Abstract
Drugs that enhance the action of serotonin (5-hydroxytrypamine, 5-HT), including several selective serotonin reuptake inhibitors (SSRIs), reduce susceptibility to seizureinduced respiratory arrest (S-IRA) that leads to death in the DBA/1 mouse model of sudden unexpected death in epilepsy (SUDEP). However, it is not clear if specific 5-HT receptors are important in the action of these drugs and whether the brain is the major site of action of these agents in this SUDEP model. The current study examined the actions of agents that affect the 5-HT3 receptor subtype on S-IRA and whether intracerebroventricular (ICV) microinjection of an SSRI would reduce S-IRA susceptibility in DBA/1 mice. The data indicate that systemic administration of SR 57227, a 5-HT3 agonist, was effective in blocking S-IRA in doses that did not block seizures, and the S-IRA blocking effect of the SSRI, fluoxetine, was abolished by co-administration of a 5-HT3 antagonist, ondansetron. Intracerebroventricular administration of fluoxetine in the present study was also able to block S-IRA without blocking seizures. These findings suggest that 5-HT3 receptors play an important role in the block of S-IRA by serotonergic agents, such as SSRIs, which is consistent with the abnormal expression of 5-HT3 receptors in the brainstem of DBA mice observed previously. Taken together, these data indicate that systemically administered serotonergic agents act, at least in part in the brain, to reduce S-IRA susceptibility in DBA/1 mice and that 5-HT3 receptors may be important to this effect.
Keywords: 5-HT, SSRIs, antagonist, audiogenic seizures, intracerebroventricular
1. Introduction
Sudden unexpected death in epilepsy (SUDEP) is a shattering consequence of epilepsy in adult and pediatric patients [1–5] and results in a major cause of lost patient years second only to stroke among neurological disorders [6]. In the majority of witnessed cases of SUDEP, a generalized convulsive seizure and severe respiratory dysfunction were observed prior to death [3]. DBA/1 and DBA/2 mice have been shown to be relevant models of SUDEP, because they exhibit generalized convulsions that lead directly to seizure-induced respiratory arrest (S-IRA), resulting in death if resuscitation is not rapidly instituted [7–9].
Several drugs that enhance the activation of serotonin (5-hydroxytryptamine, 5-HT) receptors, including selective serotonin reuptake inhibitors (SSRIs), prevent S-IRA without blocking seizures in DBA mice, and 5-HT antagonists increase S-IRA susceptibility in non-susceptible DBA mice [10–12]. Seizures induced by maximal electroshock or pilocarpine in Lmx1b(f/f) mice resulted in elevated seizure-induced mortality because of breathing cessation, which was also reduced by SSRI administration [13]. Since seizure susceptibility is not blocked by SSRIs in DBA mice at doses that block S-IRA, this suggests that these agents exert selective effects on respiratory arrest rather than a general anticonvulsant effect. Respiratory dysfunction after seizures is common in patients with epilepsy [14, 15], and patients who exhibited partial seizures and who had been taking SSRIs exhibited less respiratory dysfunction than untreated patients in a retrospective study [16].
Serotonin is known to play a role in the normal regulation of respiration, in part, by acting on neurons in the medullary respiratory centers to enhance respiration in response to elevated CO2 levels [17, 18]. Although our previous studies demonstrated that the SSRI, fluoxetine, at a dose that suppresses S-IRA, did not enhance respiratory ventilation in the absence of seizures in DBA/1 mice, SSRIs may prevent respiratory arrest via maintaining respiratory function during generalized tonic-clonic seizures [12]. Serotonin is also involved in the arousal response via ascending projections from raphe nuclei [19, 20]. Thus, deficits of serotonergic neurotransmission in both respiratory and arousal systems may contribute to S-IRA in DBA/1 mice.
Specific subtypes of 5-HT receptors are thought to be selectively relevant to control of respiration, arousal and epilepsy [21–23]. At least seven subtypes of 5-HT receptors are expressed in the brainstem [24, 25], and the absence of 5-HT2C receptors in transgenic mice is associated with seizure susceptibility that can result in seizure-induced death [26, 27], similar to that seen in DBA mice. The 5-HT2A receptors are reported to contribute importantly to S-IRA induced by electroshock [13]. Our previous studies indicate that levels of several 5-HT receptor subtype proteins, including 5-HT2B, 5-HT2C and 5-HT3 receptors, were significantly reduced in DBA/1 mice compared with those in a seizure-resistant mouse strain, although a 5-HT2B/2C receptor agonist (mCPP) was ineffective in reducing S-IRA in DBA/1 mice [28]. Therefore, the present study examined the involvement of 5-HT3 receptors, the only ionotropic receptor among all of the 5-HT receptor subtypes identified [29], in S-IRA in DBA/1 mice. The 5-HT receptors are also known to exist peripherally and can potentially be an important target for these S-IRA suppressing SSRIs, particularly by exerting effects in the lung [30]. Therefore, the present study also examined the effect of administration of an SSRI directly into the brain using intracerebroventricular (ICV) route.
