Abstract
Schwann cells (SCs) detect injury to peripheral nerves and transform phenotypically to respond to injury and facilitate repair. Cell-signaling pathways and changes in gene expression that drive SC phenotypic transformation in injury have been described; however, the SC receptors that detect PNS injury have not been identified. LDL receptor-related protein (LRP1) is a receptor for numerous ligands, including intracellular proteins released by injured cells and protein components of degenerated myelin. In certain cell types, including SCs, LRP1 is a cell-signaling receptor. Herein, we show that binding of the LRP1 ligand, tissue-type plasminogen activator (tPA), to cultured rat SCs induces c-Jun phosphorylation, a central event in activation of the SC Repair Program. The response to tPA was blocked by the LRP1 antagonist, receptor-associated protein (RAP). c-Jun phosphorylation also was observed when cultured rat SCs were treated with a recombinant derivative of matrix metalloproteinase-9 that contains the LRP1 recognition motif (PEX). The ability of LRP1 to induce c-Jun phosphorylation and ERK1/2 activation was confirmed using cultures of human SCs. When tPA or PEX was injected directly into crush-injured rat sciatic nerves, c-Jun phosphorylation and ERK1/2 activation were observed in SCs in vivo. The ability of LRP1 to bind proteins released in the earliest stages of PNS injury and to induce c-Jun phosphorylation support a model in which SC LRP1 functions as an injury detection receptor in the PNS.
Keywords: Schwann cell, LDL receptor-related protein 1, tissue-type plasminogen activator, matrix metalloproteinase-9, repair program, c-Jun
Introduction
In the peripheral nervous system (PNS), Schwann cells (SCs) serve as “first responders” to injury. Distal to the injury site, SCs de-differentiate, down-regulating myelin gene expression and substantially altering their mRNA transcriptome. This process involves: 1) up-regulation of pathways that support survival in the challenging microenvironment of the injured PNS; 2) activation of the transcription factor c-Jun; 3) orchestration of the inflammatory response; and 4) secretion of growth factors and structural proteins that guide and support axonal regeneration [1–3]. Activated SCs also participate in the first phase of myelin degradation [1, 3, 4]. Collectively, these changes are referred to as “Activation of the SC Repair Program” [3]. Considerable work has been conducted to determine the changes in cell-signaling and gene expression that drive these robust and important changes in SC phenotype. However, receptors that sense nerve injury remain unidentified.
LDL receptor-related protein 1 (LRP1) is a type I transmembrane receptor with endocytic and cell-signaling activity, which is expressed by SCs selectively in nerve injury and promotes many of the changes observed in SCs in the activated Repair Program [5–8]. When presented with specific ligands, LRP1 activates cell-signaling pathways in SCs, which support SC survival, even in the absence of axonal contact [5, 9]. By regulating ERK1/2, Rac1, and RhoA, LRP1 promotes SC migration [6–8]. Furthermore, LRP1 functions in the phagocytosis of debris that may accumulate in PNS injury [10]. Finally, by activating ERK1/2, LRP1 ligands induce expression of MCP-1/CCL2, a potent chemoattractant for inflammatory cells in PNS injury [11]. These activities position LRP1 favorably to function as a key receptor in activation of the SC Repair Program.
Unlike most other receptors, LRP1 binds over 60 ligands that are both structurally and functionally diverse [12]. LRP1 ligands that have been identified by proteomics screens include protein components of degenerated myelin and intracellular proteins that may be released into the extracellular spaces following tissue injury [13]. We hypothesized that the broad ligand-binding capacity of LRP1 may allow this receptor to survey the cellular microenvironment for evidence of injury and trigger early cell-signaling responses, required for activation of the SC Repair Program.
