Summary
Human mutations in the cytoplasmic C-terminal domain of Slack sodium-activated potassium (KNa) channels result in childhood epilepsy with severe intellectual disability. Slack currents can be increased by pharmacological activators or by phosphorylation of a Slack C-terminal residue by protein kinase C. Using an optical biosensor assay, we find that Slack channel stimulation in neurons or transfected cells produces loss of mass near the plasma membrane. Slack mutants associated with intellectual disability fail to trigger any change in mass. The loss of mass results from the dissociation of the protein phosphatase 1 (PP1) targeting protein, Phactr-1, from the channel. Phactr1 dissociation is specific to wild-type Slack channels and is not observed when related potassium channels are stimulated. Our findings suggest that Slack channels are coupled to cytoplasmic signaling pathways, and that dysregulation of this coupling may trigger the aberrant intellectual development associated with specific childhood epilepsies.
Introduction
The Na+-activated K+ channel Slack (KNa1.1, KCNT1, SLO2.2) is widely distributed throughout the nervous system (Kaczmarek, 2013). KNa currents in neurons and in Slack-transfected cells are regulated by several pathways, including phosphorylation of a serine residue (S407) in cytoplasmic C-terminal domain, which results in a stimulation of current amplitude (Barcia et al., 2012; Santi et al., 2006). The large cytoplasmic C-terminal domain of Slack also interacts with the Fragile X mental retardation protein (FMRP), a RNA-binding protein that regulates activity-dependent protein translation (Brown et al., 2010; Zhang et al., 2012). The interaction of Slack with FMRP also stimulates channel activity. The amplitude of Slack currents can also be stimulated by a number of pharmacological agents, including bithionol and niclosamide (Biton et al., 2012; Yang et al., 2006).
Mutations in ion channels can produce disorders of excitability, such as childhood epileptic seizures. Mutations in Slack have been described in malignant migrating partial seizures of infancy (MMPSI), a condition that produces infantile seizures coupled with very severe intellectual disability (Barcia et al., 2012; Kim et al., 2014). The majority of these mutations produce single amino acid substitutions within the cytoplasmic C-terminal domain of Slack. These gain-of-function mutations increase Slack current amplitude (Kim et al., 2014).
It is unclear, however, why some epilepsy-associated mutations produce little intellectual deficit, while others, such as those in Slack, result in severe intellectual disability. Similar gain-of function mutations in another FMRP-interacting channel, the closely related Ca2+-activated K+ channel BK (KCNMA1, SLO1) (Deng et al., 2013), also produce seizures but do not result in intellectual disability (Du et al., 2005; N’Gouemo, 2014). This suggests that differences in the effects of these mutations on interactions with cytoplasmic signaling pathways, rather than current amplitude, may contribute to the differences in intellectual function.
We have found that the large cytoplasmic domain of Slack interacts with a cytoplasmic signaling protein, Phactr1 (Phosphatase and Actin Regulator 1). Under normal conditions, stimulation of Slack channels causes Phactr1 to dissociate from the channel, resulting in a measurable loss of mass close to the plasma membrane of neurons. Mutant disease-causing Slack channels, however, fail to associate/dissociate with Phactr1. Our results suggest that failure to interact appropriately with its cytosolic signaling partners may underlie the severe intellectual disability associated with Slack mutations.
Results
Activation of Slack decreases mass at the plasma membrane
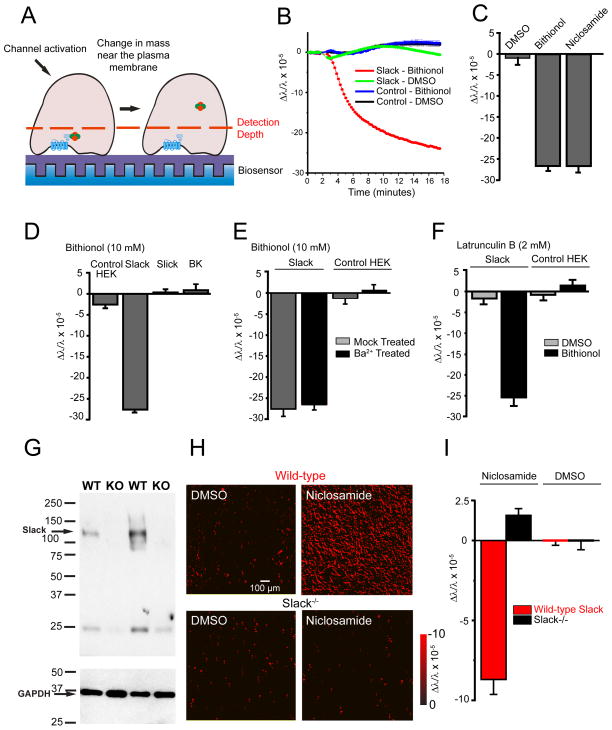
To monitor the interactions of Slack channels with its potential cytoplasmic partners in real-time within living cells, we used resonance-wavelength grating (RWG) optical biosensors, a technique that has been used to monitor the activation of G-protein coupled receptors (Fang et al., 2007; Fleming and Kaczmarek, 2009; Lee, 2009). When cells adhere to these optical biosensors, changes in mass within ~150 nm of the biosensor alter the peak intensity of the reflected wavelengths of resonant light. Decreases or increases in protein density near the plasma membrane produce decreases or increases in the relative index of refraction, respectively (Fleming et al., 2014) (Figure 1A). RWG optical biosensors are sensitive enough to detect the binding of small molecules to proteins, providing a ready assay to detect the much larger changes resulting from the association/dissociation of channels with other proteins (Daghestani and Day, 2010; Lin et al., 2002). We first tested the actions of bithionol and niclosamide, two pharmacological activators that substantially enhance Slack currents (Biton et al., 2012; Yang et al., 2006). Treatment of Slack-expressing HEK293T cells with the Slo family channel activator bithionol (10 μM) (Yang et al., 2006) produced a progressive decrease in mass at the plasma membrane over several minutes following application (Figure 1B). The decrease in mass was sustained over the course of these experiments. Bithionol had no effect on untransfected cells, and DMSO vehicle had no significant effect on either untransfected or Slack-expressing cells (Figure 1B). Similar results were obtained using niclosamide (Biton et al., 2012) (Figure 1C).
