Abstract
Objective
To provide the first longitudinal characterization of mood and psychosocial functioning in youth with comorbid bipolar (BD) and autism spectrum (ASD) disorders.
Method
The Course and Outcome of Bipolar Youth study followed 368 youth (7–17 years) with DSM-IV bipolar I (BP-I), -II, or Not Otherwise Specified (NOS) for, on average, 9 years using the Longitudinal Interval Follow-up Evaluation. This subgroup analysis compared youth with and without ASD on clinical presentation, percentage of time with mood symptomatology, and psychosocial functioning.
Results
Thirty youth (~8%) met DSM-IV criteria for Asperger disorder or pervasive developmental disorder-NOS (referred to here as ASD). Lifetime worst episode severity was similar in both groups, but youth with both BD and ASD (BD+ASD) had elevated rates of comorbid attention-deficit/hyperactivity and obsessive-compulsive disorders, were younger at intake, and had an earlier onset of mood symptoms. Over time, in both groups, the proportion of predominantly euthymic youth increased, and episode recurrence decreased. Compared to youth with BD, the clinical presentation of youth with BD+ASD more frequently involved distractibility, racing thoughts, depressed mood, social withdrawal, and low reactivity of negative mood states. ASD-related symptomatic differences were generally strongest early and decreased over time. Youth with BD+ASD had significantly greater impairment in friendships throughout follow-up.
Conclusion
Youth with BD+ASD exhibit typical BD mood symptoms but with earlier onset, mixed symptom presentation, and additive functional impairments. Significant amelioration of clinical symptoms occurred over time, suggesting that early recognition and treatment of mood disorders in youth with ASD may improve clinical outcomes.
Keywords: Autism spectrum disorder, bipolar disorders, longitudinal study, psychosocial functioning
INTRODUCTION
Autism spectrum disorder (ASD), characterized by repetitive stereotyped behaviors and social communication deficits, can be associated with several disruptive behaviors (e.g. tantrums, aggression, self-injurious behaviors [SIBs]) that often escalate during adolescence. Alongside depression with suicidal ideation (SI), disruptive behaviors represent the most common reasons for psychiatric hospitalization.1 It is increasingly recognized that comorbid psychiatric disorders significantly contribute to the morbidity associated with ASD.2 A growing body of literature depicts elevated rates of mood disorders amongst youth with ASD, including bipolar disorder (BD).3–5 Accurate identification of comorbid disorders in youth with ASD has proved difficult, however, due to significant overlaps in symptom presentation, diagnostic overshadowing, questions regarding the appropriateness of standard diagnostic criteria, and a paucity of phenomenological data. Rates of concordance between community diagnoses and those obtained on structured interviews tailored to youth with ASD are particularly low for BD,6 suggesting that it has been especially challenging to identify.
The prevalence of comorbid BD in ASD cohorts has been estimated at 7%.7 The largest study to date notes a diagnosis of BD in 5.2% of over 4,000 youth with ASD attending community mental health centers.8 Conversely, estimates of ASD prevalence in cohorts of participants with BD have varied broadly, from 1.4% in a Swedish national register data of over 54,000 adults with BD,9 to upwards of 30% in a clinical sample of youth with early-onset bipolar-I disorder (BP-I).10
Large systematic studies exploring the clinical presentation of BD in adults with ASD are lacking, and only a few cross-sectional studies explore BD in youth with ASD. In part, the lack of phenomenological data is attributed to methodological shortfalls. Most cohorts and clinical trials of participants with BD excluded participants with ASD. Conversely, studies of youth with ASD and adults generally lacked rigorous diagnostic characterization or follow-up assessments of BD mood symptoms. Case series and expert opinions suggest that adults with BD and ASD (hereinafter referred to as BD+ASD) frequently exhibit mixed or atypical features (e.g. prominent irritability, mood lability, dysthymia, and aggression, vs. euphoria and motoric activation), and are often incorrectly diagnosed with psychotic and personality disorders.11 Depressive episodes can be long-lasting and under-recognized.12 BP-I+ASD youth appear to exhibit more typical manic symptoms. When compared to BP-I youth participating in a family study, youth with BD+ASD were noted to have greater impairments in functioning, younger age of BD onset, and an elevated prevalence of grandiosity.10 The longitudinal trajectory of clinical symptoms and functional deficits has remained largely uncharacterized.
The Course and Outcome of Bipolar Youth (COBY) study included youth with Asperger disorder and pervasive developmental disorder not otherwise specified (PDD-NOS). Herein we present the results of an analysis that compared youth with BD+ASD and youth with BD on: clinical presentation, percentage of observational time with mood symptomatology, and psychosocial functioning. We predicted, based on existing literature, that youth with BD+ASD would have a more severe course of BD, with mixed and atypical symptoms, as well as additive psychosocial functioning impairments.
METHOD
Participants
The methods for the COBY study have been described previously.13,14 Briefly, youth 7–17 years with DSM-IV BP-I, -II, or operationally defined BP-NOS (all required to be episodic) were recruited at three university centers and enrolled regardless of current mood state or treatment status. Youth with schizophrenia, intellectual disability (ID), autistic disorder, and mood disorders secondary to medical conditions or substance use were excluded from the study. Each university’s institutional review board approved the study, and consent was obtained from participants. A total of 368 youth with at least 4 years (average 9 years) follow-up were included in this analysis.
Assessments
At intake, youth and primary caretakers were interviewed for psychiatric disorders and treatment with the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL).15 Functional impairment was assessed using the Child Global Assessment Scale (CGAS).16 Behavioral problems (internalizing and externalizing) were assessed with the parent-reported Child Behavior Checklist (CBCL).17
Detailed longitudinal characterization of mood symptoms was obtained through intake and biannual administration of the Mania Rating Scale (MRS)18 and the Depression Rating Scale (DRS),15 derived from the respective sections of the KSADS-PL.15 Comorbid diagnoses, weekly changes in psychiatric symptoms, SI and behaviors, and psychosocial functioning were assessed using subsections of the Longitudinal Interval Follow-up Evaluation (LIFE).14 Clinician-derived consensus scores, obtained after separately interviewing youth and their caretakers, were used in the analysis. The LIFE Psychiatric Status Rating (PSR)19 uses numerical codes linked to DSM-IV criteria for BD and comorbid disorders. Rates of comorbid disorders were based on the percentages of youth meeting full criteria at intake or follow-up. The LIFE Psychosocial Functioning Scales (PSF)19 rate functioning in each of 4 domains (work, interpersonal relations, satisfaction, recreation) on a 5-point scale (1= excellent functioning, 5 = severe impairment). Since youth with ASD are thought to have greater impairments in peer vs. family interactions, the interpersonal relations domain was split into two subdomains: friends and family. In keeping with previously published work,19 scores represent the item of greatest impairment within the domain.