Herein, we define LRP1 as an activator of the global transcription factor, c-Jun, which plays a central role in activation of the SC Repair Program. We show that different LRP1 ligands, including tissue plasminogen activator (tPA) and a GST fusion protein that includes the hemopexin domain of matrix metalloproteinase-9 (PEX) [7], activate c-Jun in primary cultures of rat SCs in vitro. This response was blocked by an LRP1 signaling antagonist, Receptor-associated protein (RAP). LRP1 demonstrated equivalent activities in studies with human SCs. When LRP1 ligands were injected into rat sciatic nerves, 24 h after crush injury, c-Jun was robustly activated in vivo. Finally, we have shown for the first time that LRP1 is expressed by human SCs in culture and initiates c-Jun activation. The function of LRP1 as a cell-signaling receptor, therefore, is conserved in human and rat SCs. Our results suggest that LRP1 may function as a key SC injury detection receptor and play a pivotal role in activating the SC Repair Program in PNS injury.
Methods
Proteins and reagents
Non-enzymatic, non-cleavable human tPA was purchased from Molecular Innovations (Novi, MI). RAP and PEX were expressed as GST-fusion proteins and purified as described previously [7, 14]. Proteins expressed in bacteria were subjected to urea treatment followed by dialysis and Detoxi-Gel endotoxin-removing columns (ThermoFisher, Waltham, MA). The following antibodies and dilutions were used for immunoblotting: Phospho-c-Jun (#9261, 1:1000), total c-Jun (#9165, 1:1000), phospho-ERK1/2 (#9101, 1:1000), total ERK1/2 (#9102, 1:1000) and HRP-linked anti-Rabbit IgG (#7074, 1:2000). These antibodies were purchased from Cell Signaling Technologies (Danvers, MA). Antibodies targeting β-actin (A5316, 1:5000) and the C-terminal light chain of LRP1 (L2170, 1:1000) were from Sigma-Aldrich (St. Louis, MO). HRP-linked anti-mouse IgG (#1706516, 1:5000) was from BioRad (Hercules, CA). Immunofluorescence (IF) microscopy was performed using antibody that detects myelin protein P0 (AB9352, 1:250) from Millipore (Billerica, MA), phosphorylated-c-Jun (#9261, 1:100) from Cell Signaling Technologies (Danvers, MA), Alexa Fluor® 568 conjugated anti-Rabbit IgG (A10042, 1:300) from ThermoFisher (Waltham, MA), and Alexa Fluor® 488 conjugated anti-Chicken IgG (703-545-155, 1:300) from Jackson ImmunoResearch Laboratories, Inc (West Grove, PA).
Cell culture
Rat SCs were isolated from sciatic nerves extracted from 1-day-old Sprague Dawley rats and purified with Thy1.1 antibody (M-7898, Sigma-Aldrich St. Louis, MO) and rabbit complement (S7764, Sigma-Aldrich St. Louis, MO) as previously described [5]. Primary cultures of > 95% rat SCs [5] were maintained in complete medium consisting of Dulbecco’s Modified Eagle Medium (DMEM; 11885, Gibco®) supplemented with 10 % heat inactivated FBS (SH30080.03, HyClone™), 100 U/ml penicillin, 100 μg/ml streptomycin, 21 μg/ml bovine pituitary extract (CC-4009, Lonza) and 4 μM forskolin (344270, Millipore) at 37 °C in humidified atmosphere with 5 % CO2. Primary cultures of human SCs, isolated from human spinal nerve, were purchased from ScienCell Research Laboratories (#1700; Carlsbad, CA) and maintained in the manufacturer’s SC Medium (#1701) at 37 °C with 5 % CO2. Experiments were performed with cells that were passaged no more than seven times.
Activation of c-Jun in vitro
SCs were seeded into 6-well plates at a density of 1–3 × 105 cells per well and grown until 70–80 % confluent. Cultures were then washed with and maintained in serum-free medium (UltraCULTURE™, 12-725F, Lonza) supplemented with 2 mM L-glutamine (25030-081, Gibco®) for a total of 35 min (rat SCs) or 45 min (human SCs). LRP1 ligands (100 nM MMP-9-PEX or 12 nM tPA) were added as indicated. In some studies, the LRP1 cell-signaling antagonist, RAP (300 nM), was added 20 min before the agonistic ligands. Cells were washed in ice cold 20 mM sodium phosphate, 150 mM NaCl, pH 7.4 (PBS) and extracted in RIPA buffer (1 % Triton X-100, 0.5 % sodium deoxycholate, 0.1 % SDS, 1 mM sodium orthovanadate) with protease inhibitors (Complete™, mini EDTA free, Roche). Protein concentrations of cell extracts were determined by bicinchoninic acid assay (BCA; 23225, Pierce) and equal amounts of protein (5–20 μg) were subjected to immunoblotting.
