Abstract
Background
Menopause is associated with significant hormonal changes that result in increased total body fat and abdominal fat, amplifying the risk for metabolic syndrome and diseases such as diabetes, cardiovascular disease and cancer in postmenopausal women. Intermittent fasting regimens hold significant health benefit promise for obese humans, however, regimens that include extreme daytime calorie restriction or daytime fasting are generally associated with hunger and irritability, hampering long-term compliance and adoption in the clinical setting. Time-restricted feeding (TRF), a regimen allowing eating only during a specific period in the normal circadian feeding cycle, without calorie restriction, may increase compliance and provide a more clinically viable method for reducing the detrimental metabolic consequences associated with obesity.
Methods
We tested TRF as an intervention in a mouse model of postmenopausal obesity. Metabolic parameters were measured using Clinical Laboratory Animal Monitoring System (CLAMS) and we carried out glucose tolerance tests. We also stained liver sections with oil red O to examine steatosis and measured gene expression related to gluconeogenesis.
Results
Preexisting metabolic disease was significantly attenuated during 7 wk of TRF. Despite having access to the same high fat diet (HFD) as ad libitum fed (ALF) mice, TRF mice experienced rapid weight loss followed by a delayed improvement in insulin resistance and a reduced severity of hepatic steatosis by having access to the HFD for only 8 h during their normal nocturnal feeding period. The lower respiratory exchange ratio in the TRF group compared with the ALF group early in the dark phase suggested that fat was the predominant fuel source in the TRF group and correlated with gene expression analyses that suggested a switch from gluconeogenesis to ketogenesis. In addition, TRF mice were more physically active than ALF fed mice.
Conclusions
Our data support further analysis of TRF as a clinically viable form of intermittent fasting to improve metabolic health due to obesity.
Keywords: Time-restricted feeding, obesity, intermittent fasting, postmenopausal, insulin resistance, hepatosteatosis, mice
1. Introduction
Obesity is a strong risk factor for type 2 diabetes and several types of cancer including breast, colon, liver, and prostate cancer. Just prior to and after menopause, women experience increased adiposity (% fat mass) that contributes to the increased incidence of obesity and the metabolic syndrome with time after menopause [1-3]. Nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), which are manifestations of the metabolic syndrome and precursors to cirrhosis of the liver, also increase following menopause [4, 5]. Furthermore, inflammation and insulin resistance that are associated with obesity are known risk factors for breast cancer especially in postmenopausal women [6]. Older women consume disproportionately more health care than other segments of the population and published data document statistically significant gender differences in chronic disease risk factors, the associations of risk factors with disease, and chronic disease rates [7-10]. Considering the increased risk that obesity poses for human disease, dietary obesity prevention and intervention strategies may be effective for mitigating obesity's harmful sequelae and reducing medical and emotional burden on society. Indeed, dieting (restricting food intake to lose weight) caloric restriction (CR: energy restriction without incurring malnutrition) and intermittent fasting (IF; cycling between fasting and non-fasting periods) can reverse many of the detrimental effects of obesity [1, 11-13]. One of the most common IF protocols is alternate day fasting (ADF) consisting of 24 h of ad libitum feeding followed by 24 h of fasting or severe CR (75-90% restriction of energy needs on 1-2 days per week) that reduces circulating cholesterol and triglycerides, and decreases blood pressure, fat mass and insulin resistance [14].
At the mechanistic level, CR reduces growth factor activation of Akt and mTORC1 and induces AMPK and the sirtuins, and CR mimetics such as metformin or everolimus are being tested in a number of cancer-related clinical trials [11, 15]. ADF reduces oxidative stress and cancer incidence in rodents [16] but has not been shown to provide any advantages over CR for weight loss in humans [17]. Despite promising data in animals [18, 19], these dietary interventions have not been adopted in the clinic, possibly reflecting difficulties patients face when trying to incorporate CR or ADF into their daily routine and/or problems with long-term compliance as these regimens are associated with hunger and irritability and the benefits can take weeks or months to materialize [20, 21]. Indeed, a recent IF study reported that while compliance was strong during the study period, the majority of participants found the fasting days made daily living more difficult and only 18% would adhere to the regimen if prescribed by a physician [22]. A nutritional intervention that has the same benefits but is easier to maintain would greatly increase long-term compliance.