A DSM-IV-based diagnostic interview was used to assess for ASD at intake and follow-up. Youth had to demonstrate a pervasive pattern of clinically significant impairment related to restricted patterns of behaviors, deficits in the development of reciprocal social interaction, and impairments in communication. For an Asperger disorder diagnosis, youth had to exhibit three or more symptom clusters: two from the social interaction domain, and at least one manifestation of restricted, repetitive, and stereotyped pattern of behaviors/interests/activities. For PDD-NOS, participants had to exhibit two symptom clusters. Assessments were conducted by trained research staff, and all diagnoses were confirmed by child psychiatrists/psychologists.
Statistical Analysis
Demographic and clinical differences between groups were evaluated using standard parametric and nonparametric statistics as appropriate. P-values were based on 2-tailed tests. As previously described,13 PSR data was aggregated across follow-up and used to calculate the proportion of time spent with subsyndromal mood symptoms. For longitudinal analyses, data from relevant measures of clinical symptoms and PSF was aggregated into 2-year intervals. In figures, the number on the x-axis denotes the end of the interval (i.e., data from the start of study through month 24 encompass the 1st time point). COBY is ongoing, and the number of participants with adequate data for the current analyses dropped off steadily after the 10th year; thus, this was chosen as the cutoff point for analysis.
Although the small number of youth with BD+ASD precluded latent class analysis (LCA), exploratory analyses were conducted to evaluate the percentage of youth falling within clinical groups as follows: “predominantly ill” youth spend <15% of time asymptomatic, while “predominantly well” youth spend >75% of time asymptomatic. Cut-points were based on overall time spent in specified states, derived from the previously published LCA of the entire COBY cohort.20
Prevalence of specific mood symptom clusters was calculated as the percentage of youth with a clinically significant level of the cluster reported at least once on the MRS and DRS. The clinical threshold was operationally defined as the previously published cutoff score for the relevant measure,15 and typically corresponded to a minimum of mild to moderate symptom severity. P-values for mood states and all longitudinal prevalence data were based on generalized linear mixed models, accounting for repeated measures, controlling for age and sex. Findings with p<.05 were considered statistically significant.
RESULTS
Diagnostic Considerations and Clinical Characteristics at Intake
Twenty-five youth met criteria for Asperger disorder and five for PDD-NOS at intake or during follow-up (~8% of the cohort). A greater percentage of youth with BD+ASD were male and non-Hispanic white (Table 1). Although duration of premorbid BD symptoms was similar for both groups, youth with BD+ASD were significantly younger at study entry (and at an earlier stage of pubertal development; see Table S1, available online). There were no other significant between-group demographic differences at intake. OCD and ADHD were significantly more common in youth with BD+ASD (Table 1). Rates of other comorbid diagnoses (anxiety, posttraumatic stress, oppositional defiant, and substance use disorders [SUD]) were comparable between groups, although a history of physical/sexual abuse was three-fold more common in youth with BD (p=.063, Table S1, available online). Analysis of family history (Table S1, available online) revealed a significantly lower rate of SUD in first-degree relatives of youth with BD+ASD (20% for BD+ASD vs. 45% for BD, p=.005), but similar rates of other disorders, including BD, ADHD, and anxiety. Consistent with greater prevalence of comorbid ADHD in youth with BD+ASD, significant differences in psychopharmacologic treatment history were limited to elevated rates of current/prior treatment with stimulants, alpha-blockers, and/or strattera (Table S1, available online).
Table 1.
Demographics and Clinical Characteristics at Intake*
| Measures | BD+ASD (n=30) |
BD (n=338) |
Effect Size | P-value | |
|---|---|---|---|---|---|
| Age, y | 11.0 ± 2.7 | 12.8 ± 3.3 | 0.55 (d) | <.001 | |
| Male, % | 83 | 51 | 4.69 (OR) | < .001 | |
| White, % | 97 | 81 | 7.58 (OR) | .033 | |
| IQ, mean ± SD | 109 ± 19 | 106 ± 15 | 0.20 (d) | .3 | |
| Onset of mood symptoms, y | 6.7 ± 2.6 | 8.7 ± 3.9 | 0.52 (d) | .007 | |
| Duration of bipolar disorder, y | 4.4 ± 2.0 | 4.3 ± 3.0 | 0.03 (d) | .84 | |
| Child Global Assessment Scale | 55.9 ± 7.8 | 54.8 ± 12.5 | 0.09 (d) | .65 | |
| History of suicidal ideation, % | 57 | 75 | 0.44 (OR) | .03 | |
| History of suicide attempts, % | 17 | 30 | 0.48 (OR) | .13 | |
| Mania Rating Scale | Current | 24.2 ± 11.5 | 22.9 ± 12.4 | 0.11 (d) | .57 |
| Most Severe Lifetime | 32.2 ± 8.2 | 34.1 ± 7.8 | 0.24 (d) | .24 | |
| Depression Rating Scale | Current | 15.3 ± 10.0 | 14.4 ± 10.2 | 0.09 (d) | .66 |
| Most Severe Lifetime | 21.3 ± 12.0 | 22.3 ± 10.7 | 0.09 (d) | .63 | |
| Diagnostic Characterization Including Comorbid Disorders (DSM-IV Criteria), % | |||||
| Bipolar subtype, most recent BP-I/-II/-NOS | 50/ 3.3/ 46.7 | 60/ 7.4/ 32.2 | N/A | .24 | |
| Anxiety disordersa | 50 | 62 | 0.61 (OR) | .21 | |
| OCD | 30 | 14 | 2.63 (OR) | .05 | |
| ADHD | 87 | 67 | 3.30 (OR) | .03 | |
| ODD | 70 | 58 | 1.69 (OR) | .21 | |
| PTSD | 10 | 21 | 0.42 (OR) | .15 | |
| Psychotic disorders | 23 | 36 | 0.53 (OR) | .18 | |
Note: ADHD = attention-deficit/hyperactivity disorder; ASD = autism spectrum disorder; BD = bipolar disorder; BP-I/-II/-NOS = bipolar disorder I/II/not otherwise specified; d = Cohen’s d; OCD = obsessive-compulsive disorder; ODD = oppositional defiant disorder; OR = odds ratio with BD as reference group; PTSD = posttraumatic stress disorder.