Figure 1. Stimulation of Slack channels alters mass distribution at the plasma membrane.
(A) Schematic diagram of Slack activation in a cell adherent to the biosensor. (B) Activation of Slack-expressing, but not untransfected, HEK cells with bithionol (10 μM) produced a sustained decreased in mass near the plasma membrane, n=32 wells/condition, p<0.001. (C) Changes in mass in Slack-expressing HEK after Slack activation with bithionol (10 μM) or niclosamide (500 nM) (n=16, p<0.001). (D) Stimulation of Slack, but not other Slo channel family members, with bithionol (10 μM) decreases mass near the plasma membrane, n=24, p<0.001. (E) Blocking ion flux through Slack channels with Ba2+ (1 mM) does not alter the signal upon channel activation, n=16. (F) Latrunculin B (2 μM) does not alter the signal upon Slack activation by bithionol (10 μM), n=12. (G) Slack protein expression is absent in brain synaptosomes isolated from Slack−/− mice (n=3/genotype). (H and I) Activation of Slack channels in WT, but not Slack−/−, murine cortical neurons decreases mass near the plasma membrane. (H) Representative images showing change in mass in cortical neurons with red representing decreases in mass and black representing no change in mass. (I) Quantification of (H), n=12, p<0.001. Error bars ±SEM.
The observed decrease in mass upon channel stimulation is specific to Slack channel stimulation and not a general consequence of increased K+ conductance. Bithionol is a potent activator of other Slo family potassium channels, including BK channels and the very closely related Na+-activated K+ channel Slick (KNa1.2, KCNT2, Slo2.1) (Yang et al., 2006). Changes in mass were, however, evoked only in Slack-, but not in BK- or Slick- expressing cells (Figure 1D). As a further test to determine if ion flux plays a role in mass change, we pretreated Slack-expressing cells with the known Slack pore blocker Ba2+ (1 mM) (Bhattacharjee et al., 2003) for one hour prior to bithionol (10 μM) addition. Blocking K+ flux had no effect on the observed signal (Figure 1E). Changes in mass at the surface of the biosensor could reflect Slack-dependent changes in cell morphology or cellular movement. To exclude the possibility that the mass change upon Slack stimulation results from such changes we pretreated Slack-expressing cells with the actin polymerization inhibitor Latrunculin B (2 μM) (Wakatsuki et al., 2001) for one hour prior to channel stimulation by bithionol. Inhibition of actin polymerization did not attenuate the observed signal, excluding the possibility that the loss of mass results from actin-mediated changes in cell morphology (Figure 1F).
To determine whether the Slack-stimulation induced loss of mass at the plasma membrane is an artifact of heterologous channel expression in HEK cells, we carried out the same experiment using primary cultures of cortical neurons from wild-type mice (Figure 1G–I). These neurons express Slack channels endogenously (Bhattacharjee et al., 2002; Brown et al., 2008). As in the cell line, a similar sustained decrease in mass was observed upon treatment with the Slack activator niclosamide (1 μM) (Biton et al., 2012) (Figure 1H–I). No significant change in mass was, however, produced on treatment of cortical neurons from animals in which the Slack gene had been deleted (Lu et al., 2015) (Figure 1G–I).
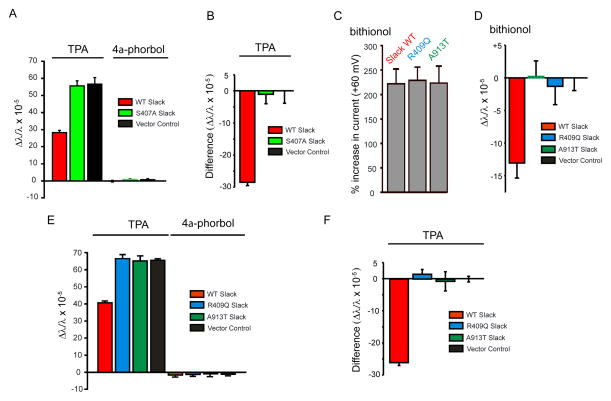
Under physiological conditions, the stimulation of Slack occurs upon phosphorylation of residue S407 in the cytoplasmic C-terminus by protein kinase C (PKC), leading to a 2–3 fold increase in current (Barcia et al., 2012; Santi et al., 2006). Even in untransfected cells, however, treatment with PKC activators such as TPA (12-O-Tetradecanoylphorbol-13-acetate, 100 nM) leads to PKC translocation to the plasma membrane, increasing mass measured at the biosensor (Figure 2A). A change in mass produced by Slack stimulation in response to PKC activation was therefore calculated as the difference between the mass change produced by TPA in untransfected- and Slack-expressing cells (Figure 2B). We found that, like bithionol-treatment, stimulation of Slack channels by TPA led to a decrease in mass at the membrane (Figure 2B) and that the stimulatory effects of bithionol and phosphorylation are additive (Figure S1A and S1B). Mutation of serine 407 to an alanine abolished differences between Slack-expressing and control cells (Figure 2A and 2B), without altering bithionol sensitivity (Figure S1C and S1D). This indicates that, like bithionol, stimulation of Slack channels by phosphorylation at S407 decreases Slack-associated mass at the plasma membrane.