There is increasing evidence that time-restricted feeding (TRF), the practice of restricting the time of calorie intake, but not the amount of calorie intake, to an 8-12 hour window that corresponds with daily circadian rhythms (e.g., eating during the night or “dark phase” for nocturnal mice), is an alternative approach for metabolic disease and cancer prevention that might be easier to implement in terms of compliance [13]. Food signals entrain peripheral clock rhythms and amplitudes. In fact, synchronizing feeding-fasting with normal circadian rhythm appears to improve oscillations in circadian clock gene expression, enhance energy metabolism, and reduce inflammation, while loss of circadian clock genes dysregulates metabolism and inflammatory responses [23, 24]. For example, loss of the core clock component protein cryptochrome (Cry) leads to constitutive elevation of proinflammatory cytokines in a cell-autonomous manner in mice [25] and loss of the clock gene period circadian clock 2 (Per2) causes systemic and liver-specific perturbations in glucose metabolism and altered food intake behavior [26].
TRF during the dark phase fully protects male mice from obesity, hyperinsulinemia, hepatic steatosis, and inflammation, despite their consuming the equivalent amount of calories as ad libitum-fed (ALF) mice [23, 27]. In female Drosophila melanogaster, TRF attenuates cardiac aging and prevents body weight gain compared with ALF, without reducing calorie intake [28]. We have found that restricting access to a pro-inflammatory, western-style high-fat diet, without restricting calorie intake, ameliorates hepatic steatosis and insulin resistance in ovariectomized (OVX) female mice, a postmenopausal model.
2. Materials and methods
2.1 Animals and diets
All animal experiments were carried out in accordance with the guidelines of the NIH and were approved by the University of California, San Diego, Institutional Animal Care and Use Committee. Mice were maintained in a facility with a 12 h light/12 h dark cycle with water and food ad libitum. Forty-five female C57BL/6N mice (Charles River, Wilmington, MA) aged 7-8 weeks were ovariectomized (OVX) and body weights measured weekly thereafter. At 10 weeks of age 15 mice were continued on normal chow (NC; 12% kcal from fat; 3.02 kcal/g; Purina 5001, LabDiet, St. Louis, MO) and the remaining 30 mice introduced to a high fat diet (HFD, 60% kcal from fat; 5.24 kcal/g) D12492, Research Diets, New Brunswick, NJ). Both diets in pellet form were initially weighed and placed into standard wire food racks. Remaining pellets were weighed the next day to calculate the amount of food consumed before additional pellets were added and the new total amount of food weighed.
At 19 weeks of age when the HFD mice reached an average of 40g (9 weeks of HFD feeding), they were divided into ALF and TRF groups of 15 mice each. The TRF group had access to the HFD for 8 h per day during the dark cycle from Zeitgeber time (ZT) 16 (10pm) to ZT time 0 (6am). ZT 0 is lights-on (6 am) and ZT 12 is lights-off (6 pm). The ambient room temperature for group housed mice was 22 ± 1°C. At 6am, the TRF group was moved to clean boxes for the fasting period to prevent foraging and coprophagia, after which time the mice were placed in their home boxes. Mice in all groups were handled daily at the same time to control for any handling stress and minimize experimental variation between groups [29]. Mice were euthanized 7 weeks after commencing the TRF intervention, the bilateral #3 and #4 mammary and parametrial fat pads were removed and weighed and the liver frozen for further analysis.
2.2 Metabolic cage assessments
Four mice per group (normal chow, ALF, TRF), matched for body weight by group where possible (TRF and ALF), were individually housed in a 12-chamber Clinical Laboratory Animal Monitoring System (CLAMS) with controlled temperature (22 ± 0.5°C), light and feeding (Columbus Instruments, Columbus, OH). CLAMS provides automated access to food and monitors the cumulative amount of food eaten as well as the amount eaten in each bout of feeding with the use of a Mettler Toledo balance with a resolution of 0.01g. Feeders are designed to account for spillage of food and to prevent foraging. Oxygen uptake, carbon dioxide output, respiratory exchange ratio (RER), horizontal and vertical ambulatory movement, feeding and drinking were measured over a 5-day period. Highly variable data from day 1 during acclimatization were excluded from further analyses.
2.3 Glucoregulatory assessments
Glucose tolerance test (GTT). At 25 weeks of age, 7 mice per group were fasted for 6 h prior to the GTT. Fasting commenced at ZT 0, when the feeding period ends for the TRF group and the dark phase ends for all of the mouse groups. Thus, all mice were in similar postprandial states during fasting assessments. Blood was collected via the tail vein at 0 min and then 1 g/Kg glucose injected intraperitoneally. Blood glucose was monitored using a hand held glucometer (One Touch Ultra, LifeScan, Milpitas, CA) at intervals up to 120 min. Terminal fasting plasma insulin was measured on 10 mice per group by ELISA; terminal fasting plasma glucose was measured by the glucose oxidase method (YSI, Model 2950, Yellow Springs, Ohio). Homeostatic model of insulin resistance (HOMA-IR) was calculated as follows: (fasting plasma insulin concentration (mU/ml)) × (fasting blood glucose levels (mg/dl))/(405) [30].