Includes: separation and generalized anxiety disorders, panic disorder, social phobia, agoraphobia
Intake and worst lifetime scores on the CGAS, MRS, and DRS were comparable, but youth with BD+ASD had fewer reported instances of SI and attempts. Intake CBCL revealed similar t-scores on anxious/depressed, somatic, and disruptive behavior subscales including aggression. Withdrawn, social, and thought and attention problem subscales were significantly higher in youth with BD+ASD, frequently falling in the high borderline to clinical range (Table S1, available online).
Longitudinal Mood Symptom Analyses
Total number of hospitalizations and percentage of youth experiencing at least one hospitalization were similar for both groups (Table 2). Youth with BD+ASD were significantly younger during their first mood episodes and first hospitalization. The prevalence of youth with mood episodes and psychotic symptoms occurring prior to or during follow-up was comparable in both groups. The prevalence of reported SI and attempts at intake and over follow-up was significantly lower in youth with BD+ASD, while SIBs were comparable in both groups.
Table 2.
Longitudinal Assessment of Mood Disorder Symptoms
| Measures | BD+ASD (n=30) |
BD (n=338) |
Effect Size | P-value |
|---|---|---|---|---|
| Length of follow-up, months | 108.2 ± 18.9 | 107.7 ± 18.2 |
0.03 (d) | .89 |
| Number of interviews | 13.9 ± 3.7 | 11.9 ± 3.9 | 0.51 (d) | .009 |
| Age at onset of first major depressive episode, y | 10.6 ± 4.2 | 12.4 ± 4.4 | 0.41 (d) | .05 |
| Lifetime major depressive episode, % | 83.3 | 84.6 | 0.91 (OR) | .79 |
| Age at onset of first manic/hypomanic episode, y | 9.6 ± 3.2 | 12.6 ± 4.8 | 0.64 (d) | <.001 |
| Lifetime manic/hypomanic episode, % | 73.3 | 74.6 | 0.93 (OR) | .88 |
| Age at onset of first mixed episode, y | 10.6 ± 3.7 | 13 ± 4.8 | 0.51 (d) | .04 |
| Lifetime mixed episode, % | 34.3 | 44.1 | 0.66 (OR) | .94 |
| Lifetime mood-related psychotic symptoms, % | 20.0 | 23.3 | 0.82 (OR) | .68 |
| Lifetime suicidal ideation, % | 63.3 | 80.8 | 0.41 (OR) | .02 |
| Lifetime suicide attempt, % | 30.0 | 50.6 | 0.42 (OR) | .03 |
| Lifetime self-injurious behaviors, % | 36.7 | 36.7 | 1 (OR) | .99 |
| Age at first psychiatric hospitalization, y | 10.0 ± 3.2 | 12.2 ± 4.1 | 0.55 (d) | .02 |
| Lifetime psychiatric hospitalization, % | 63.3 | 67.0 | 0.85 (OR) | .70 |
| Lifetime hospitalizationsa | 2.9 ± 7.3 | 2.5 ± 3.3 | 0.11 (d) | .75 |
| Percent follow-up time spent in the following mood states, p values controlled for age | ||||
| Asymptomatic | 43 ± 27.7 | 49.7 ± 27 | 0.25 (d) | .49 |
| Syndromal episodes | 11 ± 13.2 | 14.5 ± 16.2 | 0.22 (d) | .39 |
| Subsyndromal symptoms present | 46 ± 23.7 | 35.8 ± 21.7 | 0.47 (d) | .12 |
Note: ASD = autism spectrum disorder; BD = bipolar disorder; d = Cohen’s d; OR = odds ratio with BD as reference group.
Refers to the average total number of individual hospitalizations occurring prior to or during follow-up.
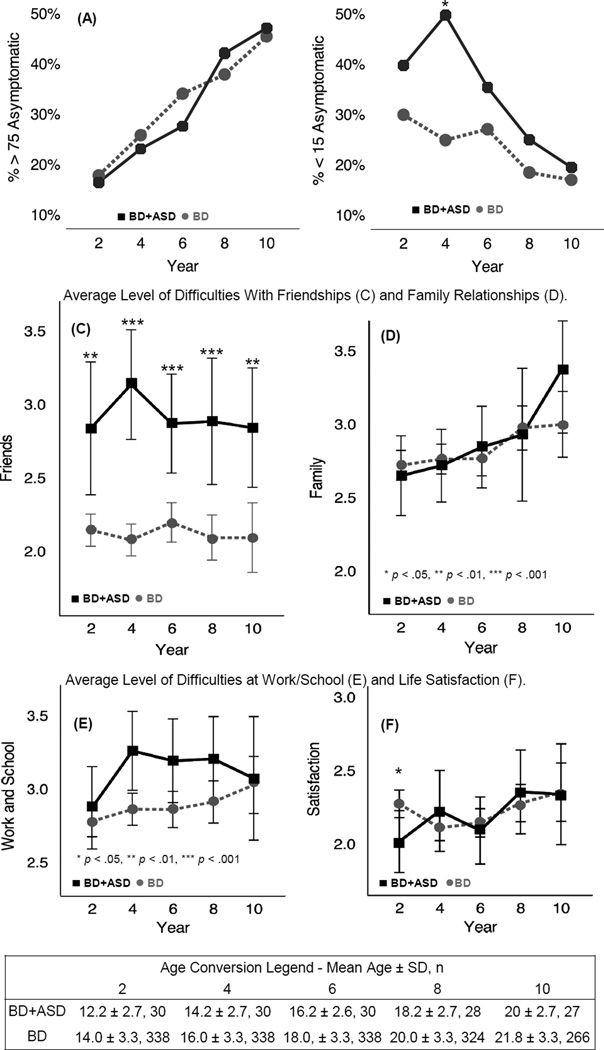
Additional characterization of the percent of follow-up time spent in different mood states is noted in Table S2 (available online). To provide a concise longitudinal perspective, data were aggregated into 2-year intervals starting at intake (Figure 1). Over follow-up in both groups, the prevalence of predominantly well youth increased from ~17 to ~47%. During the last 2 years, the average youth in both groups spent ~14/24 months asymptomatic. The prevalence of predominantly ill participants was initially higher in youth with BD+ASD, peaking to become significantly higher during study years 2–4 (50% of BD+ASD vs. 25% of youth with BD), and steadily decreasing to ~20%, comparable to that of youth with BD by years 8–10. Over the first 4 years, the average youth with BD+ASD spent ~6 additional months with subsyndromal symptoms (2.11 years for BD+ASD vs. 1.6 years for youth with BD). In both groups, the percentage of youth experiencing a recurrent mood episode decreased over follow-up (Figure S1, available online). After controlling for age, only probability of being predominantly ill during years 2–4 remained significantly elevated (p[age] = .017; p[age, sex] = .018).