Figure 2. MMPSI and phosphorylation site Slack mutations prevent decreases in mass upon channel activation.
(A) Changes in mass measured 30 min after addition of the PKC activator TPA or its inactive analog 4α-phorbol, to untransfected cells or to cells expressing wild-type (WT) Slack or its phosphorylation site mutant S407A Slack. The increase in mass produced by TPA in untransfected cells or S407A cells is significantly reduced in cell expressing WT Slack. n=16, p<0.001. (B) The contribution of Slack to the mass change in a was calculated by the difference between the positive mass change after TPA addition in untransfected (black bar, A) and Slack-containing cells (red bar, B). (C) Percentage increases in Slack current amplitude produced by bithionol (10 μM, 20 min) in Xenopus oocytes expressing WT Slack, or the MMPSI mutants R409Q Slack or A913T Slack cRNA. n = 3 oocytes/condition. (D) Slack R409Q and A913T mutant channels produce no change in mass after activation by bithionol (10 μM), n = 16, p<0.001. (E) Changes in mass produced by activation of PKC by TPA in R409Q and A913T Slack expressing cells are not different from those in untransfected (Vector Control cells). (F) Differences in mass change between untransfected and Slack-expressing cells in response to TPA treatment. Mass change in R409Q and A913T mutant Slack-expressing cells was not different from untransfected cells but significantly different from WT Slack cells (n = 16, p<0.001). Error bars ± SEM.
Slack-induced changes in mass are absent in Slack MMPSI mutants
To determine whether human Slack mutations that produce infantile seizures coupled to severe intellectual disability alter the normal pattern of mass distribution upon channel stimulation, we studied two previously described MMPSI mutations, Slack-R409Q and -A913T (Barcia et al., 2012). Expression of these mutant channels yield currents that resemble those of wild-type Slack, but their amplitude is increased by 2–3 fold and they cannot be further activated by PKC (Barcia et al., 2012). We first tested their sensitivity to pharmacological stimulation. Despite the fact that they are insensitive to TPA (Barcia et al., 2012), the currents of both mutant channels were activated by bithionol (10 μM) to the same extent as wild-type channels (Figure 2C). Surprisingly, however, we found that that both mutants failed to produce any change in mass in response to either bithionol (Figure 2D) or TPA (Figure 2E and 2F). The time course of changes in mass upon activation of PKC by TPA in cells expressing Slack-MMPSI mutant channels was identical to that in cells transfected with only control vector (Figure S2). The loss of Slack-associated changes in mass at the plasma membrane may therefore be linked to the pathogenesis of MMSPI.
Slack interacts with FMRP, Cyfip1 and Phactr1
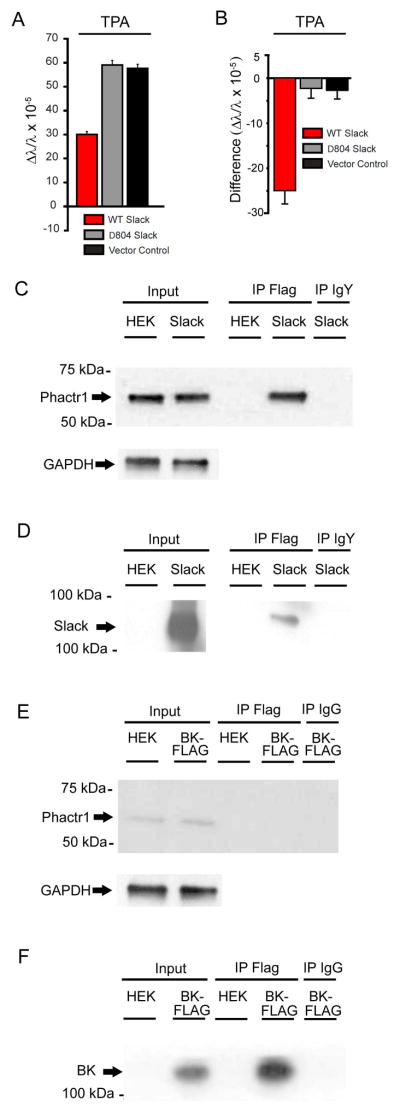
In addition to altering ion flux across the plasma membrane, the activation of ion channels can directly influence cytoplasmic events through protein-protein interactions between the channels and cellular signaling molecules (Calhoun and Isom, 2014; Deng et al., 2013; Ferron et al., 2014; Gross et al., 2011). Previous studies showed that the distal C-terminus of Slack interacts with FMRP, and that deletion of the Slack C-terminal domain distal to residue 804 results in functional channels that fail to interact with FMRP (Brown et al., 2010). We found that deletion of this same domain abolished the decrease in mass upon channel stimulation, indicating that this domain is required for the channel-protein interactions that lead to the loss of mass (Figure 3A). We therefore carried out experiments to test whether protein-protein interactions with FMRP or other cytoplasmic factors were required for the observed mass effects.