2.4 Cryosectioning and oil red O tissue staining
Liver tissue was harvested at ZT 6 following fasting from ZT 0 on the final day of the experiment. Tissue was embedded in optimal cutting temperature compound (OCT), sectioned on a cryostat at 7 μm thickness, air-dried and fixed in 10 % neutral buffered formalin (Thermoscientific, Malaga, WA). Oil red O (Sigma-Aldrich, San Louis, MO; 0.5% in isopropanol) was diluted 3:2 in water and sections stained for 30 min, washed in water and counterstained with methylene blue (Sigma-Aldrich). Samples were examined by light microscopy and imaged using a SPOT Idea digital camera (Diagnostic Instruments, Sterling Heights, MI). The area of oil red O lipid staining from 4 representative images in 3 mice per group was measured using Image J software [31].
2.5 mRNA isolation and semi-quantitative real time PCR (RT-PCR)
RNA was prepared using Trizol (Life Technologies, Grand Island, NY) and cDNA synthesized. RT-PCR was carried out using a StepOne Plus machine (Life Technologies). Primers are listed in Supplemental Table 1.
2.6 Statistics
Data were analyzed by one-way or two-way ANOVA as indicated in the figure legends followed by multiple comparisons corrected using Tukey's method. RT-PCR data were log2 transformed and analyzed by one-way ANOVA followed by Holm-Sidak's multiple comparisons test as indicated in the figure legends. All data were analyzed using Graphpad Prism software (Graphpad software, La Jolla, CA) and the level of significance was set at p<0.05.
3. Results
3.1 Obese female OVX mice lose weight with TRF
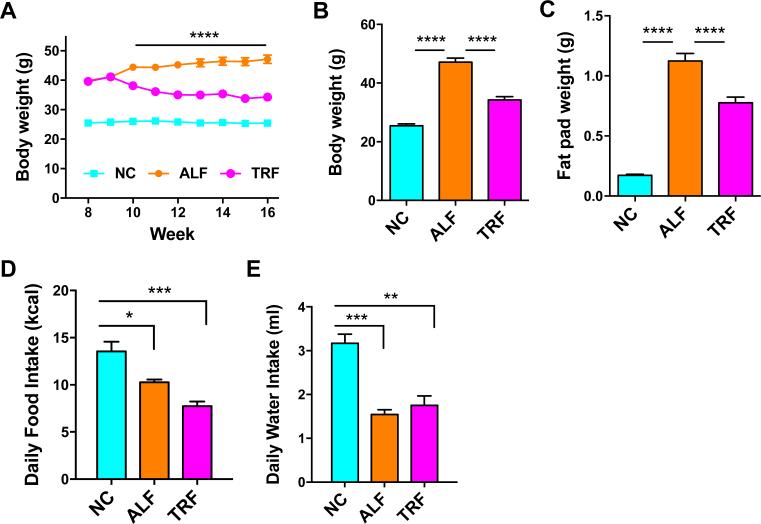
Age-matched female OVX mice were fed either normal “low fat” chow (NC) or HFD (60% kcal from fat) ad libitum. Nine weeks after initial HFD-feeding and associated body weight accumulation (average >40g total body weight), the HFD-fed mice were split to ALF and TRF HFD-fed groups. The ALF group of mice continued to steadily gain weight over this time (15% increase from 9 wk to end of study, p < 0.0005; Fig 1A). The TRF group of mice lost 17% of their body weight (p < 0.0001) within 3 wk of commencing the TRF program and then their weight stabilized thereafter (Fig 1A). While the body weight of the TRF group after 6 weeks remained significantly higher than the NC group (1.34-fold increase, p<0.0001), it was significantly lower than the ALF group (1.85-fold increase compared with NC, p<0.0001; Fig. 1A & B). A similar pattern of weight difference in the mammary fat pads emerged at the end of the study (Fig. 1C), but the magnitude of weight difference between the NC group and the ALF (6.5-fold increase, p<0.0001) and TRF (4.5-fold increase compared with NC, p<0.0001) groups was more pronounced.
Figure 1. TRF reduces obesity in HFD-fed OVX mice.
A. Body weights of mice on NC, ALF and TRF over time. TRF was initiated when the mice were 19 weeks of age (week 9 of HFD) when average body weights of HFD-fed mice were over 40g. ****p<0.0001 represents difference of TRF group from ALF and NC groups following 2way ANOVA and Tukey's multiple comparisons test. B. Final body weights. C. Thoracic and inguinal mammary fat pad weights. D. Daily food intake of mice during the final week of the study. E. Daily water intake by mice during the last week of the study. Data are means ± SEM for 10 mice per group in A, B and C and 4 mice per group in D and E. Data in B-E were analyzed by one-way ANOVA and Tukey's multiple comparisons test. **p<0.01, ***p<0.001, ****p<0.0001.