Figure 1.
Characterization of clinical state and psychosocial functioning across follow-up. Note: (A) In both groups, the prevalence of predominantly euthymic youth, who spend ≥75% of the interval asymptomatic, increases over time. (B) Compared to youth with bipolar disorder (BD), prevalence of predominantly ill (with <15% of follow-up time asymptomatic) is initially higher, and peaks in BD+ASD youth during years 2–4 of follow-up, then decreases to be comparable. (C) Youth with bipolar and autism spectrum disorders (BD+ASD) have significantly higher impairments in friendships across follow-up. (D) Both groups had consistently moderate levels of impairment in relationships with family members. (E) Moderate impairments are seen in work/school functioning, with transient slight elevations (not significant after controlling for age) in BD+ASD youth during years 2–4. (F) Satisfaction for both groups was similar across follow-up, with the exception of the first 2 years, when it was slightly better (lower score reflects higher satisfaction) for BD+ASD youth. *p < .05, **p < .01, ***p < .001.
Psychosocial Functioning
Youth in both groups had comparable functioning with, on average, slight–moderate impairments in global satisfaction and family relationships (Figure 1). Youth with BD+ASD had a moderate level of dysfunction in school/work, which was slightly elevated compared to youth with BD; this trend was not significant after correcting for participant age. Youth with BD+ASD had significantly greater impairment in friendships throughout follow-up. Between-group separation was greatest during years 2–4 (moderate for BD+ASD vs. slight impairment for youth with BD, p<.001). Total PSF scores (data not shown due to space constraints) reflected elevations in the friendships domain. Youth with BD+ASD had slightly but significantly better life satisfaction during the first 2 years of follow-up, with satisfaction decreasing to a level comparable to that of youth with BD throughout the rest of the study. Comparable functioning in recreational activities, with slight impairment that was stable over time, was seen in both groups (data not shown).
Mood Symptom Characterization
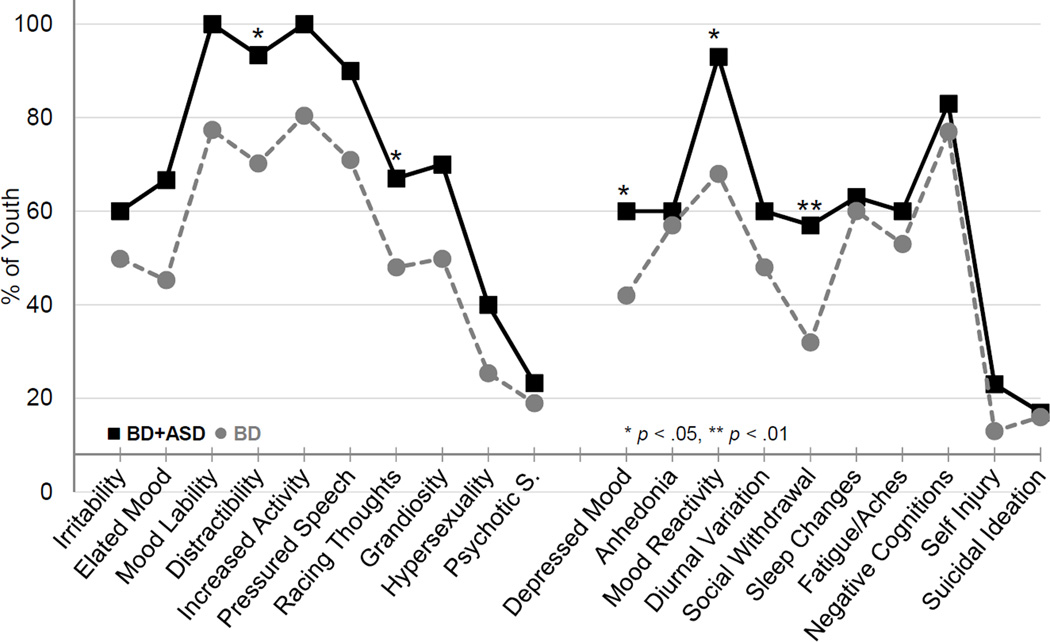
Overall, BD+ASD youth exhibited a mood symptom profile similar to youth with BD (Figure 2). Although the prevalence of clinically significant symptoms on the MRS was high for both groups, nearly all youth with BD+ASD experienced mood lability, distractibility, and increased goal-directed activity during the first 2 years of follow-up (vs. 70–80% of youth with BD). After controlling for age, only elevations in distractibility remained significant (p=.037). Elevations in the rate of racing thoughts were significant after controlling for age and sex. There were no significant between group differences in any sub-category of psychotic symptoms. Prevalence of decreased need for sleep, impaired judgment, and behavioral disinhibition were comparable for both groups (data not shown).
Figure 2.