Figure 3. Slack interacts with Phacr1.
(A) The changes in mass produced by PKC activation in cells expressing wild type Slack differs from that in cells expressing Δ804 Slack, which lacks the distal C-terminus, or untransfected cells (n = 16, p<0.001). Error bars ±SEM. (B) Data in (A) are plotted as the difference between untransfected cells and Slack-expressing cells. (C–F) Phactr1 binds to Slack channels but not BK channels. Cell lysates were prepared from Slack-Flag (C and D) or BK-Flag (E and F) expressing cells and coimmunoprecipitation was performed using anti-Flag antibodies followed by western blotting with anti-Phactr1 (C and E) antibodies or anti-Slack (D and F) antibodies (n=2/condition). GAPDH loading controls for cell lysates of Slack-Flag (C) and BK-Flag (E) inputs were performed.
To identify proteins other than FMRP that might interact with the C-terminus of Slack, we used the yeast 2-hybrid approach to identify two additional Slack-interacting proteins, Phactr1 (Phosphatase and Actin Regulator 1) and Cyfip1 (Cytoplasmic FMR1-Interacting Protein 1) (Figure S3). The interaction of Phactr1 with Slack was confirmed with co-immunopurification of Slack from transfected HEK cells (Figure 3C and 3D) and mouse brain (Figure S4), followed by Western blotting. Phactr1 did not co-immunoprecipitate with BK channels, indicating the specificity of this interaction with Slack (Figure 3E).
Phactr1 is required for Slack-dependent changes in mass at the plasma membrane
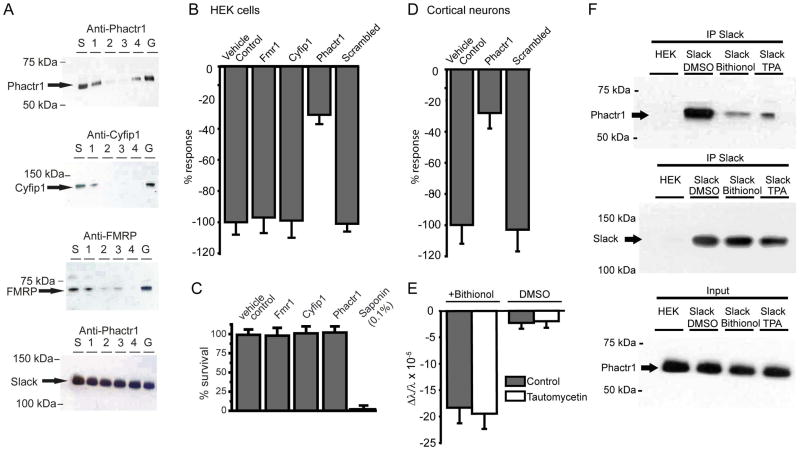
FMRP, Phactr1 and Cyfip1 are all expressed endogenously in HEK cells. To determine if one of these proteins is required for the decrease in mass at the plasma membrane upon Slack stimulation, we suppressed expression of each protein using RNAi. Conditions were optimized by detecting decreased protein expression of the target protein with individual or siRNAs in combination (Figure 4A). A decrease in levels of Phactr1, but not those of FMRP or Cyfip1, strongly suppressed the ability of Slack stimulation to produce a decrease in mass at the membrane (Figure 4B). RNAi treatments had no effect on cell viability (Figure 4C). To confirm that Phactr1 is required for changes in mass produced by Slack stimulation in real neurons, we repeated this experiment using mouse primary cortical neurons (Figure 4D). In these neurons, as in the HEK cells, down-regulation of Phactr1 strongly suppressed the effects of Slack stimulation.
Figure 4. Phactr1 is required for Slack-induced changes in mass following phosphorylation or pharmacological stimulation.
(A) Blot showing decreased expression of Slack-associated proteins following RNAi against FMRP, Cyfip1, or Phactr1 in WT Slack-expressing cells. Lanes are s=scrambled siRNA negative control, 1 – 3 = three different individual siRNAs alone, 4 = equimolar combination of the 3 siRNAs, G=GAPDH positive control. Bottom blot shows that RNAi against Phactr1 did not decrease Slack expression. (B) RNAi against Phactr1, but not FMRP or Cyfip1, significantly reduced the mass change produced by bithionol(10 μM) in WT Slack cells (n=8, p<0.001, Error bars ± SEM). (C) RNAi against Slack-associated proteins, does not decrease cell viability in a Resazurin assay. A standard curve of cell survival was generated by utilizing 10 fold dilutions of Saponin from 0.1% to 0%, with 0.1% Saponin treated cells considered no cell survival, and 0% Saponin treated cells considered complete cell survival, n = 3 wells/condition, Z’ for assay = 0.60 (D) Changes in mass produced in mouse primary cortical neurons by the Slack activator niclosamide (1 μM) are suppressed in neurons treated with RNAi against Phactr1(n=12, p<0.01). (E) Pre-treatment of Slack-B expressing cells with the PP1 inhibitor tautomycetin (2 μM) does not alter bithionol-induced changes in mass in WT Slack cells (n=8). (F) Western blots demonstrating that treatment of WT Slack cells with either bithionol (10 μM) or TPA (100 nM) strongly reduces levels of Phactr1 that can be co-immunoprecipitated with Slack channels (n=2/condition).