Immunoblotting
Protein extracts were subjected to SDS-PAGE (10 %) and transferred to nitrocellulose membranes using the Mini Trans-Blot® system (BioRad, Hercules, CA). Membranes were blocked with 5 % nonfat dry milk (#170-6404, Biorad) in 10 mM Tris/HCl, 150 mM NaCl, pH 7.5, 0.1 % Tween® 20 (TBST) for 1 h at room temperature. Primary antibodies diluted in 5 % BSA/TBST were incubated overnight at 4 °C. Membranes were washed with TBST and incubated with secondary HRP-coupled antibodies in TBST with 5 % nonfat dry milk for 1 h at room temperature. Protein bands were visualized using Pierce ECL 2 Substrate (#80196, Fisher Scientific) and X-ray films on a Kodak X-Omat 2000A and digitized with a CanoScan 5600F (Canon) scanner. Membranes were re-probed with additional antibodies after incubation in stripping buffer (62 mM Tris/HCl, pH 6.8, 2 % SDS, 100 mM β-mercaptoethanol) for 30 min at 50 °C followed by extensive washes with TBST.
Activation of c-Jun in SCs by LRP1 ligands in vivo
All animal procedures were performed according to protocols approved by the University of California, San Diego Committee on Animal Research, and conform to the NIH Guidelines for Animal Use. Sprague-Dawley rats (200 g) from Harlan Laboratories (San Diego, CA) were housed in pairs with 12-h-light/dark cycles and ad libitum access to food and water. Surgeries were performed as described previously [15]. Briefly, animals were anesthetized with 5 % isoflurane (Fluriso™, VetOne, Boise, ID) and anesthesia was maintained at 3 % isoflurane. Using sterile technique, the right sciatic nerve was exposed at mid-thigh level by separating the muscle layers and crushed for 15 s with a flat forceps. The muscle layer was closed with silk sutures and the skin with small surgical staples. 24 h after injury, rats were anesthetized and 2 μL of LRP1 ligand (12 μM tPA or 20 μM PEX) or vehicle (PBS) was injected into the sciatic nerve at the crush injury site. After 15 min, sciatic nerve was collected distal to the crush injury site (0–3 mm). Contralateral nerve was collected as a control. Tissue samples were extracted in RIPA buffer with subsequent sonication (Fisher Scientific 500 Sonic Dismembrator). Protein concentrations were determined and immunoblotting was performed as described above. For immunofluorescence (IF) microscopy studies, sciatic nerve tissue samples were rinsed with PBS, embedded in Tissue-Tek® optimum cutting temperature compound, and fresh-frozen on dry ice.
Immunofluorescence microscopy
Fresh-frozen nerve tissues was sectioned (transversal, 10 μm thick) on a cryostat (Leica) and transferred to microscopy slides (VWR® superfrost® plus, 4811-703). Tissue sections were washed with PBS and blocked in 5 % BSA/PBS with 0.3 % Triton-X100 for 30 min at room temperature. Primary antibodies diluted in blocking solution were incubated overnight at 4 °C. Slides were washed with PBS and incubated with fluorescent secondary antibodies in blocking solution for 1 h at room temperature. Sections were sealed with mounting medium (ProLong Gold Antifade Reagent with DAPI; P36935, Molecular Probes™) and dried overnight. Images (800 × 800 pixel, 12 bits) were acquired using an Olympus FV1000 confocal microscope with an oil-immersion objective (60 X, 1.42 NA) and analyzed with ImageJ.
Statistical analysis
Data are represented as mean ± standard deviation (SD). Densitometry was performed with ImageJ (version 1.50i) and statistical comparisons were done by one-way ANOVA and Tukey’s post-hoc analysis in Prism (Version 6, GraphPad).