Food and water intake was measured at 13 min intervals by the CLAMS apparatus over a four day period in four mice per group. Daily intake is the average of the cumulative intake from all the intervals on a particular day. After accounting for differences in the energy density of the NC and HFD diets, daily caloric intake was lower in the ALF and TRF groups versus the NC group (Fig. 1D). Although there was a trend towards lower daily calories consumed in the TRF group compared with the ALF group, this difference was not statistically significant. It should be noted that food intake can be different in mice housed individually in metabolic cages compared with group-housed mice and this may account for some discrepancies compared with previous studies which find no difference in calories consumed per day between NC and ALF groups [23, 32]. Daily water intake was lower in the ALF and TRF groups compared with the NC group (Fig. 1E), most likely due to moisture and texture differences in the diets, the NC diet being much drier than the HFD.
3.2 Metabolic assessment following TRF
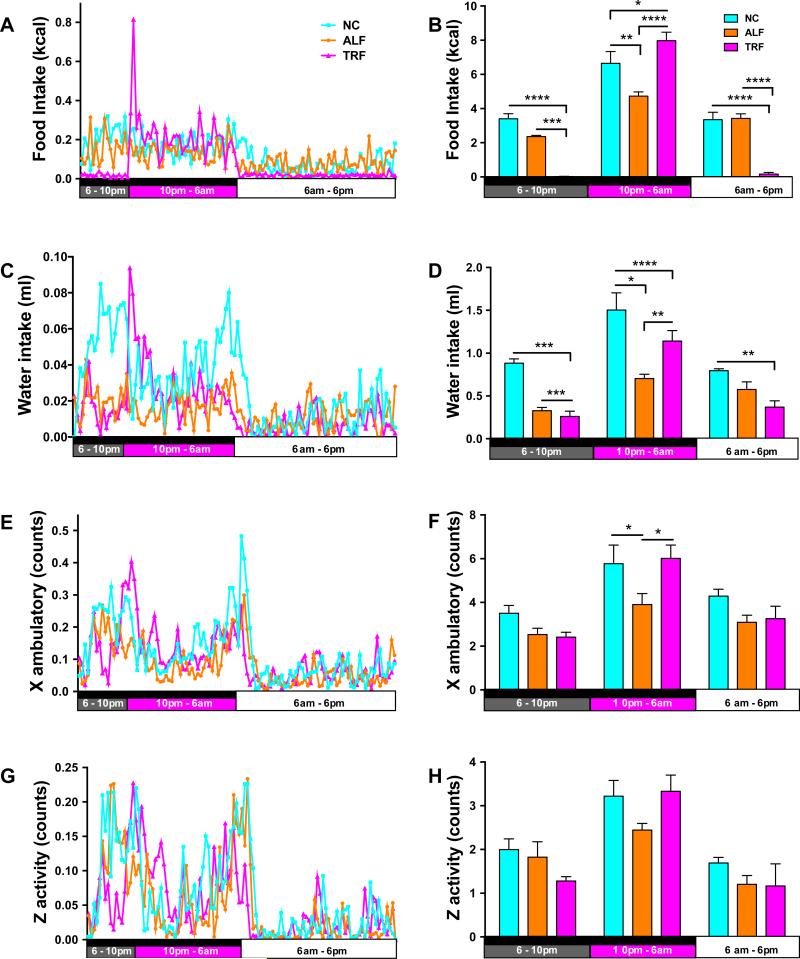
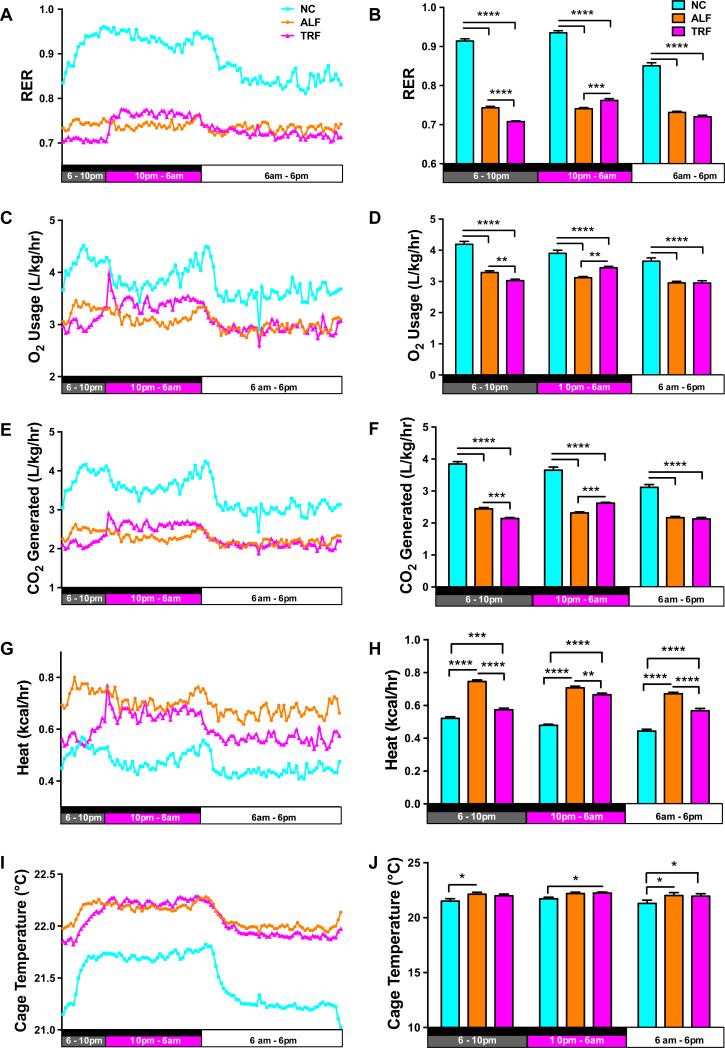
To investigate the physiological alterations that might explain TRF-induced changes, mice were subjected to a CLAMS analysis to assess activity, respiration, feeding and drinking during the last week of the study. After the first 24 h period of acclimatization, mice on the TRF regimen showed a spike in food and water intake when food first became available during the dark phase of each 24 h cycle (Fig. 2A,C) and this contributed to a significant increase in calories consumed by these mice during the 8 h TRF feeding period compared with the NC and ALF mice (Fig. 2B). NC mice ate and drank predominantly during the dark phase, while feeding in the ALF group was spread more evenly over the whole 24 h cycle (Fig. 2B,D). Calculations of the food energy consumed showed that the NC mice consumed more total calories than the ALF mice. The NC-fed mice showed greater physical activity during the dark phase than ALF mice; TRF mice exhibited the same level of physical activity as the NC mice during the 8 h TRF period (Fig. 2E-H). As expected the respiratory exchange ratio (RER) for the ALF mice was approximately 0.75 and did not vary, indicating that the ALF mice were in an oxidative state