Phactr1 regulates the cellular localization of protein phosphatase 1 (PP1) (Allen et al., 2004; Wiezlak et al., 2012), and dissociation of Phactr1, presumably together with PP1, is likely to promote and maintain phosphorylation of the Slack channel. To determine whether the activity of PP1 is required for changes in mass at the plasma membrane, we tested the effects of the PP1 inhibitor tautomycetin (5 μM), which selectively inhibits PP1 over Protein Phosphatase 2A (Mitsuhashi et al., 2003). This agent did not, however, alter changes in mass at the plasma membrane on Slack stimulation, indicating that PP1 activity per se is not required (Figure 4E).
The requirement for expression of Phactr1 for the observed change in mass on stimulation of Slack channels could represent the physical dissociation of Phactr1 from the channel, or could reflect Phactr1-dependent dissociation of some other factor. To measure changes in Phactr1/Slack association directly, we immunoprecipitated Slack channels before and after activation by TPA and bithionol. Levels of Phactr1 that were detected in the immunoprecipitate were very greatly reduced after channel stimulation (Figure 4F), confirming that loss of mass at the plasma membrane occurs when Phactr1 dissociates from Slack channels. Thus, PKC translocates to the membrane and phosphorylates Slack channels at S407 causing the release the Phactr1 and subsequent loss of mass at the membrane.
Discussion
Our findings indicate that the cytoplasmic C-terminal of Slack KNa channels interacts with several cytoplasmic signaling molecules including the phosphatase targeting protein Phactr1. On stimulation of the activity of Slack channels, either by the activation of PKC or by pharmacological agents, Phactr1 dissociates from the channel complex. It is possible that the change in mass at the plasma membrane that we measure both in transfected cells and cortical neurons in response to channel stimulation reflects the dissociation of other factors in addition to Phactr1. Nevertheless, suppression of the expression of either FMRP or Cyfip1 did not alter changes in mass at the membrane following Slack stimulation, indicating that these interacting proteins are not required for the observed effects.
We have shown that the distal C-terminus of Slack is required for Phactr1/Slack interactions. This sequence of this region of Slack differs substantially from the corresponding regions in Slick and BK (Bhattacharjee et al., 2003; Salkoff et al., 2006), which apparently do not interact with Phactr1. Although the overall structure of Slack channels has recently been solved by cryo-electron microscopy (Hite et al., 2015), the distal cytoplasmic C-terminal region could not be resolved clearly, providing no insights into how this region may contribute to Phactr1 biding.
The dissociation of Phactr1 is likely to regulate phosphorylation of the Slack channel. It may, however, potentially also regulate other channel-associated proteins such as FMRP. Alternatively, its dissociation from the channel may allow it to interact with other membrane or cytoplasmic targets. The disassociation of Phact1 on channel stimulation may therefore provide a signal that links neuronal excitability to downstream signaling pathways. The finding that the Phactr1/Slack interaction is entirely abolished in mutant Slack channels that produce childhood epilepsy with very severe developmental delay (Kim and Kaczmarek, 2014), suggests that this pathway may be crucial to normal neuronal development.
Experimental Procedures
Animals
Rodents were handled in accordance with protocols approved by the Yale University Institutional Animal Care Committee.
Expression Slack-B or Mutant Expression Vectors
Slack-B S407A, R409Q, A913T, and Δ804 C-terminal truncation mutants were constructed as previously described (Barcia et al., 2012; Brown et al., 2010). Vector DNA concentrations were determined by a flourometric Qubit dsDNA Broad Range assay kit (Life Technologies). Transfections were performed with FugeneHD (Promega) transfection reagent. BIND Biosensor plates were seeded with 10,000 HEK293T cells/well. 0.2 μg DNA and 0.6 μL FugeneHD were diluted in 10 μL low serum OPTI-MEM medium (Life Technologies) for 15 minutes to allow DNA/FugeneHD complexes to form and added to HEK293T seeded plates and incubate under 95% Air/5% CO2 at 37° C for 48 hours.
Two-electrode voltage clamp in Xenopus oocytes
cRNA was created from Slack-B and mutant channel cRNA in pOX oocytes expression vector with a mMessage mMachine T3 kit (Ambion) and aliquoted in sterile water. Oocytes were isolated from Xenopus laevis frogs, injected with 50 – 100 ng of cRNA, and incubated in MND96 (Brown et al., 2010) at 18° C for up to 7 days before experiments were performed. Whole-oocyte currents were measured by a two-electrode voltage clamp amplifier (OC-725C, Warner Instruments.) Electrodes were filled with 3 M KCl and had resistance 0.1 – 1 MΩ. Standard bath solution was MND96. Ooctyes were depolarized by 400 ms pulses from a holding potential of −90 mV to test pulses of between −80 and +60 mV in 10 mV increments for 5 seconds. Data recording and analysis were performed using pClamp (Molecular Devices), Excel (Microsoft) and Origin 8.1 (OriginLab Corporation) software packages.