Characterization of clinically significant mood symptoms reported on Mania Rating Scale (MRS) and Depression Rating Scale (DRS) over the initial 2 years of follow-up. Note: Symptoms measured on MRS are shown on the left side of the graph, while DRS symptoms are on the right. Youth with bipolar and autism spectrum disorders (BD+ASD) had a higher prevalence of distractibility, depressed mood, poor mood reactivity, and social withdrawal. *p < .05, **p < .01.
On the DRS, BD+ASD youth had a higher prevalence of depressed mood (60% for BD+ASD vs. 42% for youth with BD), social withdrawal (57% for BD+ASD vs. 32% for youth with BD), and poor mood reactivity (93% for BD+ASD vs. 68% for youth with BD), which refers to the lack of temporary improvement in negative mood states associated with pleasant activities. All differences remained significant after controlling for age (p=.032, .01, and .018, respectively). The prevalence of other depressive symptoms was largely comparable between groups. There were no significant differences in the prevalence of SIBs.
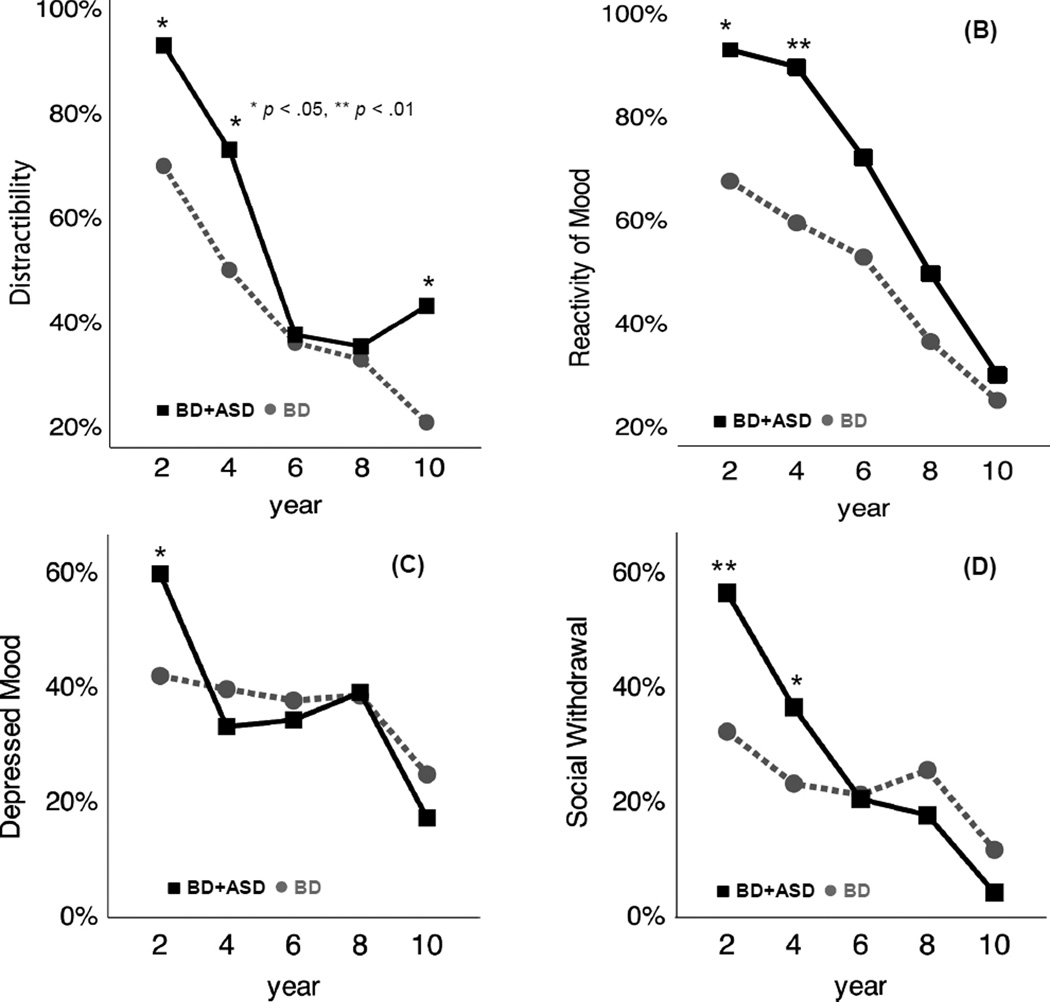
Over time, the overall prevalence of symptoms and between-group differences in the prevalence of most symptoms decreased (Figure 2; Figure S2 for Racing Thoughts, available online). The prevalence of distractibility initially increased in youth with BD+ASD, transiently dipped to be comparable to youth with BD during years 4–8, then again increased during years 8–10. A similar trend was observed for Grandiosity (Figure S2, available online).
While none of the group*time interactions were significant at the 0.05 level, it is possible to have an omnibus test that fails to reject the null hypothesis of no overall interaction effect (i.e., the group effects do not vary with time) while one or more of the post hoc comparisons is deemed significant. Additional characterization of the exploratory longitudinal comparisons for Figures 1–3, and Figures S1–2 (available online), is noted in Table S3 (available online).
Figure 3.
Time-course of the prevalence of select mood symptoms. Note: (A) Distractibility was initially more frequent in youth with bipolar and autism spectrum disorders (BD+ASD), decreased to levels comparable to youth with BD over years 4–8, then increased to again be significantly more prevalent in BD+ASD youth. Reactivity of negative mood states (B), depressed mood (C), and social withdrawal (D) were more prevalent in BD+ASD youth during the initial years, with overall symptom prevalence, as well as differences between groups, decreasing over follow-up. *p < .05, **p < .01
DISCUSSION
Given the complex emotional and behavioral presentation of youth with ASD at intake, delineation of bipolar symptom phenomenology in youth with BD+ASD is needed to inform differential diagnosis. This study utilized a well-characterized cohort of youth with BD to provide the first systematic, longitudinal evaluation spanning 10 years of clinical presentation and PSF in youth with BD+ASD. Overall severity and clinical trajectory of syndromal mood episodes in youth with BD+ASD looked remarkably similar to that of youth with BD.