for the duration of the study consistent with the composition of the diet and the constant food intake (Fig. 3A,B). The RER for the NC mice varied between 0.95 (reflecting primarily carbohydrate oxidation) during the dark eating phase to 0.85 during the light phase, as the mice switched to a combination of carbohydrate and fat oxidation. The mice on NC eat less during the light phase but never show a fasting switch to oxidative metabolism. The TRF mice fasted for 16 h per day as reflected in RER values of 0.7 (reflecting primarily fat oxidation) from 6am to 10pm. The RER increased in the TRF mice during the 8 h TRF period from 10pm to 6am due to their consumption of carbohydrate and protein in the HFD during this time. Consistent with the higher RER, the NC-fed mice exhibit greater O2 usage (Fig. 3C,D) and CO2 generation (Fig. 3E,F), and less heat generation (Fig. 3G,H) compared to the ALF and TRF mice. This results in lower cage temperatures (Fig. 3I,J), since diurnal changes in cage temperature are indicative of physical activity. ALF-fed mice generate more heat due to greater mitochondrial fatty acid oxidation and hence have slightly higher cage temperatures than NC-fed mice. The TRF mice generate the same heat as the ALF animals during the feeding phase but less during the fasting phase (Fig. 3G,H).
Figure 2. TRF increases physical activity.
Four mice per group were placed in Comprehensive Lab Animal Monitoring System (CLAMS) housing for a 5-day period to measure metabolic parameters. After 1 day of acclimatization, data were averaged over the 24-hour period for 4 days (left panels) with the black bar representing the dark phase, the open bar representing the light phase and the magenta bars representing the period of feeding for the TRF group in each figure. A. Food intake, C. Water intake E. X Ambulatory (e.g., walking/running movement in the X-axis plane) and G. Z activity (e.g., reaching/rearing in the Z-axis plane). Measurement averages for each time period are presented in bar graphs (right panels). B. Food intake, D. Water intake F. X Ambulatory (e.g., walking/running movement in the X-axis plane) , and H. Z activity (e.g., reaching/rearing in the Z-axis plane). Data are means ± SEM for 4 mice per group analyzed by two-way ANOVA and Tukey's multiple comparisons test. *p<0.05, **p<0.01, ***p<0.005 and **** p<0.001.