BIND Scanner Assays
Poly-D-lysine/Laminin coated TiO2 384-well BIND biosensor plates were utilized for all Scanner assays. For HEK293 cell based experiments, cells were counted with a hemocytometer and plated at a density of 10k cells/well in growth media and incubated for 24–48 hours. For primary cortical neuron based assays, neurons from postnatal day 2 or 3 male mice were prepared as described (Brewer and Torricelli, 2007) and incubated for 7 days. After 7 days incubation, growth media was removed and HBSS with 0.1% DMSO was added. Cells were allowed to adjust to room temperature for 1 hour before a baseline measurement was recorded.
Measurements were recorded at 3.75 μm/pixel resolution for the central 1.0 mm2 area of each well. Compounds or DMSO controls were added, and 30 minutes later a second measurement was recorded. A density gradient map was generated for both records, and individual cells and the local background selected utilizing BIND Scanner analysis software. The change in the index of refraction for each cell and the local background as a function of the difference between the first and second images was calculated. The change in index of refraction for each cell was normalized to the local background, and a mean value calculated for each well. The change in peak wavelength of refraction was divided by the initial peak wavelength to give a physically relevant change in the index of refraction. For primary cortical neuron experiments, niclosamide addition was normalized to DMSO vehicle alone addition. Results were exported to Microsoft Excel and Origin Statistical Package for analysis.
Statistical Analysis
Data recording and analysis were performed using pClamp (Molecular Devices), Excel (Microsoft), Origin (Origin Lab Corporation), and BIND Scanner and BIND View (X-BODY Biosciences). The mean±SEM values are plotted. Where 2 groups were compared, 2-tailed student’s t-test was performed to compare statistical significance; where 3 or more groups were compared, 1-way ANOVA test was performed.
See Supplemental Experimental Procedures for chemicals, cell culture, creation of mammalian expression vectors, animals, yeast two hybrid assay, RNAi against candidate Slack-B binding partners, cell viability assay, protein purification of RNAi samples, co-immunopurification experiments, western blotting, as well as detailed experimental procedures.
Supplementary Material
Figure S1 (Related to Figure 2): Activation of Slack-B by the small molecule activator bithionol and by channel phosphorylation are independent, and the effect of bithionol activation is unchanged in Slack-S407A phosphorylation mutants.
Slack transfected A) Xenopus oocytes or B) HEK cells were treated with either bithionol (10 μM), TPA (100 nM), or bithionol followed 20 minutes later by TPA. After 20 minutes incubation, either (A) two-electrode voltage clamp electrophysiology or (B) resonance wavelength grating optical biosensor assay was performed. For (A), the maximum percent increase in current from potentials of −80 mV to +60 mV was calculated (n= 3–4 oocytes/condition, p<0.01), and for (B) change in mass near the plasma was calculated (n=16 wells/condition, p<0.001).
Slack or Slack-S407A transfected (C) Xenopus oocytes or (D) HEK cells were treated with bithionol (10 μM) and either (C) two-electrode voltage clamp electrophysiology or (D) resonance wavelength grating optical biosensor assay was performed 20 minutes after bithionol treatment. For (C), the maximum percent increase in current from potentials of −80 mV to +60 mV was calculated (n= 4 oocytes/condition), and for (D) change in mass near the plasma was calculated (n=16 wells/condition). Error bars ± SEM.
Figure S2 (Related to Figure 2): TPA-induced mass redistribution in cells expressing MMPSI associated Slack mutant channels, is identical to that of cells transfected with only with vector.
Slack, Slack-R409Q, or Slack-A913T transfected HEK cells and those transfected with vector alone were treated with the PKC activator TPA (100 nM) and the change in mass near the plasma membrane recorded (n=32 wells/condition). Error bars ± SEM.
Figure S3 (Related to Figure 3): Protein-protein interactions between Slack-Phactr1 and Slack-Cyfip1 were identified by a yeast-two hybrid assay.
Using a Gal-4 based yeast two-hybrid assay, the C-terminus of Slack was inserted into pGBKT7 and screened against a mouse brain cDNA library in pGADT7 vector. AH109 yeast cells were sequentially transformed with Slack-pGBKT7 and then the mouse cDNA library. Two of these clones from the initial screen containing (a) Phactr1 (aa1-435 NP_001289564) and (b) Cyfip1 (aa 1-326, NP_035500) demonstrated grown in restrictive conditions (-Leu, -Trp, -His).
Figure S4 (Related to Figure 3): Phactr1 co-immunoprecipitates with Slack in mouse brain.
Cell lysates were prepared from mouse brain in either wild-type or Slack knock-out mice. Co-immunoprecipitation was performed using anti-Slack antibodies or chicken IgY antibodies followed by western blotting with anti-Phactr1 antibodies. Left, middle, and right panels are from the same blot but were separated, reordered and adjusted in width for clarity of presentation. Also shown are GAPDH loading controls for cell lysates of wild-type or Slack knock-out mice (n=2/condition).