The most prominent differences in this study were an earlier onset, mixed symptom presentation, and additive functional impairments in youth with BD+ASD. Study outcomes were similar in many respects to those of prior cross-sectional studies. Demographic differences between BD and BD+ASD youth, as well as the 8% overall prevalence of comorbid ASD, were consistent with those observed in a cohort of youth with mixed BD subtype.21 Overall, IQ was comparable in the two groups. Population-based studies suggest that comorbidity for BD is four-fold more common in youth with ASD without vs. with ID.22 The reasons for this trend are unclear, but the average provider appears more likely to encounter BD in youth with ASD similar to those in COBY. Rates of BD, depression, and anxiety in first-degree relatives of both groups were also comparable to those noted in a cohort of youth with BP-I.10 Our finding of earlier onset in BD+ASD youth offers a possible explanation for the wide range of estimated ASD comorbidity in studies of individuals with BP.7 Specifically, cohorts of youth with BD may be enriched for ASD when compared to population studies, which typically include adults with BD independently of age of BD onset.
As anticipated, intake CBCL revealed higher levels of several internalizing and externalizing symptoms in youth with BD+ASD. Aggression was notably higher than typically observed in other ASD cohorts, but comparable to that of COBY youth with BD, and both BD and BP+ASD youth from another clinical sample.23 It has been proposed that comorbid disorders could underlie SI and challenging behaviors in a subset of youth with ASD.5,24 An unexpected finding was that BD+ASD youth had a lower prevalence of SI and attempts at intake and follow-up vs. youth with BD. It is worth noting, however, that our rates were higher than has been reported in other ASD cohorts.25 In comparison to youth with BD, prevalence of SI in youth with ASD without comorbid BD was noted to be much lower and comparable to that of typically developing youth, with BD+ASD youth having an intermediate rate.23 It has been suggested that youth with ASD may be less likely to report SI and behaviors.26 Additionally, child abuse, a well-characterized risk factor for suicidal behaviors,27 was nearly three times less common in our BD+ASD youth than youth with BD. Communication deficits in youth with ASD may interfere with reporting, leading to the underestimation of abuse prevalence.29 The 7% rate of child abuse reported in this study is slightly lower than rates reported for youth with ASD in community settings (16.5–18.5%28), which may be an artifact of this study.
Our longitudinal approach enabled exploration of symptoms from adolescence to young adulthood. Regardless of ASD, mood episode occurrence in COBY youth was most frequent during the early years and steadily decreased over time. The pattern differed for subsyndromal symptoms. During the first 4 years of follow-up, youth with BD+ASD spent a greater percentage of time (six additional months) with subsyndromal symptoms. Since time spent in syndromal episodes was comparable between groups, the data suggests that inter-episode periods for youth with BD+ASD during the early years of COBY was characterized by subsyndromal symptoms (most commonly mixed type). Curiously, differences between the groups become non-significant after controlling for age, suggesting that ASD+BD youth appear to spend more time in subsyndromal mixed states because of their younger age rather than comorbid ASD. As expected based on our clinical experience, youth with BD+ASD were significantly more likely to be predominantly ill during their early teenage years.
Youth with BD+ASD exhibited a wide array of typical manic and depressive symptoms. Our findings contrast trends observed in case studies of BD+ASD participants11,12 but are consistent with observations from larger cohorts.10 Although youth with BD+ASD had a very high prevalence of numerous manic symptoms, the observation that these findings (except distractibility and racing thoughts) became non-significant after controlling for age suggests that they are more reflective of the typical course of early-onset BD rather than resulting from comorbidity for ASD.
During the first few years of follow-up, depressed mood, social withdrawal, and poor mood reactivity were significantly more prevalent in youth with BD+ASD. Between-group differences peaked during the first 4 years, then progressively decreased. Social withdrawal, more common at intake, may preferentially increase in youth with ASD during periods of stress or low mood.30 Therefore, increases in the prevalence of this measure were expected, and it was particularly encouraging to observe steady resolution of withdrawal over time. Poor mood reactivity could indicate a more severe depression and fits with a higher prevalence of depressed mood in youth with BD+ASD during the first 2 years of follow-up. However, differences in mood reactivity persisted beyond this period. An alternative explanation is that these activities could be perceived as stressful rather than pleasant by youth with ASD due to social skill deficits, sensory differences, and difficulties coping with routine disruption.
Rates of psychotic symptoms were comparable in both groups throughout follow-up, supporting the hypothesis that some mood symptoms in youth with ASD may be misdiagnosed as psychosis, thereby artificially elevating this comorbidity in community settings.11 Coincidentally, grandiose beliefs, which occurred more frequently in our youth with BD+ASD (although this trend was not significant after controlling for participant age), as well as those studied by Joshi et al,10 could easily be mistaken for psychosis if accompanying mood symptoms are absent or missed.
As expected and in keeping with ASD-related deficits in social communication, comorbidity for ASD most affected the quality of friendships. youth with BD+ASD had worse friendships throughout the majority of follow-up, while PSF in other domains was generally similar to youth with BD. Differences between groups were greatest during years 2–4, when youth with BD+ASD were ~12–14 years old. During the same period, youth with BD+ASD were significantly more likely to be predominantly ill. Many experienced prolonged periods with mixed subsyndromal symptoms, suggesting a possibly reciprocal interaction between mood symptoms and problematic friendships. Moderate functional impairments in family relationships and work have previously been observed in youth with BD regardless of mood episode status,31 and shown to worsen in COBY youth during mixed and depressive episodes.32 The observed differences in work functioning between BD and BD+ASD youth were most likely due to their younger age.