Figure 3. Fat is the fasting source of fuel for TRF mice.
An RER of 0.7 during the fasting period for TRF mice indicated that fat was the major source of fuel, whereas ALF mice burn a mixture of fats and carbohydrates, and NC mice burn mainly carbohydrates. Data for the respiratory exchange ratio (RER) (panels A and B); the volume of oxygen consumed (panels C and D), the volume of carbon dioxide produced panels (E and F), the heat generated (panels G and H), and the cage temperature (panels I and J) were collected and analyzed by two-way ANOVA and Tukey's multiple comparisons test. *p<0.05, **p<0.01, ***p<0.005 and **** p<0.001.
3.3 TRF improves glucose tolerance and insulin resistance
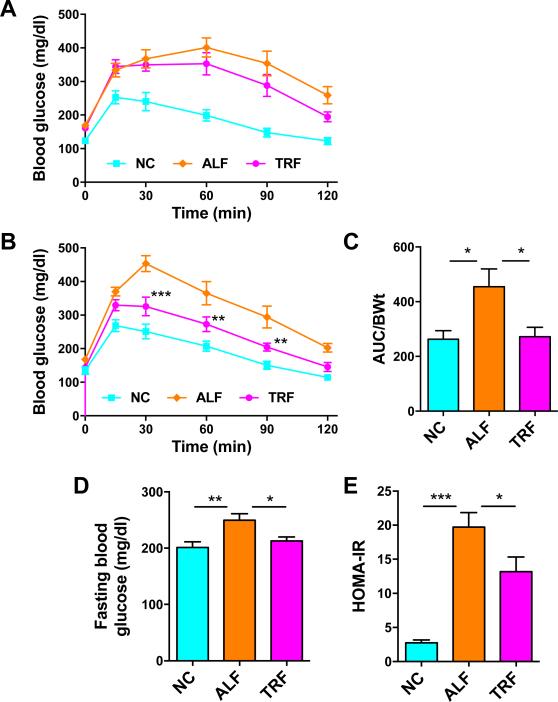
Our interventional TRF approach resulted in glucose tolerance that was only slightly improved after 4 weeks, despite this being the period of greatest weight loss during the study. After 2 additional weeks of the TRF regimen, a significant improvement in glucose tolerance was observed in the TRF group compared with the ALF group, suggesting that improvements in insulin resistance continued to occur in the TRF group in the absence of significant weight loss (Fig. 4A-C). Mice in the ALF and TRF groups were matched for body weight (~40g) for the GTT, since body weight differences alone can affect the GTT. Terminal fasting plasma glucose was elevated in ALF mice compared to NC mice, but fasting plasma glucose in TRF mice had normalized to levels observed in NC mice (Fig. 4D). HOMA-IR was significantly reduced by the TRF regimen (Fig. 4E), indicative of improved insulin sensitivity.
Figure 4. TRF improves glucose tolerance in HFD-fed OVX mice.
A. IP-GTT on mice after 4 weeks of TRF. B & C. IP-GTT after 6 weeks of TRF (panel B) with corresponding area under the curve (AUC) graph of glucose normalized to body weight (BWt). Data are means ± SEM for 7 mice per group **p<0.01, ***p<0.005 vs. ALF. D. Terminal fasting blood glucose. E. HOMA-IR at the end of the study. Data for panels D and E are means ± SEM for 10 mice per group analyzed by one-way ANOVA and Tukey's multiple comparisons test. *p<0.05, **p<0.01, ***p<0.005.
3.4 Amelioration of hepatosteatosis with TRF
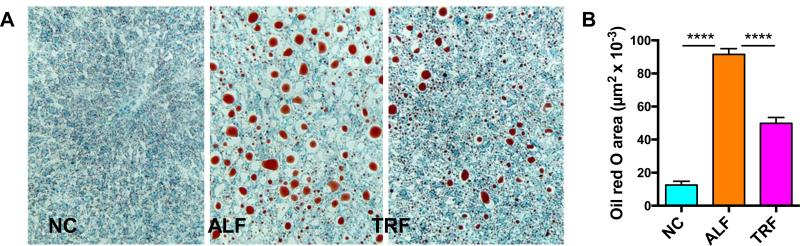
Hepatic steatosis develops in OVX mice fed a HFD and has been used to model NAFLD [33]. In our study, lipid content in the liver was visualized using oil red O staining of cryosections (Fig. 5A). The ALF mouse group showed a 6-fold increase in the amount of fat in the liver (% area) compared to the NC group, while the TRF group showed only a 3.5-fold increase (Fig. 5B). We postulate that this partly explains our observation of better glucose tolerance in the TRF mice compared to ALF mice (Fig. 4A, B) as hepatic steatosis is associated with liver insulin-resistance and glucose intolerance.