Acknowledgments
The authors are grateful to Yangyang Yan and Fred Sigworth at Yale University for donation of Slack-B, Slick and BK cell lines and technical assistance with protein purification, and to Steven Shamah and Brandt Binder at X-BODY Biosciences for the usage of the BIND Scanner system and technical assistance. This work is supported by grants from the NIH (HD067517) and from FRAXA to L.K.K.
Footnotes
Author contributions: M.R.F. and L.K.K designed research. M.R.F., M.R.B, J.K., Y.Z. and D.P.G. performed research; G.H and R.N. generated MMPSI mutants; A.E.B, P.R and R.L generated Slack−/− animals; D.S.N carried out yeast-two hybrid experiments. M.R.F, M.R.B and L.K.K wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen PB, Greenfield AT, Svenningsson P, Haspeslagh DC, Greengard P. Phactrs 1–4: A family of protein phosphatase 1 and actin regulatory proteins. Proc Natl Acad Sci U S A. 2004;101:7187–7192. doi: 10.1073/pnas.0401673101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia G, Fleming MR, Deligniere A, Gazula VR, Brown MR, Langouet M, Chen H, Kronengold J, Abhyankar A, Cilio R, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44:1255–1259. doi: 10.1038/ng.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Gan L, Kaczmarek LK. Localization of the Slack potassium channel in the rat central nervous system. J Comp Neurol. 2002;454:241–254. doi: 10.1002/cne.10439. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci. 2003;23:11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biton B, Sethuramanujam S, Picchione KE, Bhattacharjee A, Khessibi N, Chesney F, Lanneau C, Curet O, Avenet P. The antipsychotic drug loxapine is an opener of the sodium-activated potassium channel slack (Slo2.2) J Pharmacol Exp Ther. 2012;340:706–715. doi: 10.1124/jpet.111.184622. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protoc. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci. 2010;13:819–821. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Spilianakis CG, Flavell RA, von Hehn CA, Bhattacharjee A, Kaczmarek LK. Amino-termini isoforms of the Slack K+ channel, regulated by alternative promoters, differentially modulate rhythmic firing and adaptation. J Physiol. 2008;586:5161–5179. doi: 10.1113/jphysiol.2008.160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun JD, Isom LL. The role of non-pore-forming beta subunits in physiology and pathophysiology of voltage-gated sodium channels. Handb Exp Pharmacol. 2014;221:51–89. doi: 10.1007/978-3-642-41588-3_4. [DOI] [PubMed] [Google Scholar]
- Daghestani HN, Day BW. Theory and applications of surface plasmon resonance, resonant mirror, resonant waveguide grating, and dual polarization interferometry biosensors. Sensors (Basel) 2010;10:9630–9646. doi: 10.3390/s101109630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS, Klyachko VA. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77:696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Luders HO, Shi J, Cui J, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- Fang Y, Li G, Ferrie AM. Non-invasive optical biosensor for assaying endogenous G protein-coupled receptors in adherent cells. J Pharmacol Toxicol Methods. 2007;55:314–322. doi: 10.1016/j.vascn.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Ferron L, Nieto-Rostro M, Cassidy JS, Dolphin AC. Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat Commun. 2014;5:3628. doi: 10.1038/ncomms4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MR, Kaczmarek LK. Use of optical biosensors to detect modulation of Slack potassium channels by G protein-coupled receptors. J Recept Signal Transduct Res. 2009;29:173–181. doi: 10.1080/10799890903056883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MR, Shamah SM, Kaczmarek LK. Use of label-free optical biosensors to detect modulation of potassium channels by G-protein coupled receptors. J Vis Exp. 2014:e51307. doi: 10.3791/51307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Yao X, Pong DL, Jeromin A, Bassell GJ. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci. 2011;31:5693–5698. doi: 10.1523/JNEUROSCI.6661-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hite RK, Yuan P, Li Z, Hsuing Y, Walz T, MacKinnon R. Cryo-electron microscopy structure of the Slo2.2 Na(+)-activated K(+) channel. Nature. 2015;527:198–203. doi: 10.1038/nature14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK. Slack, Slick and Sodium-Activated Potassium Channels. ISRN Neurosci. 2013 doi: 10.1155/2013/354262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GE, Kaczmarek LK. Emerging role of the KCNT1 Slack channel in intellectual disability. Front Cell Neurosci. 2014;8:209. doi: 10.3389/fncel.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GE, Kronengold J, Barcia G, Quraishi IH, Martin HC, Blair E, Taylor JC, Dulac O, Colleaux L, Nabbout R, et al. Human slack potassium channel mutations increase positive cooperativity between individual channels. Cell Rep. 2014;9:1661–1672. doi: 10.1016/j.celrep.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH. Label-free optical biosensor: a tool for G protein-coupled receptors pharmacology profiling and inverse agonists identification. J Recept Signal Transduct Res. 2009;29:146–153. doi: 10.1080/10799890903064390. [DOI] [PubMed] [Google Scholar]