Results
LRP1 activates c-Jun in rat SCs in vitro
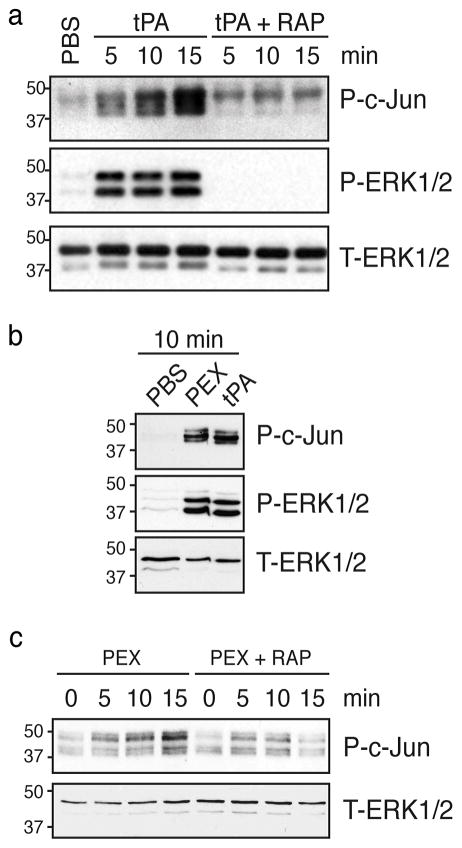
Rat SCs in primary culture were treated with the LRP1 ligand, tPA. Within 5 min, ERK1/2 was activated (Fig. 1a), as anticipated [15]. Robust c-Jun phosphorylation also was observed in the same timeframe. To confirm that c-Jun phosphorylation by tPA requires LRP1, we added the LRP1 cell-signaling antagonist, RAP. In the absence of tPA, RAP had no effect on c-Jun phosphorylation or ERK1/2 activation. When RAP was added together with tPA, ERK1/2 activation and c-Jun phosphorylation in response to tPA were blocked (Fig. 1a).
Figure 1. LRP1 ligands induce phosphorylation of c-Jun in primary cultures of rat SCs.
(a) Rat SCs were pretreated with RAP (300 nM) or vehicle and then with tPA (12 nM) for 5–15 min. Control cells were treated with vehicle (PBS). (b) Rat SCs were treated with PEX (100 nM) or tPA (12 nM) for 10 min. (c) Rat SCs were pretreated with RAP (300 nM) or vehicle and then with PEX for 5–15 min. Equal amounts of cellular protein were loaded into each lane and subjected to SDS-PAGE and electrotransfered to nitrocellulose. Membranes were probed to detect phosphorylated c-Jun (P-c-Jun), phosphorylated ERK1/2 (P-ERK1/2), and total ERK1/2 (T-ERK1/2) as a loading control. Each blot represents at least 2–4 independent studies.
Next, we tested the ability of a second LRP1 ligand, PEX, to induce c-Jun phosphorylation in cultured rat SCs. PEX includes the LRP1 binding motif in MMP-9 but lacks the protease active site [7, 16]. Fig. 1b shows that PEX induced c-Jun phosphorylation and ERK1/2 activation similarly to tPA (Fig. 1b). Time course experiments for the PEX response are shown in Fig. 1c. Importantly, RAP blocked c-Jun phosphorylation in response to PEX (Fig. 1c).
LRP1 ligands activate c-Jun in human SCs
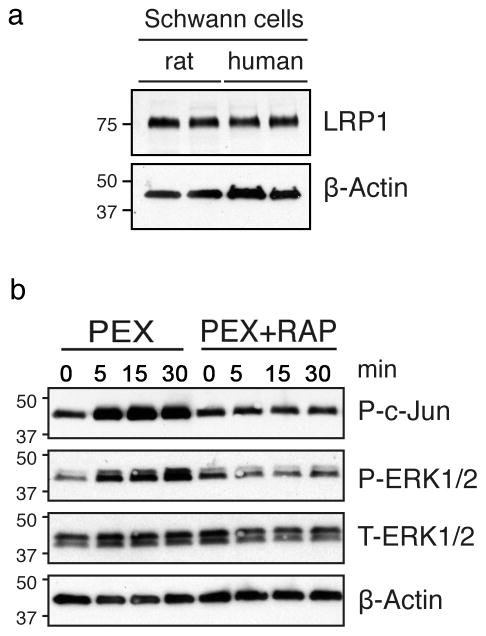
LRP1 has not been previously studied in human SCs. We identified LRP1 in cultured human SCs by immunoblot analysis, using a species cross-reactive antibody that detects the 85-kDa LRP1 light chain (Fig. 2a). The abundance of LRP1 in human SCs was similar to that detected in cultured rat SCs.