2. Materials and methods
2.1. Animals
The male and female DBA/1 mice were obtained from Harlan Laboratories (Indianapolis, IN) and were housed and or bred in the animal facility with food pellets and water available ad libitum. Multiple groups of DBA/1 mice were screened for susceptibility to S-IRA induced by audiogenic seizures (AGSz), beginning on postnatal day (PND) 24–30, and acoustic stimulation was presented daily for 3–4 days at which time 90 to 100% of DBA/1 mice in each group developed S-IRA susceptibility, as previously described [8]. This “priming” process at a young age is important to developing the chronic consistent S-IRA susceptibility that allows the animals to model SUDEP and the testing of SUDEP prevention treatments [8, 31]. A total of 97 mice were used in the current study. The operational definition of S-IRA is described below. Only those mice that consistently exhibited S-IRA were used in these experiments. The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Southern Illinois University School of Medicine and Massachusetts General Hospital, which are in accordance with the National Institute of Health guidelines for care and use of laboratory animals. Measures were included in the protocols to minimize the pain and discomfort of the animals and to minimize animal usage.
2.2. Seizure induction and resuscitation
All DBA/1 mice were subjected to an acoustic stimulation paradigm, consisting of a broad-band acoustic stimulus generated by an electrical bell (Heath Zenith Model #172C-A or FOS 4771L, Tecumseh, MI) at an intensity of 96–110 dB SPL. Mice were individually placed in a plastic cylinder within a sound-isolation room. The stimulus was given for a maximum duration of 60 s or until the mouse exhibited a tonic seizure, which ended in tonic hindlimb extension convulsions and consistently resulted in S-IRA. Behaviors were recorded on videotape, and seizure-related behaviors were quantified visually off-line. After S-IRA was evoked, all DBA/1 mice received respiratory support to assist in recovery of respiration, as described below. The operational criteria for S-IRA were defined by the appearance of a deep respiratory gasp and relaxation of the pinnae determined visually, which were invariant indicators in previous studies that S-IRA had begun and death was imminent [8]. Although S-IRA was behaviorally determined by qualitative (visual) breathing assessment, our previous quantitative studies using plethysmography indicated that S-IRA leads to terminal asystole and death in DBA/1 mice [32]. Resuscitation was accomplished by placing the outflow polyethylene tube (4.4 mm external diam.) of a rodent respirator (Harvard Apparatus 680, Holliston, MA) over the nostrils of the supine mice. The respirator was previously in operation pumping room air (180 strokes/min), and when the outflow tube was placed over the nostrils, the one cc volume induced visible displacement of the chest. Initiation of resuscitation began within 5 s after the final deep respiratory gasp to effectively revive the mice [7, 8]. The mice were re-subjected to the acoustic stimulation paradigm 24 h and 48 h after drug administration and at 24 h intervals thereafter, if necessary, to determine if the susceptibility to S-IRA had returned.
2.3. Intracerebroventricular (ICV) injection
The ICV cannulation was carried out as described in earlier studies [33]. In brief, mice were anesthetized with ketamine/xylazine (100/10 mg/kg, i.p.). A guide cannula (26G, Plastics One, Roanoke, VA) was stereotaxically implanted based on coordinates from Paxinos and Franklin (1997) [34] (AP − 0.4 mm; ML − 1.0 mm; and V −2.0 mm). The guide cannula was fixed to the skull using mounting screws (BASi, West Lafayette, IN) and dental cement (A–M Systems, Sequim, WA). A stainless steel stylet was used to occlude the guide cannula when not in use. The animals were then allowed to recover for a week during which period tetracycline (1 g/l) was administered in the drinking water to reduce infection. One week after implant surgery, each mouse was tested to verify that it remained susceptible to seizures and S-IRA and then, resuscitated. The following day microinjections were made using a Hamilton syringe and a pump (11 Elite Nanomite, Harvard Apparatus), which was connected to the internal cannula (33G, Plastics One) by a polyethylene tubing, and 1.0 µl of fluoxetine or the vehicle, dimethyl sulfoxide (DMSO), was administered at a rate of 0.5 µl/min into the left lateral ventricle. The injection cannula was left in place for a further one min before being slowly withdrawn to avoid back flow.
2.4. Histology
Verification of the placement of the ICV guide cannula was done at the end of experiments. Fast green was injected ICV to mark the ventricular space. Each mouse was euthanized with an overdose of ketamine/xylazine and transcardially perfused with 30 ml PBS (pH 7.4), followed by 30 ml 4% paraformaldehyde. The brains were removed and stored in 4% paraformaldehyde at 4°C. Each brain was sectioned into 50-µm thickness of coronal slices using a freezing microtome (CM 1850 UV, Leica, Buffalo Grove, IL). The placement of the guide cannula was observed using a light microscope. Only data from animals with correct cannula placement were used for statistical analysis.
2.5. Drugs
Because of the very small volume used for ICV injection, the dose of fluoxetine (120 nmol) was not soluble in saline, and dimethyl sulfoxide (DMSO) was used as the vehicle for these experiments. The DBA/1 mice received the following drugs acutely: the SSRI, fluoxetine, administered i.p. (40 mg/kg in saline) 30 min prior to AGSz induction or ICV (60, 90, or 120 nmol in DMSO) 15 min prior to AGSz. The selective 5-HT3 agonist SR 57227 (20–40 mg/kg in saline) or the 5-HT3 antagonist ondansetron (0.5–1 mg/kg in distilled water) was administered 30 and 35 min prior to AGSz, respectively. All drugs were obtained from Sigma-Aldrich (St. Louis, MO).