The above-noted results need to be interpreted in the context of study limitations. First, COBY was not designed to assess symptoms in youth with ASD and utilized DSM-IV criteria. Although intake assessments did not include specialized ASD diagnostic evaluations, results from the CBCL, which has previously been shown to have good utility in identifying youth with ASD,33 were felt to provide auxiliary support for the diagnoses. The pattern of elevations in withdrawn behaviors, thought, and social problems was distinct from that seen in youth with BD, but similar to that observed in other ASD cohorts.17,33 The impact of revisions to diagnostic criteria (of ASD and other disorders) in DSM-5 on these findings may warrant future attention34. Second, this study did not explore or control for effects related to ADHD comorbidity. Phenotypically, over half of individuals with one of the two developmental disorders show clinically significant symptoms of the other.35,36 Given the overlap and extremely high rate of comorbidity within both groups, it was felt that controlling for comorbid ADHD could introduce additional biases into the analysis. It is also possible, however, that due to this high rate of comorbidity, the reported findings may be attributable to ADHD, or to common shared features of ADHD and ASD, rather than the co-occurrence of ASD and BD. Third, although our sample of youth with BD+ASD was comparable in size to previous studies, its size limited the use of more complex statistical analyses. A larger cohort would allow multivariate analysis to explore the impact of episode status on symptoms and PSF. Fourth, we cannot address pre-BD intake irritability because the nature of the study design was such that they were identified as participants when they already had confirmable BD. Finally, the exclusion of youth with autistic disorder and ID limits the generalizability of the findings. Given that such youth constitute about 30% of individuals with ASD,37 the presentation of BD in this population warrants further inquiry.
In conclusion, our results provide support for the continued use of standard criteria to diagnose BD in youth with ASD. Although younger at BD onset, the overall clinical course for youth with BD+ASD was favorable. During their preteen to early teenage years, most youth with BD+ASD had inter-episode periods characterized by a mixture of subsyndromal symptoms. Future directions for research would include assessment of emotional regulation in a longitudinal ASD sample, elucidating differences between intake emotion regulation deficits and subsyndromal inter-episode symptoms.
Significant amelioration of clinical symptoms occurred over time, suggesting that early recognition and treatment may improve clinical outcomes. In other cohorts of youth with early-onset BD, long-term engagement in treatment, regardless of modality, was associated with ongoing improvement in functioning38. Future work is needed to explore the impact of specific pharmacologic and psychosocial treatments on the clinical course of BD in youth with ASD.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Mental Health grants MH059929 (B.B.), MH59691 (M.B.K./S.Y.), and MH59977 (M.S.).
Mr. Rooks served as the statistical expert for this research.
The authors thank the families for their participation, the COBY research staff, Brian Rooks, BA, of the University of Pittsburgh Department of Statistics, for his statistical expertise, and Shelli Avenevoli, PhD, from the National Institutes of Mental Health, for her support.
Dr. Strober has received support from the Resnick Endowed Chair in Eating Disorders. Dr. B. Goldstein has received grant or research support from Brain Canada, the Canadian Institutes of Health Research, the Brain and Behavior Research Foundation (NARSAD), the National Institute of Mental Health, the Ontario Mental Health Foundation, and the Ontario Ministry of Research and Innovation. Dr. T. Goldstein has received royalties from Guilford Press. Dr. Ryan has received grant or research support from the National Institute of Mental Health. He has served on the Scientific Advisory Board of the Child Mind Institute. Dr. Hunt serves as the Senior Editor of the Brown Psychopharm Newsletter published by Wiley Publishers. Dr. Dickstein has received grant or research support from the National Institute of Mental Health and an independent investigator grant from the National Alliance for Research on Schizophrenia and Depression: the Brain and Behavior Research Foundation. Dr. Birmaher has served as a consultant to Schering-Plough, has participated in a forum sponsored by Forest, and has or will receive royalties for publications from Random House, Inc., UpToDate, and Lippincott Williams and Wilkins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Disclosure: Drs. Borue, Mazefesky, Keller, Yen, Diler, Axelson, Mr. Rooks, and Mss. Hower, Gill, and Liao report no biomedical financial interests or potential conflicts of interest.
References
- 1.Guinchat V, Cravero C, Diaz L, et al. Acute behavioral crises in psychiatric inpatients with autism spectrum disorder (ASD): recognition of concomitant medical or non-ASD psychiatric conditions predicts enhanced improvement. Res Dev Disabil. 2015;38:242–255. doi: 10.1016/j.ridd.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Chiang H-L, Gau SS-F. Comorbid psychiatric conditions as mediators to predict later social adjustment in youths with autism spectrum disorder. J Child Psychol Psychiatry. 2016;57:103–111. doi: 10.1111/jcpp.12450. [DOI] [PubMed] [Google Scholar]
- 3.Munesue T, Ono Y, Mutoh K, Shimoda K, Nakatani H, Kikuchi M. High prevalence of bipolar disorder comorbidity in adolescents and young adults with high-functioning autism spectrum disorder: a preliminary study of 44 outpatients. J Affect Disord. 2008;111:170–175. doi: 10.1016/j.jad.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 4.DeLong R. Autism and Familial Major Mood Disorder: Are They Related? J Neuropsychiatry Clin Neurosci. 2004;16:199–213. doi: 10.1176/jnp.16.2.199. [DOI] [PubMed] [Google Scholar]