Figure 2. LRP1 activates c-Jun in human SCs.
(a) Extracts of human and rat SCs were probed by immunoblot analysis to detect the 85-kDa LRP1 light chain. (b) Immunoblot analysis was performed to detect phosphorylated c-Jun (P-c-Jun) and phosphorylated ERK1/2 (P-ERK1/2) in cultured human SCs treated with PEX (100 nM). Cultures were pretreated with RAP (300 nM) or vehicle as indicated. Equal amounts of cellular protein (5 μg) were loaded into each lane and subjected to SDS-PAGE and electrotransferred to nitrocellulose membranes. β-actin and total ERK1/2 were determined as loading controls. Each blot represents at least 2–3 independent experiments.
When PEX was incubated with cultured human SCs for 5–30 min, c-Jun was phosphorylated and ERK1/2 was activated (Fig. 2b). Both cell-signaling events appeared to occur concurrently. When human SCs were pre-treated with RAP, c-Jun phosphorylation and ERK1/2 activation were blocked. These results show that the ability of LRP1 to activate c-Jun is conserved in rat and human SCs.
LRP1 ligands activate c-Jun in SCs in vivo
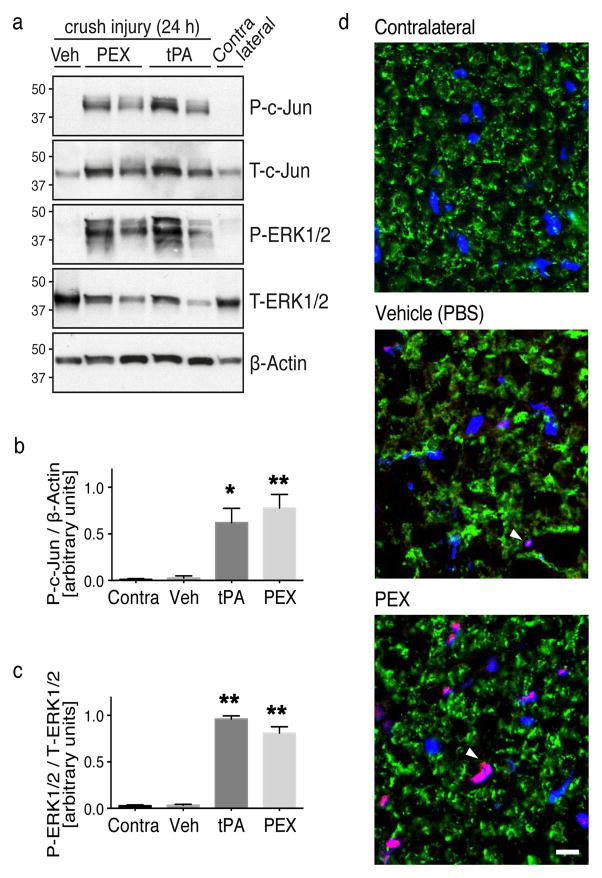
To test whether LRP1 promotes c-Jun phosphorylation in injured sciatic nerves in vivo, we injected tPA (12 μM), PEX (20 μM), or vehicle directly into crush-injured rat sciatic nerves. Injections were performed 24 h after crush injury when LRP1 expression is substantially up-regulated in SCs [5, 17]. Nerve tissue 0–3 mm distal to the injury site was collected 15 min after injection. Immunoblot analysis demonstrated a significant increase in c-Jun phosphorylation in nerves injected with either tPA (p < 0.05) or PEX (p < 0.01), compared with vehicle-treated controls (Fig. 3a,b). c-Jun phosphorylation was not observed in contralateral nerves.