2.6. Statistics
The incidence of S-IRA between drug and vehicle groups was compared using Mann-Whitney U test. Statistical significance was inferred if p<0.05.
3. Results
3.1. A 5-HT3 receptor agonist reduces S-IRA
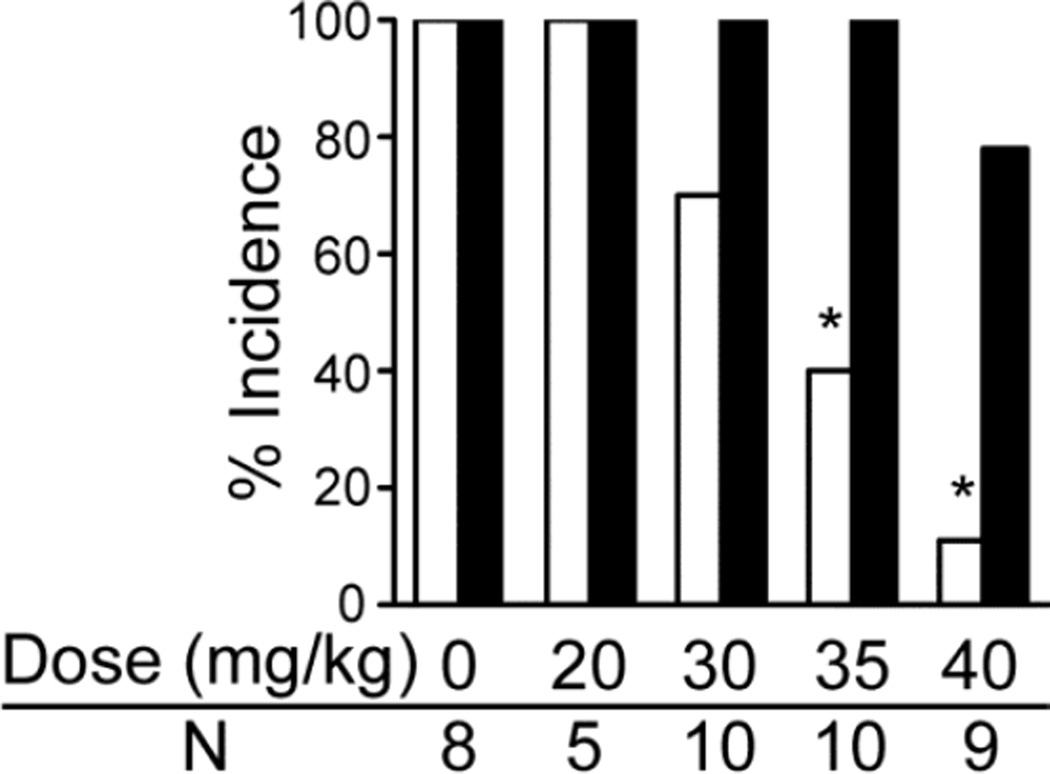
The effects of systemic administration of the selective agonist for the 5-HT3 receptor (SR 57227) in primed DBA/1 mice that consistently exhibited S-IRA are shown in Fig. 1. SR 57227 significantly reduced S-IRA at doses that did not affect the severity or susceptibility to AGSz. Thus, at doses of 35 and 40 mg/kg, the 5-HT3 agonist was effective in inhibiting S-IRA without changing AGSz susceptibility. The saline vehicle and lower doses of SR 57227 (20 and 30 mg/kg) were ineffective in reducing S-IRA. The suppressive effect of this 5-HT3 agonist on S-IRA was reversible, and most mice returned to S-IRA susceptibility 24 h after drug administration. Susceptibility to S-IRA did not return in one DBA/1 mouse likely because of delayed sequelae of the S-IRA, including nasal exudates and labored breathing, suggestive of pulmonary edema. This mouse was euthanized at 72 hr after drug administration because of its compromised health status. Another mouse was not successfully resuscitated.
Fig. 1.
Effect of a 5-HT3 agonist (SR 57227) on the incidence of audiogenic seizures (AGSz) and seizure-induced respiratory arrest (S-IRA) in DBA/1 mice.
The effects of systemic (i.p.) administration of SR 57227 [20, 30, 35 or 40 mg/kg in saline (vehicle) or saline shown as zero dose] were examined in S-IRA susceptible DBA/1 mice. Thirty min after SR 57227 injection, the 35 and 40 mg/kg dose significantly reduced the incidence of S-IRA evoked by acoustic stimulation as compared with vehicle control. The incidence of AGSz was not significantly affected by any of the other injections. White bars indicate the incidence of S-IRA; black bars indicate the incidence of AGSz.
*Significantly different from vehicle control, p < 0.05. N is the number of animals for each dose.