Figure 5. Hepatosteatosis is reduced in HFD-fed OVX mice after TRF.
A. Livers were frozen and cryosections stained with oil red O (red) for lipid. B. The area of oil red O was quantitated using Image J. Data are means ± SEM for 6 mice per group analyzed by one-way ANOVA and Tukey's multiple comparisons test. ****p<0.0001.
3.5 Modulation of hepatic gene expression reflects beneficial effects of TRF
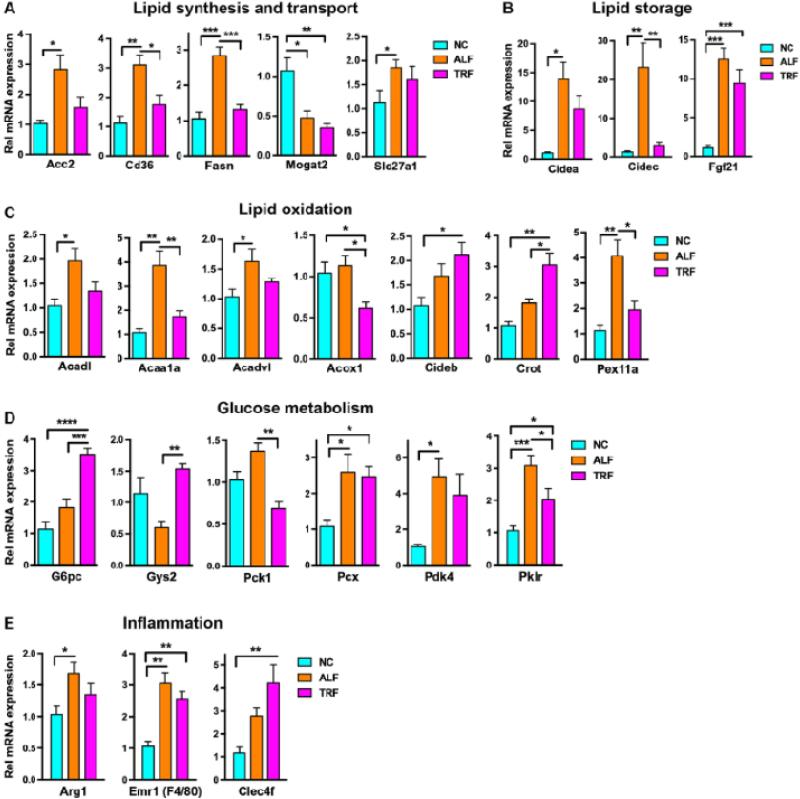
Mice were sacrificed at ZT 6 after a 6 h fast and RNA extracted from the liver for assessment of the expression of genes involved in lipid and glucose metabolism. As expected from the increased adiposity, the lipid biosynthetic genes Acc2 and Fasn, and the lipid transport genes Cd36 and Slc27a1 were elevated in the ALF mice compared to NC mice despite being fasted for 6 h (Fig. 6A). These genes were not elevated in the TRF group however, indicating less lipogenesis in the liver. The lipid storage genes Cidea and Fgf21 were elevated by the HFD in both the ALF and TRF groups, but surprisingly the Cidec gene was only elevated in the ALF group and not the TRF group, consistent with the reduced steatosis (Fig. 6B). Other lipid storage genes such as Fitm2, Lpin1, Plin1, and Plin2 were unchanged (Suppl Fig. 1). The lipid oxidation genes Acadl, Acaa1a, and Acadvl were elevated in the ALF group as were the peroxisomal genes Pex11a and Crot (Fig. 6C). Interestingly unlike the other genes Crot expression was much higher in the TRF group suggesting increased lipid transport into peroxisomes for oxidation (Fig. 6C). Similarly, Cideb expression was higher in the TRF group. The first enzyme in the β-oxidation pathway Acox1 was expressed at a slightly lower level in the TRF group (Fig. 6C) but other genes involved in peroxisomal oxidation were unchanged (Supp Fig. 1). Pyruvate carboxylase (Pcx), pyruvate dehydrogenase kinase 4 (Pdk4) and pyruvate kinase (Pklr) were increased in the ALF and TRF groups indicating that pyruvate is being utilized for oxaloacetate production to maintain mitochondrial β-oxidation (Fig. 6D). Glucose-6-phosphatase (G6pc) is markedly elevated in the TRF group but PEPCK (Pck1) is slightly reduced (Fig. 6D) suggesting increased gluconeogenesis. The livers of ALF mice show mild elevation of inflammatory genes such as Arg1 and Emr1 (F4/80) and TRF mice show higher expression of the Kupffer cell marker Clec4f (Fig. 6E), but cytokine levels are unchanged by the ALF or TRF regimes (Suppl Fig. 1). Hence hepatic inflammation was not a feature of NAFLD in our model and was not significantly altered by TRF.