Figure 3. Activation of c-Jun in SCs by LRP1 ligands in vivo.
(a) Immunoblot analysis was performed to detect phosphorylated c-Jun (P-c-Jun) and activated ERK1/2 (P-ERK1/2) in crush-injured rat sciatic nerves injected with 2 μl of PEX (20 μM), tPA (12 μM), or vehicle. Uninjured contralateral nerve was studied as a control. Nerves were injected 24 h after crush-injury and harvested 15 min after injecting ligands. Equal amounts of nerve protein (20 μg) were loaded into each lane and subjected to SDS-PAGE. Immunoblot analysis was performed to detect β-actin and total ERK1/2 as loading controls. Each lane represents a different rat. (b) Quantification of phosphorylated c-Jun relative to β-actin by densitometry (n=4 rats/group; * denotes p < 0.05; ** denotes p < 0.01 compared to contralateral nerve). (c) Quantification of phosphorylated ERK1/2 relative to total ERK1/2 by densitometry (n=4 rats/group; ** denotes p < 0.01 compared to contralateral). (d) IF microscopy to detect phosphorylated-c-Jun (red). Nerves were injected with vehicle, or PEX, 24 h after crush injury. Nerves were collected 15 min later. Contralateral nerves were examined as uninjured controls. Transverse cryosections (10 μm) of sciatic nerve tissue, 1–3 mm distal to the injury site, were examined. The compact myelin protein, P0 (green), was stained to highlight SC architecture. Images are at 60x magnification (scale bar 10 μm). Note c-Jun immunoreactivity co-localized with DAPI-stained nuclei (blue) in SC crescents (pink). Images represent n=2/group.
The absence of detectable c-Jun phosphorylation in vehicle-injected, crush-injured nerves may have reflected the short exposure times we used to demonstrate the robust response to PEX. Our protocol was not designed to detect changes in baseline cell-signaling (compared with contralateral nerve). We did observe an increase in total c-Jun in vivo after injection of tPA or PEX (Fig. 3a). This is consistent with previous reports showing rapid up-regulation of c-Jun protein expression by trophic factors [18].
In addition to c-Jun phosphorylation, ERK1/2 activation was observed in crush-injured sciatic nerves in response to LRP1 ligands (Fig 3a), as anticipated [7, 15]. The increase in phosphorylated (activated) ERK1/2 was similar when tPA or PEX was injected (Fig. 3c). Next, we performed dual labeling IF microscopy to detect phosphorylated c-Jun and the compact myelin marker, P0, in PEX-treated crush-injured sciatic nerves. As controls, we also studied vehicle-injected crush-injured nerves and contralateral nerves. IF microscopy showed abundant phosphorylated c-Jun localized mainly to SC nuclei in PEX-treated nerves (Fig. 3d). Little to no c-Jun phosphorylation was detected in vehicle-treated or contralateral sciatic nerve. Collectively, these findings indicate that c-Jun activation is regulated by SC LRP1 in vivo.
Discussion
Normal SC function and activation of the SC Repair Program are key in facilitating functional recovery from PNS injury and in preventing neuropathic pain [1, 19]. The transformation in SC phenotype, associated with activation of the Repair Program, is profound. Changes in the SC mRNA transcriptome have been identified. Considerable information is available regarding the cell-signaling factors in SCs that are activated in order to execute the Repair Program. The transcription factor c-Jun plays a central role in this process, but is supported by other factors such as ERK1/2, which may be particularly important in regulating expression of inflammatory mediators by SCs in PNS injury [20–23].