3.2. Fluoxetine effect on S-IRA is blocked by a 5-HT3 receptor antagonist
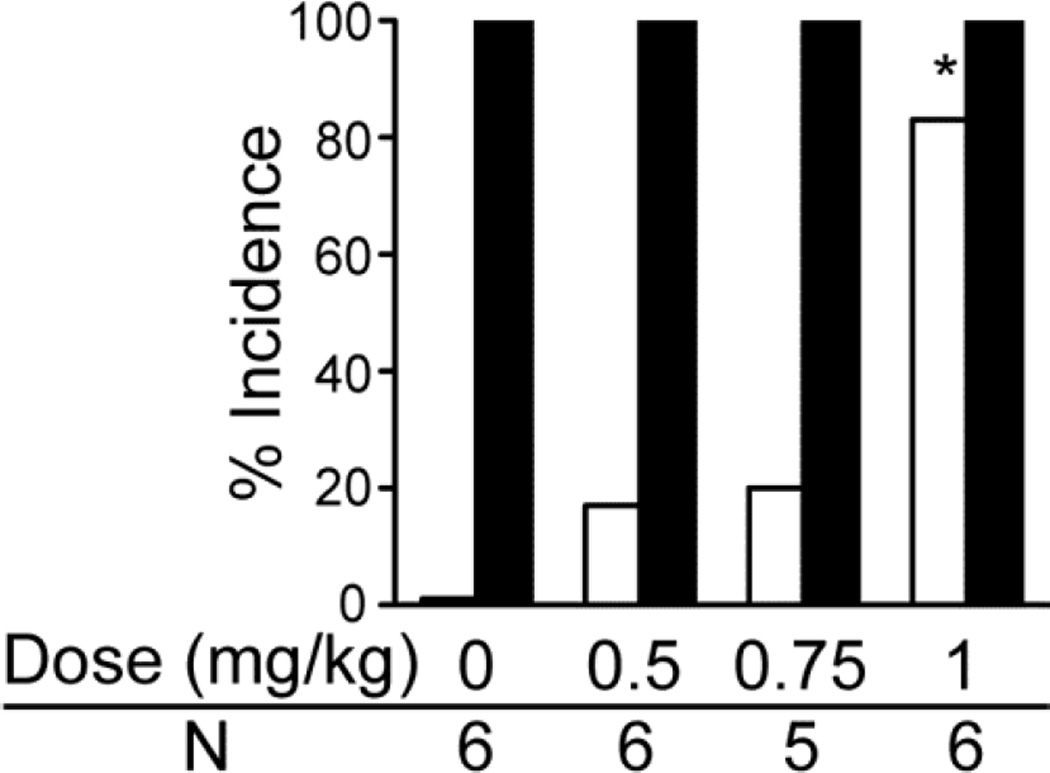
The role of 5-HT3 receptors in the occurrence of S-IRA in primed DBA/1 mice was further evaluated by examining the ability of a selective 5-HT3 antagonist, ondansetron (OND), to alter the ability of the SSRI, fluoxetine, to suppress S-IRA. When given sequentially, this 5-HT3 antagonist blocked the ability of fluoxetine to reduce S-IRA (Fig. 2). Thus, OND (0.5, 0.75, 1.0 mg/kg or vehicle i.p.) was administered 5 min prior to fluoxetine (40 mg/kg, i.p.), and 30 min later, AGSz were induced (Note, OND in doses up to 2.0 mg/kg had no effect on AGSz or S-IRA in DBA/1 mice in preliminary studies). Thirty min after fluoxetine administration, a complete suppression of S-IRA was induced in mice pre-treated with vehicle or the lower doses of OND, but AGSz susceptibility was not affected. A dose-dependent reduction in the suppressive effect of fluoxetine was induced by OND, which reached statistical significance at 1.0 mg/kg (p < 0.05). The incidence of AGSz was not significantly affected by any of the pre-injections.
Fig. 2.
Effect of a 5-HT3 antagonist (ondansetron) on S-IRA suppressing action by fluoxetine in DBA/1 mice.
Systemic (i.p.) administration of ondansetron (OND), 0.5, 0.75 or 1.0 mg/kg or vehicle shown as zero dose, 35 min prior to audiogenic seizures (AGSz) on the ability of fluoxetine (40 mg/kg, i.p.), 30 min prior to AGSz, to reduce the incidence of AGSz and seizure-induced respiratory arrest (S-IRA) in primed DBA/1 mice that consistently exhibited S-IRA before treatment. Fluoxetine plus vehicle induced a complete suppression of S-IRA without blocking AGSz. A dose-dependent reduction in the suppressive effect of fluoxetine was induced by OND, which reached statistical significance at 1.0 mg/kg. The incidence of AGSz was not significantly affected by any of the injections. White bars indicate the incidence of S-IRA; black bars indicate the incidence of AGSz.
*Significantly different from fluoxetine alone, p < 0.05. N is the number of animals for each dose.
