tumor |
| Seizure frequency per day | 7 | 4 | 4 |
| Social effect | Was in electronics, lost his job and was incarcerated |
Was a pilot, relocated to work as a flight instructor |
Was a cashier, lost her job because of seizures |
| Aura | Fear | Laughing or whoopee noise | Sensation of freezing |
| Ictal seminologic findings | Starts with ictal cry and sudden flexation of both elbows; followed by bilateral violent flailing movements in all 4 extremities, followed by a short postictal period if any |
Can occur out of wakefulness or sleep and consist of sudden rocking, flailing, sometimes running and vocalization with rapid recovery |
Starts with hyperventilation, then grabs forehead with left hand, positioning of right arm and leg, with jerky movements of right arm and leg |
| Seizure duration | Seconds | Seconds | Minutes |
| Behavioral changes | Agitation, aggression, and paranoia; more pronounced postictally; aggressive nature and paranoid thinking interictally |
Motor aggression, poor judgment, especially after a cluster of spells; no significant interictal manifestations |
None |
| Interictal EEG | Independent bifrontal sharp waves during sleep |
Normal | Normal except no deep sleep was attained |
| Ictal EEG | Obscured by muscle artifact | Obscured by muscle artifact, postictal delta wave slowing maximal over the frontal regions |
Left frontocental slowing |
| MR imaging | Left anterocingulate arteriovenous malformation vs cavernoma |
2 Cavernomas with hemorrhage in the cingulate gyrus |
Nonenhancing mass in the left cingulate gyrus |
| Surgical procedure | Lesionectomy | Lesionectomy | Left cingulate and superofrontal gyrus resection |
| Pathologic findings | Remote hemorrhage and reactive astrocytosis |
Reactive gliosis hemosiderin, cavernous angioma |
Infiltrating glioma |
| Seizure outcome | Seizure free in 3-mo follow-up; 2 seizures in 9-mo follow-up |
Seizure free since 1998, receiving no medications since 2003 |
3–4/d Down to 1/mo after therapy with tiagabine was started; seizure free for 8 mo, then recurrence then seizure free for about 2 y |
| Behavioral outcome | Resolved | Resolved | Not applicable |
Abbreviations: EEG, electroencephalogram; MR, magnetic resonance.
RESULTS
Table 1 gives details of the cases. The behavioral changes associated with seizures in case 1 and in case 2 were unusual and profound. Case 1 demonstrated aggression and paranoia; the patient’s postictal behavioral changes were long lasting. Behavioral problems in the setting of a normal interictal EEG had previously led to the diagnosis of nonepileptic spells and a personality disorder. He used to trade in electronics and lost his job as a consequence of the seizures. At one point, his postictal aggression led to imprisonment. In case 2, motor aggression, poor judgment, and paranoia were noted, mainly postictally. These signs were more profound after prolonged clusters of seizures. The patient previously worked as a commercial pilot and as a passenger had a series of seizures that resulted in the flight’s being diverted for a medical emergency landing; during the flight, he was uncontrollably wandering up and down the aisle of the plane, often running up against the bulkhead. He was placed on medical leave but was able to return to work as a flight instructor after epilepsy surgery controlled his seizures.
Two patients (one with an aura of intense fear and the other with an aura of laughter without mirth) had simple partial seizure symptoms that were previously linked to the cingulate gyrus. The third patient described a “freezing” aura. To our knowledge, this is the first time an aura of freezing has been reported in association with cingulate gyrus epilepsy.
All 3 patients had complex ictal motor behavior, suggesting a frontal origin of these seizures before radiological or electrophysiological methods of diagnosis.
Ictal EEGs in 2 patients were obscured by muscle artifacts, while 1 patient had an EEG with left frontocentral slowing only and no specific spikes or sharp waves during the event.
Lesionectomy was performed in all of our patients. The surgical procedures produced good outcomes in terms of seizure control and resolution of behavioral problems. Case 1 was almost seizure free at 9 months after surgery, with only 2 seizures during this period (the frequency before surgery was 6–10 per day). A better outcome was achieved in case 2, who as of this writing has been seizure free for 11 years and not receiving antiepileptic drugs for the past 7 years. Case 3 was seizure free for 8 months before recurrence, but with better control and more than 98% reduction in seizure frequency. We believe that the only reason she is not seizure free is the recurrence of her glioma after surgical resection.