An important gap in our understanding of the SC Repair Program is the SC receptor that senses nerve injury and triggers the injury response. The current study was predicated on the hypothesis that LRP1 is that missing receptor. When we initiated the current study, abundant data were already available to support this hypothesis. First, LRP1 is up-regulated rapidly in SCs in vivo in nerve injury and capable of activating ERK1/2 [5, 7]. Activation of LRP1-dependent cell-signaling induces expression of monocyte chemoattractant protein-1 (MCP-1/CCL2), a central chemokine involved in recruitment of inflammatory cells to the injured PNS [11]. Furthermore, LRP1 possesses an unusually broad and diverse set of ligands, which remains incompletely characterized but includes proteins released after PNS injury, in degenerated myelin and from injured and dying cells [10, 13]. These characteristics position LRP1 favorably to function as an injury response receptor.
We have now shown that ligand-binding to LRP1 activates c-Jun, a central event in activation of the SC Repair Program. This is consistent with previous reports showing that LRP1 modulates c-Jun signaling cascades in other cell types [24]. c-Jun activation was observed in both rat and human SCs in culture and in SCs in vivo in crush-injured rat sciatic nerves. The ability of LRP1 to trigger c-Jun phosphorylation in conjunction with ERK1/2 activation supports the hypothesis that LRP1 is a key injury detection receptor in SCs in the PNS.
If LRP1 and c-Jun function as components of a single system in activation of the SC Repair Program, then one might assume that conditional deletion of either gene in SCs in mice would generate similar phenotypes. This is not the case. Mice with conditional c-Jun deletion in SCs experience slower myelin degeneration in PNS injury, unlike mice in which LRP1 is deleted in SCs, which demonstrate accelerated myelin degeneration. We think this difference reflects the activity of LRP1 in SC viability. In LRP1-deficient SCs, accelerated demyelination following nerve injury is not viewed as resulting from activation of the SC Repair program but instead, compromise to the health and function of SCs. Irrespective of whether this hypothesis is correct, our present results highlight the principle that loss-of-function model systems may not model the function of a gene product, when that gene product is expressed, especially for a product as complicated as LRP1, which works in conjunction with a family of ligands and co-receptors [12]. This is however, an unsettled problem that may be best tackled by comparing the mRNA transcriptomes of LRP1-deficient SCs with those of SCs in which the Repair Program is activated and immature SC precursors in developing nerves. The latter two transcriptomes are not equivalent [25].
Conclusion
We have shown that binding of ligands to LRP1 in SCs results in activation of c-Jun. In the context of previous results regarding the function of SC LRP1, our results support a model in which LRP1 functions as an injury detection receptor in SCs.
Acknowledgments
This work was supported by the Swiss National Science Foundation [grant number P2ZHP3_158764]; the National Institutes of Health [grant numbers HL-060551, NS-057456]; and the Italian Ministry of Education, Universities, and Research, FIRB 2013 [grant number RBFR13BPK9].
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- 1.Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75(4):633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campana WM. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun. 2007;21(5):522–527. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594(13):3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry VH, Tsao JW, Fearn S, Brown MC. Radiation-induced reductions in macrophage recruitment have only slight effects on myelin degeneration in sectioned peripheral nerves of mice. Eur J Neurosci. 1995;7(2):271–280. doi: 10.1111/j.1460-9568.1995.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 5.Campana WM, Li X, Dragojlovic N, Janes J, Gaultier A, Gonias SL. The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J Neurosci. 2006;26(43):11197–11207. doi: 10.1523/JNEUROSCI.2709-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantuano E, Henry K, Yamauchi T, Hiramatsu N, Yamauchi K, Orita S, et al. The unfolded protein response is a major mechanism by which LRP1 regulates Schwann cell survival after injury. J Neurosci. 