3.3. Fluoxetine ICV blocks S-IRA
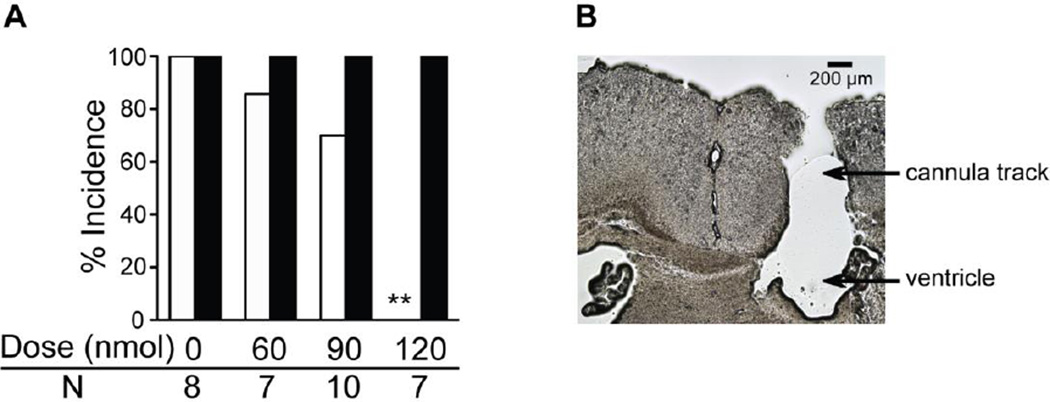
Administration of fluoxetine directly into the brain reduced the incidence of S-IRA in primed DBA/1, and this effect was dose-related (Fig. 3A). In the 60 nmol ICV group, only one of the 7 mice did not develop S-IRA after AGSz, which was not significantly different from the DMSO control (S-IRA occurrence 85.7% vs. 100%). In the 90 nmol ICV group, the S-IRA rate was reduced to 70% (n = 10) but still did not reach statistical significance. In the 120 nmol ICV group, fluoxetine blocked only S-IRA but not AGSz activity in 2/7 of DBA/1 mice tested. While fluoxetine blocked both S-IRA and tonic seizures in the rest 5/7 of mice, these mice still exhibited AGSz (wild running and/or clonic seizures). Therefore, fluoxetine at this dose blocked S-IRA in all mice tested, which is significantly different from control (p < 0.01). Two of the animals that received the 120-nmol dose exhibited tremor, hypothermia, hunched back and bradypnea, which are consistent with the serotonin syndrome that is seen in rodents [35]. These two mice were found dead 24–72 h after fluoxetine microinjection. The rest of the mice returned S-IRA susceptibility by 96 h after fluoxetine microinjection. In 32 of 40 implanted DBA/1 mice, the cannula hit the ventricle (Fig. 3B). Intracranial injections of fluoxetine in those mice in which the cannula did not hit the ventricle (8 mice) exerted no effect on S-IRA.
Fig. 3.
Intracerebroventricular (ICV) injection of fluoxetine reduces S-IRA in DBA/1 mice.
A: The effects of fluoxetine [1.0 µl, 60, 90 or 120 nmol in DMSO or the DMSO (vehicle) shown as zero dose] administered ICV on the incidence of audiogenic seizures (AGSz) and seizure-induced respiratory arrest (S-IRA) in DBA/1 mice. Fifteen min after fluoxetine injection, the 120-nmol dose significantly reduced the incidence of S-IRA evoked by acoustic stimulation as compared with vehicle control. The incidence of AGSz was unaffected by any of the other fluoxetine doses. White bars indicate the incidence of S-IRA; black bars indicate the incidence of AGSz. B: a representative example of histology showing that the drug and vehicle were successfully delivered into the ventricle.
** Significantly different from vehicle control at p<0.01. N is the number of animals for each dose.
4. Discussion
The present study indicates that a selective 5-HT3 agonist can suppress S-IRA without affecting seizure susceptibility, which is the first selective 5-HT agonist that was effective in DBA/1 mice, since neither a 5-HT2B/2C agonist nor a 5-HT7 agonist was effective in this model of SUDEP [28, 31]. The current findings with agents that act selectively on the 5-HT3 receptor, which is the only ionotropic receptor among the 7 classes of 5-HT receptor subtypes [29], are consistent with previous studies that showed abnormal levels of 5-HT3 receptor protein in both DBA/1 and DBA/2 mouse models of SUDEP [28, 36]. The ability of a selective 5-HT3 antagonist, ondansetron, to block the effect of fluoxetine in the present study is further evidence of the importance of 5-HT3 receptors in the ability of SSRIs to suppress S-IRA that was also seen previously [8, 11, 12].
The ability of a selective 5-HT3 receptor agonist to block S-IRA in DBA/1 mice seen in the current study is consistent with the effectiveness of other selective 5-HT agonists to block S-IRA in DBA/2 mice (5-HT2B/2C) [28] as well as in seizure-induced mortality in Lmx1b(f/f) mice treated with pilocarpine or by maximal electroshock (5-HT2A) [13]. The 5-HT2C receptors may also play a role in seizure-related respiratory dysfunction, since 5-HT2C knockout mice exhibit AGSz that can lead to death [26]. These findings suggest that the specific 5-HT receptor that modulates S-IRA susceptibility may vary among the different strains of animals. Further studies are needed to examine if other 5-HT receptor subtypes are involved in S-IRA in DBA/1 mice.
The present finding that a selective 5-HT3 antagonist can block the effects of fluoxetine on S-IRA supports the idea that the ability of SSRIs to suppress S-IRA [8, 11–13] is due to inhibition of 5-HT re-uptake rather than other actions exerted by SSRIs, such as effects on cholinergic, noradrenergic and sigma receptors, and transient receptor potential vanilloid type 1 channels as well as TREK-2 [tandem of pore domains in a weak inwardly rectifying K+ channel (TWIK)-related K+ channel (TREK)-2] potassium channels [9, 37–40]. Further support of an important role of 5-HT in the susceptibility to S-IRA is the ability of non-selective 5-HT antagonists to increase the susceptibility to S-IRA of both DBA/1 and DBA/2 mouse models of SUDEP [7, 31]. Selective serotonin reuptake inhibitors can also reduce electroshock or pilocarpine seizure-induced death, but not in mice with genetic deletion of 5-HT neurons [13]. 5-HT3 receptors are highly expressed in the interneurons of the forebrain structures [41]. It is not known how 5-HT3 receptor activation maintains breathing after seizures. However, it was reported that activation of 5-HT3 receptors increases the levels of norepinephrine in the locus coeruleus, a structure that is involved in arousal response [42]. It is possible that stimulation of 5-HT3 receptors reduces S-IRA in DBA/1 mice via enhancing arousal response. Future studies are needed to examine this possibility. It should be noted that the expression of 5-HT3 receptors is also significantly reduced in the brainstem of DBA/2 mice compared with that of audiogenic seizure-resistant C57BL/6J mice [36]. It is possible that activation of 5-HT3 receptors may suppress S-IRA in DBA/2 mice. Further studies are needed to confirm this.