- Lin B, Qiu J, Gerstenmeier J, Li P, Pien H, Pepper J, Cunningham B. A label-free optical technique for detecting small molecule interactions. Biosens Bioelectron. 2002;17:827–834. doi: 10.1016/s0956-5663(02)00077-5. [DOI] [PubMed] [Google Scholar]
- Lu R, Bausch AE, Kallenborn-Gerhardt W, Stoetzer C, Debruin N, Ruth P, Geisslinger G, Leffler A, Lukowski R, Schmidtko A. Slack channels expressed in sensory neurons control neuropathic pain in mice. J Neurosci. 2015;35:1125–1135. doi: 10.1523/JNEUROSCI.2423-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S, Shima H, Tanuma N, Matsuura N, Takekawa M, Urano T, Kataoka T, Ubukata M, Kikuchi K. Usage of tautomycetin, a novel inhibitor of protein phosphatase 1 (PP1), reveals that PP1 is a positive regulator of Raf-1 in vivo. J Biol Chem. 2003;278:82–88. doi: 10.1074/jbc.M208888200. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P. BKCa channel dysfunction in neurological diseases. Front Physiol. 2014;5:373. doi: 10.3389/fphys.2014.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Santi CM, Ferreira G, Yang B, Gazula VR, Butler A, Wei A, Kaczmarek LK, Salkoff L. Opposite regulation of Slick and Slack K+ channels by neuromodulators. J Neurosci. 2006;26:5059–5068. doi: 10.1523/JNEUROSCI.3372-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki T, Schwab B, Thompson NC, Elson EL. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J Cell Sci. 2001;114:1025–1036. doi: 10.1242/jcs.114.5.1025. [DOI] [PubMed] [Google Scholar]
- Wiezlak M, Diring J, Abella J, Mouilleron S, Way M, McDonald NQ, Treisman R. G-actin regulates the shuttling and PP1 binding of the RPEL protein Phactr1 to control actomyosin assembly. J Cell Sci. 2012;125:5860–5872. doi: 10.1242/jcs.112078. [DOI] [PubMed] [Google Scholar]
- Yang B, Gribkoff VK, Pan J, Damagnez V, Dworetzky SI, Boissard CG, Bhattacharjee A, Yan Y, Sigworth FJ, Kaczmarek LK. Pharmacological activation and inhibition of Slack (Slo2.2) channels. Neuropharmacology. 2006;51:896–906. doi: 10.1016/j.neuropharm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brown MR, Hyland C, Chen Y, Kronengold J, Fleming MR, Kohn AB, Moroz LL, Kaczmarek LK. Regulation of neuronal excitability by interaction of fragile X mental retardation protein with slack potassium channels. J Neurosci. 2012;32:15318–15327. doi: 10.1523/JNEUROSCI.2162-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (Related to Figure 2): Activation of Slack-B by the small molecule activator bithionol and by channel phosphorylation are independent, and the effect of bithionol activation is unchanged in Slack-S407A phosphorylation mutants.
Slack transfected A) Xenopus oocytes or B) HEK cells were treated with either bithionol (10 μM), TPA (100 nM), or bithionol followed 20 minutes later by TPA. After 20 minutes incubation, either (A) two-electrode voltage clamp electrophysiology or (B) resonance wavelength grating optical biosensor assay was performed. For (A), the maximum percent increase in current from potentials of −80 mV to +60 mV was calculated (n= 3–4 oocytes/condition, p<0.01), and for (B) change in mass near the plasma was calculated (n=16 wells/condition, p<0.001).
Slack or Slack-S407A transfected (C) Xenopus oocytes or (D) HEK cells were treated with bithionol (10 μM) and either (C) two-electrode voltage clamp electrophysiology or (D) resonance wavelength grating optical biosensor assay was performed 20 minutes after bithionol treatment. For (C), the maximum percent increase in current from potentials of −80 mV to +60 mV was calculated (n= 4 oocytes/condition), and for (D) change in mass near the plasma was calculated (n=16 wells/condition). Error bars ± SEM.
Figure S2 (Related to Figure 2): TPA-induced mass redistribution in cells expressing MMPSI associated Slack mutant channels, is identical to that of cells transfected with only with vector.
Slack, Slack-R409Q, or Slack-A913T transfected HEK cells and those transfected with vector alone were treated with the PKC activator TPA (100 nM) and the change in mass near the plasma membrane recorded (n=32 wells/condition). Error bars ± SEM.
Figure S3 (Related to Figure 3): Protein-protein interactions between Slack-Phactr1 and Slack-Cyfip1 were identified by a yeast-two hybrid assay.
Using a Gal-4 based yeast two-hybrid assay, the C-terminus of Slack was inserted into pGBKT7 and screened against a mouse brain cDNA library in pGADT7 vector. AH109 yeast cells were sequentially transformed with Slack-pGBKT7 and then the mouse cDNA library. Two of these clones from the initial screen containing (a) Phactr1 (aa1-435 NP_001289564) and (b) Cyfip1 (aa 1-326, NP_035500) demonstrated grown in restrictive conditions (-Leu, -Trp, -His).
Figure S4 (Related to Figure 3): Phactr1 co-immunoprecipitates with Slack in mouse brain.
Cell lysates were prepared from mouse brain in either wild-type or Slack knock-out mice. Co-immunoprecipitation was performed using anti-Slack antibodies or chicken IgY antibodies followed by western blotting with anti-Phactr1 antibodies. Left, middle, and right panels are from the same blot but were separated, reordered and adjusted in width for clarity of presentation. Also shown are GAPDH loading controls for cell lysates of wild-type or Slack knock-out mice (n=2/condition).