COMMENT
The cingulate gyri are situated in the medial pericallosal aspect of each frontal lobe. Because of diffusely projecting connectivity, the cingulate gyrus is an important structure in seizure propagation. It is composed of the anterocingulate cortex and the posterocingulate cortex. The anterocingulate gyrus is part of the Papez circuit and has the following 3 functional subdivisions: (1) The premotor division has projections to the spinal cord, red nucleus, and motor cortex. It receives projections from different thalamic nuclei. (2) The affect division has extensive connections with amygdala, periaqueduct gray matter, and autonomic brainstem nuclei and helps in controlling emotional autonomic responses. Electrical stimulation of this division may result in fear, pleasure, and agitation. (3) The cognitive division is found in the caudal areas and helps in avoidance learning memory. The posterocingulate cortex represents a separate entity that has different connections, histologic features, and function. It has a role in visuospatial and memory functions.6–8
Although some authors discussed the futility of trying to distinguish cingulate gyrus epilepsy from other frontal lobe epilepsy syndromes based on semiological results alone,5 we believe that lesional cingulate gyrus epilepsy can be considered a specific frontal lobe epilepsy syndrome or a distinctive constellation of findings. However, we acknowledge that in nonlesional cases it is hard to make such a differentiation without intracranial ictal recordings, leading to limited corticectomy and excellent seizure outcome.
Diagnosis of neocortical epilepsies was facilitated after the introduction of MR imaging and other neuroimaging modalities. As of 2009, 14 cases of confirmed cingulate gyrus epilepsy were reported.8–20 Our study results are consistent with previous findings about cingulate gyrus epilepsy (Table 2). Not surprising, all of our patients had complex motor activity during their seizures, similar to what has been previously reported. Twelve of 14 reviewed cases had complex motor activity; the 3 most commonly reported activities are thrashing and kicking, grasping, and running. There is a long list of other reported activities, including crying, spitting, grasping, hair fixing, lip smacking, head touching, mouth puckering, hand shaking or clapping, and repeated kissing or sucking.8–19 Some cases demonstrated myoclonus without other marked or noticeable motor activity.17
Table 2.
Comparison Between Our Series and 14 Reviewed Cases of Confirmed Cingulate Gyrus Epilepsy in the Literature
| Variable | Our Series (n=3) |
Literature (n=14)8–20 |
|---|---|---|
| Male-female ratio | 2:1 | 10:4 |
| Right-left seizure ratio | 0:3 | 10:3a |
| Age at onset | Range, 31 to 38 y | Range, 6 mo to 31 y |
| Seizure frequency | All experienced several per day | 14 Experienced several per day, 1 experienced >100/d |
| Seizure duration | Lasted seconds in 3, occasionally lasted minutes in 1 | Lasted seconds in 11, lasted up to a few minutes in 2b |
| 2GTC seizures | 0 | 2 |
| Treatment result | All failed different antiepileptic drugs | 12 Failed, 2 responded |
| MR imaging | All with lesions | 9 With lesions |
| LOC | 0 | Not available |
| Surgical outcome | 2 Seizure free, 1 with >98% reduction | 7 Who underwent lesionectomy had >90% reduction |
Abbreviations: 2GTC, secondary generalized tonic-clonic; LOC, loss of consciousness; MR, magnetic resonance.
One patient with bilateral hyperfusion on single-photon emission tomography.
Data were unavailable for the 14th case.