It has been well established that SSRIs act in humans primarily via actions in the brain even in single doses [43], as used in the present and most of the previous studies. The present study examined this issue in DBA/1 mice by administering fluoxetine directly into the brain. These experiments indicated that ICV administration of fluoxetine could block S-IRA without affecting seizure susceptibility, supporting the hypotheses that this effect of SSRIs is largely mediated via actions in the brain, since the amount of fluoxetine administered ICV is considerably below that required to block S-IRA when administered systemically [10].
As noted earlier, 5-HT release from the neurons in the brainstem raphe nuclei mediates the response to elevated CO2 by stimulation of respiration [22] and by inducing arousal under normal conditions [19]. Mice with a genetic deletion of 5-HT neurons are not aroused from sleep by elevated CO2 levels but can be aroused by other stimuli [19]. Selective 5-HT2A but not 5-HT2C receptor antagonists blocked CO2-induced arousal during sleep in normal mice [23]. Thus, defects in serotonergic mechanisms in the raphe nuclei may be important in hypercarbic conditions, which commonly occur peri-ictally in patients and animals undergoing epileptic seizures [7, 14, 15, 44]. The ability of an SSRI to significantly alter neural activity in certain raphe nuclei post-ictally in DBA/1 mice seen in our recent preliminary data suggests a role of these nuclei in the prevention of S-IRA by this agent [45]. Other deficits in serotonergic neurotransmission in DBA mice may also contribute to S-IRA [9]. Although the doses of SSRIs that prevent S-IRA in the DBA mouse models of SUDEP exceed the doses used in the treatment of depression clinically, the antidepressant effects of SSRIs were originally discovered in rodents, and the doses used in those experiments and in recent rodent depression studies (5–20 mg/kg) [39, 46] are in a similar dosage range to that used to block S-IRA in these DBA mouse models of SUDEP (15–70 mg/kg) [7, 10–12]. Although it remains to be evaluated whether these agents that enhance the activation of 5-HT receptors are relevant to a potential use in the prevention of SUDEP in patients [47], one retrospective study observed that patients who experienced partial onset seizures and had been under treatment with SSRIs exhibited a significantly lesser degree of respiratory dysfunction than an age-matched group who were not under SSRI treatment [16]. This potentially exciting finding has not yet been evaluated in a prospective study. Interpretations of these human results are made more complex because the specific SSRIs used in each of the patient groups were not specified and subsequent animal studies in DBA/1 mice showed that not all SSRIs are effective in suppressing S-IRA [31]. Further epidemiological studies are needed to investigate if the SSRIs that are effective in suppressing S-IRA in animal models also reduce the incidence of SUDEP in patients [9].
The existentially vital function of control of respiration is a multifaceted process, involving multiple neurotransmitters, neuromodulators and ion channels, including adenosine [5, 44, 48, 49]. Dravet syndrome is a form of human epilepsy that exhibits an extremely high incidence of SUDEP, and this syndrome is thought to be mainly due to a loss of function mutation of sodium channels [50, 51]. Two drugs that alter the availability of 5-HT have been shown to have potential usefulness in treating Dravet syndrome in patients, including an SSRI and d-fenfluramine, which enhances 5-HT release from nerve endings in the brain [52]. Seizures in an animal model of Dravet syndrome are also inhibited by D-fenfluramine [53]. Interestingly, 5-HT3 receptors are known to flux sodium ions, which have the potential to mediate the positive effects of serotonergic drugs seen in Dravet syndrome [54].
In summary, the present findings suggest that agents that enhance the activation of 5-HT receptors, including agents that are agonists at 5-HT3 receptors, appear to act largely within the brain, providing potentially useful information in the quest to prevent SUDEP in patients.
Highlights.
5-HT3 receptor agonist reduces S-IRA in DBA/1 mice
5-HT3 receptor antagonist reduces the suppressing effect of fluoxetine on S-IRA
Fluoxetine administered into the brain reduces S-IRA
Acknowledgments
This study was supported by Epilepsy Foundation (CF) and R03NS078591, CURE (Citizens United for Research in Epilepsy) foundation grant and fund from the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital (HJF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors has any conflict of interest to disclose.