2011;31(38):13376–13385. doi: 10.1523/JNEUROSCI.2850-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, et al. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci. 2008;28(45):11571–11582. doi: 10.1523/JNEUROSCI.3053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantuano E, Jo M, Gonias SL, Campana WM. Low density lipoprotein receptor-related protein (LRP1) regulates Rac1 and RhoA reciprocally to control Schwann cell adhesion and migration. J Biol Chem. 2010;285(19):14259–14266. doi: 10.1074/jbc.M109.085126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J Neurosci. 1996;16(19):6107–6118. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaultier A, Wu X, Le Moan N, Takimoto S, Mukandala G, Akassoglou K, et al. Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis. J Cell Sci. 2009;122(Pt 8):1155–1162. doi: 10.1242/jcs.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Yamauchi T, Gaultier A, Takimoto S, Campana WM, Gonias SL. Regulation of cytokine expression by Schwann cells in response to alpha2-macroglobulin binding to LRP1. J Neurosci Res. 2011;89(4):544–551. doi: 10.1002/jnr.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonias SL, Campana WM. LDL receptor-related protein-1: a regulator of inflammation in atherosclerosis, cancer, and injury to the nervous system. Am J Pathol. 2014;184(1):18–27. doi: 10.1016/j.ajpath.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Castaneda A, Arandjelovic S, Stiles TL, Schlobach RK, Mowen KA, Gonias SL, et al. Identification of the low density lipoprotein (LDL) receptor-related protein-1 interactome in central nervous system myelin suggests a role in the clearance of necrotic cell debris. J Biol Chem. 2013;288(7):4538–4548. doi: 10.1074/jbc.M112.384693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991;266(31):21232–21238. [PubMed] [Google Scholar]
- 15.Mantuano E, Lam MS, Shibayama M, Campana WM, Gonias SL. The NMDA receptor functions independently and as an LRP1 co-receptor to promote Schwann cell survival and migration. J Cell Sci. 2015;128(18):3478–3488. doi: 10.1242/jcs.173765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Steen PE, Van Aelst I, Hvidberg V, Piccard H, Fiten P, Jacobsen C, et al. The hemopexin and O-glycosylated domains tune gelatinase B/MMP-9 bioavailability via inhibition and binding to cargo receptors. J Biol Chem. 2006;281(27):18626–18637. doi: 10.1074/jbc.M512308200. [DOI] [PubMed] [Google Scholar]
- 17.Gaultier A, Arandjelovic S, Li X, Janes J, Dragojlovic N, Zhou GP, et al. A shed form of LDL receptor-related protein-1 regulates peripheral nerve injury and neuropathic pain in rodents. J Clin Invest. 2008;118(1):161–172. doi: 10.1172/JCI32371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiel G, Rossler OG. Immediate-early transcriptional response to angiotensin II in human adrenocortical cells. Endocrinology. 2011;152(11):4211–4223. doi: 10.1210/en.2011-1243. [DOI] [PubMed] [Google Scholar]
- 19.Orita S, Henry K, Mantuano E, Yamauchi K, De Corato A, Ishikawa T, et al. Schwann cell LRP1 regulates remak bundle ultrastructure and axonal interactions to prevent neuropathic pain. J Neurosci. 2013;33(13):5590–5602. doi: 10.1523/JNEUROSCI.3342-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166(2):392–402. doi: 10.1006/exnr.2000.7508. [DOI] [PubMed] [Google Scholar]
- 21.Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23(15):3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer S, Weishaupt A, Troppmair J, Martini R. Increase of MCP-1 (CCL2) in myelin mutant Schwann cells is mediated by MEK-ERK signaling pathway. Glia. 2008;56(8):836–843. doi: 10.1002/glia.20657. [DOI] [PubMed] [Google Scholar]
- 23.Groh J, Heinl K, Kohl B, Wessig C, Greeske J, Fischer S, et al. Attenuation of MCP-1/CCL2 expression ameliorates neuropathy in a mouse model for Charcot-Marie-Tooth 1X. Hum Mol Genet. 2010;19(18):3530–3543. doi: 10.1093/hmg/ddq269. [DOI] [PubMed] [Google Scholar]
- 24.Lutz C, Nimpf J, Marcel J, Boecklinger K, Enzinger C, Utermann G, Baier-Bitterlich G, Baier G. Evidence of functional modulation of the MEKK/JNK/c-Jun signaling cascade by the low density lipoprotein receptor-related protein (LRP) J Biol Chem. 2002;277(45):43143–43151. doi: 10.1074/jbc.M204426200. [DOI] [PubMed] [Google Scholar]
- 25.Jessen KR, Mirsky R, Lloyd AC. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb Perspect Biol. 2015;7(7):a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]