Behavioral problems and personality changes are associated with cingulate gyrus epilepsies.4,8,21 However, we report herein striking behavioral alterations that manifested mainly as motor and verbal aggression, as well as paranoid delusions. Personality and behavioral changes may persist interictally and may resolve after lesionectomy. We believe that these behavioral phenomena in our patients were postictal and interictal manifestations of seizures. This conclusion is supported by several lines of evidence. The severity of our patients’ behavioral problems correlated with the frequency and severity of seizures. Behavioral alterations almost completely resolved after surgery. Previously described patients demonstrated personality changes characterized as autistic, reclusive, sociopathic, self-mutilating, and obsessive-compulsive.8–16 Most of these changes were reversed after surgery. Major behavioral alterations were reported in 5 cases among 14 reviewed herein, which we believe to be an underreporting.8,10,14,16 The changes were postictal or interictal or were categorized as personality disorders. In our series, one patient (case 2) demonstrated laughter without mirth, which was reported in 5 cases8,11,13,15,18 among 14 reviewed. Our search for published cases with gelastic seizures revealed 12 gelastic epilepsy cases originating from the frontal lobe without a hypothalamic lesion and 5 cases originating from the cingulate gyrus.22 Thirty-four gelastic cases of temporal origin were reported before 1997; some were associated with a sense of mirth.22 Similar to our results, poorly localizing interictal and ictal EEG findings are commonly reported in seizures originating from the cingulate gyrus. Ictal EEG suggested the correct lateralization in 7 cases among 14 reviewed9,11–13,16–18 and a frontal origin in 6 cases,8,11,12,16,18,19 while it suggested both the correct lateralization and a frontal origin in only 3 cases.12,16,18
We report herein prolonged periods of aggression (case 1 and case 2), paranoia (case 1), and an aura of freezing (case 3) in cingulate gyrus epilepsy. Lesional cingulate gyrus epilepsy can be diagnosed by MR imaging of the brain, nonspecific interictal EEG, poorly localizing or nonspecific ictal EEG with or without behavioral or personality disorder, and seizures with unusual motor manifestations. Recognizing such a pattern will assist in correct early diagnosis and help avoid misdiagnosis of nonepileptic seizures. Recognition of nonlesional cases would be more difficult without intracranial recordings.
In summary, cingulate gyrus epilepsy is a rare entity. It should be suspected in patients who are seen with a frontal lobe epilepsy syndrome (short lasting, frequent, and complex stereotyped prominent motor seizures with or without vocalization and a minimal postictal phase) and with 1 or more of the following: (1) laughter without mirth, especially at the beginning of the seizure; (2) a sense of fear with or without facial expression, especially at the beginning of the seizure; or (3) striking and profound behavioral or personality changes that may last for weeks. Neuroimaging is useful in lesional cases, whereas scalp EEG is usually not helpful. Our experience shows that lesional patients have good outcomes after surgical resection, which is somewhat dependent on the nature of the lesion (eg, neoplasm vs stable lesion).
Footnotes
Author Contributions: Study concept and design: Alkawadri and Van Ness. Acquisition of data: Alkawadri, Mickey, Madden, and Van Ness. Analysis and interpretation of data: Alkawadri and Van Ness. Drafting of the manuscript: Alkawadri. Critical revision of the manuscript for important intellectual content: Alkawadri, Mickey, Madden, and Van Ness. Administrative, technical, and material support: Alkawadri, Mickey, Madden, and Van Ness. Study supervision: Van Ness.
Financial Disclosure: None reported.