References
- 1.Devinsky O. Sudden, unexpected death in epilepsy. N Engl J Med. 2011;365:1801–1811. doi: 10.1056/NEJMra1010481. [DOI] [PubMed] [Google Scholar]
- 2.Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia. 2012;53:227–233. doi: 10.1111/j.1528-1167.2011.03358.x. [DOI] [PubMed] [Google Scholar]
- 3.Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 4.Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 2014;10:271–282. doi: 10.1038/nrneurol.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faingold CL, Randall M, Long X, Uteshev VV, Kommajosyula SP, Tupal S. Neurotransmitters implicated in control of sudden unexpected death in epilepsy in animal models. In: Lathers CM, Schraeder PL, Leestma JE, Wannamaker BB, Verrier RL, Schachter SS, editors. Sudden Unexpected Death in Epilepsy: Mechanisms and New Methods for Analyzing Risks. Boca Raton: CRC Press/Taylor and Francis; 2015. pp. 251–267. [Google Scholar]
- 6.Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia. 2014;55:1479–1485. doi: 10.1111/epi.12666. [DOI] [PubMed] [Google Scholar]
- 7.Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia. 2006;47:21–26. doi: 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 8.Faingold CL, Randall M, Tupal S. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy Behav. 2010;17:436–440. doi: 10.1016/j.yebeh.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Feng HJ, Faingold CL. Abnormalities of serotonergic neurotransmission in animal models of SUDEP. Epilepsy Behav. 2015 doi: 10.1016/j.yebeh.2015.06.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faingold CL, Tupal S, Randall M. Prevention of seizure-induced sudden death in a chronic SUDEP model by semichronic administration of a selective serotonin reuptake inhibitor. Epilepsy Behav. 2011;22:186–190. doi: 10.1016/j.yebeh.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Faingold CL, Randall M. Effects of age, sex, and sertraline administration on seizure-induced respiratory arrest in the DBA/1 mouse model of sudden unexpected death in epilepsy (SUDEP) Epilepsy Behav. 2013;28:78–82. doi: 10.1016/j.yebeh.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Zeng C, Long X, Cotten JF, Forman SA, Solt K, Faingold CL, Feng HJ. Fluoxetine prevents respiratory arrest without enhancing ventilation in DBA/1 mice. Epilepsy Behav. 2015;45:1–7. doi: 10.1016/j.yebeh.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol. 2014;592:4395–4410. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131:3239–3245. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy JD, Seyal M. Respiratory pathophysiology with seizures and implications for sudden unexpected death in epilepsy. J Clin Neurophysiol. 2015;32:10–13. doi: 10.1097/WNP.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 16.Bateman LM, Li CS, Lin TC, Seyal M. Serotonin reuptake inhibitors are associated with reduced severity of ictal hypoxemia in medically refractory partial epilepsy. Epilepsia. 2010;51:2211–2214. doi: 10.1111/j.1528-1167.2010.02594.x. [DOI] [PubMed] [Google Scholar]
- 17.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sowers LP, Massey CA, Gehlbach BK, Granner MA, Richerson GB. Sudden unexpected death in epilepsy: fatal post-ictal respiratory and arousal mechanisms. Respir Physiol Neurobiol. 2013;189:315–323. doi: 10.1016/j.resp.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gharedaghi MH, Seyedabadi M, Ghia JE, Dehpour AR, Rahimian R. The role of different serotonin receptor subtypes in seizure susceptibility. Exp Brain Res. 2014;232:347–367. doi: 10.1007/s00221-013-3757-0. [DOI] [PubMed] [Google Scholar]
- 22.Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep. 2014;9:2152–2165. doi: 10.1016/j.celrep.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchanan GF, Smith HR, MacAskill A, Richerson GB. 5-HT2A receptor activation is necessary for CO2-induced arousal. J Neurophysiol. 2015;114:233–243. doi: 10.1152/jn.00213.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raul L. Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc. Cell Mol Neurobiol. 2003;23:709–726. doi: 10.1023/A:1025096718559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan D. Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp Physiol. 2005;90:175–181. doi: 10.1113/expphysiol.2004.029058. [DOI] [PubMed] [Google Scholar]
- 26.Applegate CD, Tecott LH. Global increases in seizure susceptibility in mice lacking 5-HT2C receptors: a behavioral analysis. Exp Neurol. 1998;154:522–530. doi: 10.1006/exnr.1998.6901. [DOI] [PubMed] [Google Scholar]
- 27.Sejourne J, Llaneza D, Kuti OJ, Page DT. Social Behavioral Deficits Coincide with the Onset of Seizure Susceptibility in Mice Lacking Serotonin Receptor 2c. PLoS One. 2015;10:e0136494. doi: 10.1371/journal.pone.0136494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faingold CL, Randall M, Mhaskar Y, Uteshev VV. Differences in serotonin receptor expression in the brainstem may explain the differential ability of a serotonin agonist to block seizure-induced sudden death in DBA/2 vs. DBA/1 mice. Brain Res. 2011;1418:104–110. doi: 10.1016/j.brainres.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 29.Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadirci E, Halici Z, Bayir Y, Albayrak A, Karakus E, Polat B, et al. Peripheral 5-HT7 receptors as a new target for prevention of lung injury and mortality in septic rats. Immunobiology. 2013;218:1271–1283. doi: 10.1016/j.imbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Faingold CL, Kommajosyula SP, Long X, Plath K, Randall M. Serotonin and sudden death: Differential effects of serotonergic drugs on seizure-induced respiratory arrest in DBA/1 mice. Epilepsy Behav. 2014;37:198–203. doi: 10.1016/j.yebeh.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Zhao H, Yang X, Xue Q, Cotten JF, Feng HJ. 5-Hydroxytryptophan, a precursor for serotonin synthesis, reduces seizure-induced respiratory arrest. Epilepsia. 2016;57:1228–1235. doi: 10.1111/epi.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng HJ, Faingold CL. Modulation of audiogenic seizures by histamine and adenosine receptors in the inferior colliculus. Exp Neurol. 2000;163:264–270. doi: 10.1006/exnr.2000.7382. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Franklin K. Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates. 4th. New York: Academic Press; 2013. [Google Scholar]