- 5.Matson JL, Nebel-Schwalm MS. Comorbid psychopathology with autism spectrum disorder in children: an overview. Res Dev Disabil. 2007;28(4):341–352. doi: 10.1016/j.ridd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Mazefsky CA, Oswald DP, Day TN, Eack SM, Minshew NJ, Lainhart JE. ASD, a psychiatric disorder, or both? Psychiatric diagnoses in adolescents with high-functioning ASD. J Clin child Adolesc Psychol. 2012;41(4):516–523. doi: 10.1080/15374416.2012.686102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skokauskas N, Frodl T. Overlap between Autism Spectrum Disorder and Bipolar Affective Disorder. Psychopathology. 2015;48(4):209–216. doi: 10.1159/000435787. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg RE, Kaufmann WE, Law JK, Law PA. Parent Report of Community Psychiatric Comorbid Diagnoses in Autism Spectrum Disorders. Autism Res Treat. 2011;2011:1–10. doi: 10.1155/2011/405849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song J, Bergen SE, Kuja-Halkola R, Larsson H, Landén M, Lichtenstein P. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;17(2):184–193. doi: 10.1111/bdi.12242. [DOI] [PubMed] [Google Scholar]
- 10.Joshi G, Biederman J, Petty C, Goldin RL, Furtak SL, Wozniak J. Examining the comorbidity of bipolar disorder and autism spectrum disorders: a large controlled analysis of phenotypic and familial correlates in a referred population of youth with bipolar I disorder with and without autism spectrum disorders. J Clin Psychiatry. 2013;74(6):578–586. doi: 10.4088/JCP.12m07392. [DOI] [PubMed] [Google Scholar]
- 11.Vannucchi G, Masi G, Toni C, Dell’Osso L, Erfurth A, Perugi G. Bipolar disorder in adults with Asperger’s Syndrome: a systematic review. J Affect Disord. 2014;168:151–160. doi: 10.1016/j.jad.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 12.Frazier JA, Doyle R, Chiu S, Coyle JT. Treating a Child With Asperger’s Disorder and Comorbid Bipolar Disorder. Am J Psychiatry. 2002;159(1):13–21. doi: 10.1176/appi.ajp.159.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Shaffer D. A Children’s Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;40:1228. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder J, Weiss J, Bebko J. CBCL Profiles of Children and Adolescents with Asperger Syndrome: A Review and Pilot Study. 2011;17:26–37. [Google Scholar]
- 18.Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- 19.Keller MB. The Longitudinal Interval Follow-up Evaluation. Arch Gen Psychiatry. 1987;44:540. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 20.Birmaher B, Gill MK, Axelson DA, et al. Longitudinal trajectories and associated baseline predictors in youths with bipolar spectrum disorders. Am J Psychiatry. 2014;171(9):990–999. doi: 10.1176/appi.ajp.2014.13121577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wozniak J, Biederman J, Faraone SV, et al. Mania in Children With Pervasive Developmental Disorder Revisited. J Am Acad Child Adolesc Psychiatry. 1997;36(11):1552–1559. doi: 10.1016/S0890-8567(09)66564-3. [DOI] [PubMed] [Google Scholar]
- 22.Selten J-P, Lundberg M, Rai D, Magnusson C. Risks for Nonaffective Psychotic Disorder and Bipolar Disorder in Young People With Autism Spectrum Disorder: A Population-Based Study. JAMA Psychiatry. 2015;72:483–489. doi: 10.1001/jamapsychiatry.2014.3059. [DOI] [PubMed] [Google Scholar]
- 23.Weissman AS, Bates ME. Increased clinical and neurocognitive impairment in children with autism spectrum disorders and comorbid bipolar disorder. Res Autism Spectr Disord. 2010;4:670–680. [Google Scholar]
- 24.Cassidy S, Bradley P, Robinson J, Allison C, McHugh M, Baron-Cohen S. Suicidal ideation and suicide plans or attempts in adults with Asperger’s syndrome attending a specialist diagnostic clinic: a clinical cohort study. The Lancet Psychiatry. 2014;1(2):142–147. doi: 10.1016/S2215-0366(14)70248-2. [DOI] [PubMed] [Google Scholar]
- 25.Segers M, Rawana J. What do we know about suicidality in autism spectrum disorders? A systematic review. Autism Res. 2014;7(4):507–521. doi: 10.1002/aur.1375. [DOI] [PubMed] [Google Scholar]
- 26.Richa S, Fahed M, Khoury E, Mishara B. Suicide in autism spectrum disorders. Arch Suicide Res. 2014;18(4):327–339. doi: 10.1080/13811118.2013.824834. [DOI] [PubMed] [Google Scholar]
- 27.Hannon G, Taylor EP. Suicidal behaviour in adolescents and young adults with ASD: findings from a systematic review. Clin Psychol Rev. 2013;33(8):1197–1204. doi: 10.1016/j.cpr.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Mandell DS, Walrath CM, Manteuffel B, Sgro G, Pinto-Martin JA. The prevalence and correlates of abuse among children with autism served in comprehensive community-based mental health settings. Child Abuse Negl. 2005;29(12):1359–1372. doi: 10.1016/j.chiabu.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Howlin P, Clements J. Is it possible to assess the impact of abuse on children with pervasive developmental disorders? J Autism Dev Disord. 1995;25:337–354. doi: 10.1007/BF02179372. [DOI] [PubMed] [Google Scholar]
- 30.Stewart ME, Barnard L, Pearson J, Hasan R, O’Brien G. Presentation of depression in autism and Asperger syndrome: a review. Autism. 2006;10(1):103–116. doi: 10.1177/1362361306062013. [DOI] [PubMed] [Google Scholar]
- 31.Wozniak J, Spencer T, Biederman J, et al. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord. 2004;82(Suppl 1):S59–S69. doi: 10.1016/j.jad.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein TR, Birmaher B, Axelson D, et al. Psychosocial functioning among bipolar youth. J Affect Disord. 2009;114(1–3):174–183. doi: 10.1016/j.jad.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ooi YP, Rescorla L, Ang RP, Woo B, Fung DSS. Identification of autism spectrum disorders using the Child Behavior Checklist in Singapore. J Autism Dev Disord. 2011;41(9):1147–1156. doi: 10.1007/s10803-010-1015-x. [DOI] [PubMed] [Google Scholar]
- 34.Smith IC, Reichow B, Volkmar FR. The Effects of DSM-5 Criteria on Number of Individuals Diagnosed with Autism Spectrum Disorder: A Systematic Review. J Autism Dev Disord. 2015;45(8):2541–2552. doi: 10.1007/s10803-015-2423-8. [DOI] [PubMed] [Google Scholar]
- 35.Thomas S, Sciberras E, Lycett K, Papadopoulos N, Rinehart N. Physical Functioning, Emotional, and Behavioral Problems in Children With ADHD and Comorbid ASD: A Cross-Sectional Study. J Atten Disord. 2015 May; doi: 10.1177/1087054715587096. [DOI] [PubMed] [Google Scholar]
- 36.Antshel K, Hier B. Attention Deficit Hyperactivity Disorder (ADHD) in Children with Autism Spectrum Disorders. In: Patel VB, Preedy VR, Martin CR, editors. Comprehensive Guide to Autism. New York, NY: Springer New York; 2014. pp. 1013–1029. [Google Scholar]
- 37.Christensen DL, Baio J, Braun KVN, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jerrell JM, Prewette ED. Outcomes for youths with early- and very-early-onset bipolar I disorder. J Behav Health Serv Res. 2008;35(1):52–59. doi: 10.1007/s11414-007-9081-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.