REFERENCES
- 1.Andy OJ, Chinn RM. Cingulate gyrus seizures; correlation of electroencephalographic and behavioral activity in the cat. Neurology. 1957;7(1):56–68. doi: 10.1212/wnl.7.1.56. [DOI] [PubMed] [Google Scholar]
- 2.Mazars G. Criteria for identifying cingulate epilepsies. Epilepsia. 1970;11(1):41–47. doi: 10.1111/j.1528-1157.1970.tb03865.x. [DOI] [PubMed] [Google Scholar]
- 3.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30(4):389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 4.Chauvel P, Delgado-Escueta AV, Halgren E, et al. Clinical semiology of frontal lobe seizures. In: Bancaud J, Talairach J, editors. Advances in Neurology. Vol. 57. New York, NY: Raven Press; 1992. pp. 3–58. [PubMed] [Google Scholar]
- 5.Williamson PD, Siegel AM, Roberts DW, Thadani VM. Neocortical Epilepsies. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 6.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 7.Parasuraman R. The Attentive Brain. Cambridge, England: MIT Press; 1998. [Google Scholar]
- 8.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 9.Imataka G, Ogino M, Nakagawa E, Yamanouchi H, Arisaka O. Electroencephalography-guided resection of dysembryoplastic neuroepithelial tumor: case report. Neurol Med Chir (Tokyo) 2008;48(7):318–321. doi: 10.2176/nmc.48.318. [DOI] [PubMed] [Google Scholar]
- 10.Korkmaz B, Benbir G, Demirbilek V. Migration abnormality in the left cingulate gyrus presenting with autistic disorder. J Child Neurol. 2006;21(7):600–604. doi: 10.1177/08830738060210070601. [DOI] [PubMed] [Google Scholar]
- 11.Chassagnon S, Minotti L, Kremer S, et al. Restricted frontomesial epileptogenic focus generating dyskinetic behavior and laughter. Epilepsia. 2003;44(6):859– 863. doi: 10.1046/j.1528-1157.2003.60802.x. [DOI] [PubMed] [Google Scholar]
- 12.Madhavan D, Liebman T, Nadkarni S, Devinsky O. Anterior cingulate epilepsy in an 18-year-old woman. Rev Neurol Dis. 2007;4(1):39–42. [PubMed] [Google Scholar]
- 13.Mohamed IS, Otsubo H, Shroff M, Donner E, Drake J, Snead OC., III Magneto-encephalography and diffusion tensor imaging in gelastic seizures secondary to a cingulate gyrus lesion. Clin Neurol Neurosurg. 2007;109(2):182–187. doi: 10.1016/j.clineuro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 14.San Pedro EC, Mountz JM, Ojha B, Khan AA, Liu HG, Kuzniecky RI. Anterior cingulate gyrus epilepsy: the role of ictal rCBF SPECT in seizure localization. Epilepsia. 2000;41(5):594–600. doi: 10.1111/j.1528-1157.2000.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 15.Iwasa H, Shibata T, Mine S, et al. Different patterns of dipole source localization in gelastic seizure with or without a sense of mirth. Neurosci Res. 2002;43(1):23–29. doi: 10.1016/s0168-0102(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 16.Levin B, Duchowny M. Childhood obsessive-compulsive disorder and cingulate epilepsy. Biol Psychiatry. 1991;30(10):1049–1055. doi: 10.1016/0006-3223(91)90124-5. [DOI] [PubMed] [Google Scholar]
- 17.Lim EC, Tan JJ, Ong BK, Wilder-Smith EP. Generalised myoclonus evolving into epilepsia partialis continua due to a cingulate gyrus lesion: case report and review of the literature. Parkinsonism Relat Disord. 2004;10(7):447–449. doi: 10.1016/j.parkreldis.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 18.McConachie NS, King MD. Gelastic seizures in a child with focal cortical dysplasia of the cingulate gyrus. Neuroradiology. 1997;39(1):44–45. doi: 10.1007/s002340050365. [DOI] [PubMed] [Google Scholar]
- 19.Schindler K, Gast H, Bassetti C, et al. Hyperperfusion of anterior cingulate gyrus in a case of paroxysmal nocturnal dystonia. Neurology. 2001;57(5):917–920. doi: 10.1212/wnl.57.5.917. [DOI] [PubMed] [Google Scholar]
- 20.Vetrugno R, Mascalchi M, Vella A, et al. Paroxysmal arousal in epilepsy associated with cingulate hyperperfusion. Neurology. 2005;64(2):356–358. doi: 10.1212/01.WNL.0000149530.07703.FA. [DOI] [PubMed] [Google Scholar]
- 21.Talairach J, Bancaud J, Geier S, et al. The cingulate gyrus and human behaviour. Electroencephalogr Clin Neurophysiol. 1973;34(1):45–52. doi: 10.1016/0013-4694(73)90149-1. [DOI] [PubMed] [Google Scholar]
- 22.Pearce JM. A note on gelastic epilepsy. Eur Neurol. 2004;52(3):172–174. doi: 10.1159/000081858. [DOI] [PubMed] [Google Scholar]