- 35.Haberzettl R, Fink H, Bert B. The murine serotonin syndrome - evaluation of responses to 5-HT-enhancing drugs in NMRI mice. Behav Brain Res. 2015;277:204–210. doi: 10.1016/j.bbr.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 36.Uteshev VV, Tupal S, Mhaskar Y, Faingold CL. Abnormal serotonin receptor expression in DBA/2 mice associated with susceptibility to sudden death due to respiratory arrest. Epilepsy Res. 2010;88:183–188. doi: 10.1016/j.eplepsyres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Arias HR, Feuerbach D, Targowska-Duda KM, Russell M, Jozwiak K. Interaction of selective serotonin reuptake inhibitors with neuronal nicotinic acetylcholine receptors. Biochemistry. 2010;49:5734–5742. doi: 10.1021/bi100536t. [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto Y, Tagawa N, Kobayashi Y, Mitsui-Saito K, Hotta Y, Yamada J. Involvement of the sigma1 receptor in the antidepressant-like effects of fluvoxamine in the forced swimming test in comparison with the effects elicited by paroxetine. Eur J Pharmacol. 2012;696:96–100. doi: 10.1016/j.ejphar.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Manna SS, Umathe SN. Paracetamol potentiates the antidepressant-like and anticompulsive-like effects of fluoxetine. Behav Pharmacol. 2015;26:268–281. doi: 10.1097/FBP.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 40.Dong YY, Pike AC, Mackenzie A, McClenaghan C, Aryal P, Dong L, et al. K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science. 2015;347:1256–1259. doi: 10.1126/science.1261512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev. 2012;16:187–197. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klaassens BL, van Gorsel HC, Khalili-Mahani N, van der Grond J, Wyman BT, Whitcher B, et al. Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. Neuroimage. 2015;122:440–450. doi: 10.1016/j.neuroimage.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Kommajosyula SP, Randall ME, Faingold CL. Inhibition of adenosine metabolism induces changes in post-ictal depression, respiration, and mortality in genetically epilepsy prone rats. Epilepsy Res. 2016;119:13–19. doi: 10.1016/j.eplepsyres.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Faingold CL, Randall ME, Brozoski TJ, Odintsov B, Kommajosyula SP. The periaqueductal gray and other brainstem structures are critical nuclei in seizure-induced sudden death in the DBA/1 mouse model of SUDEP. AES Annual Meeting. 2015;69:3.204. Abstract. [Google Scholar]
- 46.Fuller RW, Perry KW, Molloy BB. Effect of an uptake inhibitor on serotonin metabolism in rat brain: studies with 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine (Lilly 110140) Life Sci. 1974;15:1161–1171. doi: 10.1016/s0024-3205(74)80012-3. [DOI] [PubMed] [Google Scholar]
- 47.Richerson GB, Boison D, Faingold CL, Ryvlin P. From unwitnessed fatality to witnessed rescue: Pharmacologic intervention in sudden unexpected death in epilepsy. Epilepsia. 2016;57(Suppl 1):35–45. doi: 10.1111/epi.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen HY, Li T, Boison D. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): role of impaired adenosine clearance. Epilepsia. 2010;51:465–468. doi: 10.1111/j.1528-1167.2009.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulkey DK, Hawkins VE, Hawryluk JM, Takakura AC, Moreira TS, Tzingounis AV. Molecular underpinnings of ventral surface chemoreceptor function: focus on KCNQ channels. J Physiol. 2015;593:1075–1081. doi: 10.1113/jphysiol.2014.286500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Catterall WA. Sodium channels, inherited epilepsy, and antiepileptic drugs. Annu Rev Pharmacol Toxicol. 2014;54:317–338. doi: 10.1146/annurev-pharmtox-011112-140232. [DOI] [PubMed] [Google Scholar]
- 51.Chopra R, Isom LL. Untangling the dravet syndrome seizure network: the changing face of a rare genetic epilepsy. Epilepsy Curr. 2014;14:86–89. doi: 10.5698/1535-7597-14.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meador KJ. Seizure Reduction with Fluoxetine in Dravet Syndrome. Epilepsy Behav Case Rep. 2014;2:54–56. doi: 10.1016/j.ebcr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Kecskes A, Copmans D, Langlois M, Crawford AD, Ceulemans B, et al. Pharmacological characterization of an antisense knockdown zebrafish model of Dravet syndrome: inhibition of epileptic seizures by the serotonin agonist fenfluramine. PLoS One. 2015;10:e0125898. doi: 10.1371/journal.pone.0125898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gunthorpe MJ, Lummis SC. Conversion of the ion selectivity of the 5-HT(3a) receptor from cationic to anionic reveals a conserved feature of the ligand-gated ion channel superfamily. J Biol Chem. 2001;276:10977–10983. [PubMed] [Google Scholar]