Figure 6. Normalization of metabolic genes following TRF.
Genes involved in metabolism in the liver were assayed by real-time PCR. A. Liver synthesis and transport genes. B. Lipid storage genes. C. Lipid oxidation genes. D. Glucose metabolism genes and E. Inflammation genes. Data are means ± SEM of samples from 6 mice per group analyzed by one-way ANOVA followed by Holm-Sidak's multiple comparisons test. *p<0.05, **p<0.01, ***p<0.005 and **** p<0.001.
4. Discussion
Efforts to address the obesity epidemic have focused on dieting because reducing calorie intake overcomes the deleterious effects of obesity by ameliorating insulin resistance and impaired glucose tolerance in both rodents and humans [34, 35]. Unfortunately restricting calorie intake induces hunger and irritability in humans and these fasting regimes may require active intervention by nutritionists or clinical researchers to ensure compliance [20]. While these interventions may work in the short-term to correct metabolic dysfunction, they are not suitable for long-term health improvements due to low compliance in the obese population. Recent studies show that a prolonged daily fast (> 12 h) occurring predominantly during the inactive or sleeping phase, without calorie restriction is sufficient to prevent obesity and insulin resistance in male mice [23, 36]. Such a fasting regiment implemented on weekdays only is sufficient to maintain the metabolic improvements, suggesting that TRF has the potential to be an adoptable, feasible lifestyle modification for humans [36].
Sex hormones influence adipose tissue deposition and function, and females differ in their distribution of adipose tissues pre and post menopause. After menopause, fat deposition shifts from the subcutaneous depot to the visceral depot. Obesity causes insulin resistance and tissue inflammation in male mice, but is much less detrimental in intact, estrogenized female mice [37-39]. The protective effect of estrogen is lost in whole body ERα knockout mice [40] or OVX mice lacking ovarian steroids, that respond to obesity similar to male mice [39]. As OVX mice are often used as a model for postmenopausal hormone changes, we wanted to ask whether TRF would be effective in improving the metabolic profile of obese OVX female mice. The current studies show that in female obese OVX mice, TRF is effective as an interventional program to reduce obesity and improve metabolic profiles.
OVX mice in the ALF and TRF groups showed 6.5 and 4.5-fold increases in the weight of the subcutaneous mammary fat pads, respectively, compared with mice eating NC. Parametrial adipose tissue showed similar changes to the mammary fat pads with significant increases in both the ALF and TRF groups (data not shown), however the data were not included due to a concern that the ovariectomy surgery which disrupts the parametrial adipose tissue, would confound the interpretation. TRF led to a 17% reduction in the body weight of the OVX mice by 4 weeks and prevented the further increase seen for the ALF mice, but did not reverse the obesity. This difference in adiposity could be partially explained by a trend towards a lower food intake in the TRF group compared with the ALF group. While this is in contrast to young male mice that show no difference in food intake on a TRF regimen [23], middle aged male mice (12 months of age) on a similar TRF regimen have been shown to have reduced caloric intake and gain significantly less weight than age and sex-matched ALF mice [41]. Other potential contributing factors include an initial reduction in food intake leading to a loss of fat mass in the TRF group, a longer fasting duration leading to enhanced use of fatty acids as fuel and depletion of fat stores, and increased locomotor activity leading to increased calorie expenditure and depletion of fat stores. The TRF group showed increased physical activity during the nocturnal feeding period but similar activity during other times. This increased activity may contribute to the early weight stabilization but does not explain the metabolism, which continued to improve over the next 6 weeks in the face of constant body weight.
The liver is a central organ in lipogenesis, gluconeogenesis and cholesterol metabolism. The availability of Western diets is increasing the prevalence of obesity and metabolic syndrome and promoting pathophysiological changes that result in NAFLD, the most common liver disorder in developed countries [42]. Insulin resistance in the liver can cause glucose intolerance, failure to suppress gluconeogenesis, and steatosis [43] and HFD-induced obesity causes hepatic insulin resistance. We found that TRF reduced the severity of hepatic steatosis and improved insulin resistance, despite the consumption of similar quantities of HFD. One potential explanation is that the 16 h fast forces the mouse to switch to oxidative metabolism of lipid as an energy source causing the liver to halt lipogenesis and increase lipid oxidation, effectively clearing and preventing steatosis. Chylomicrons deliver lipids to the adipose tissue during the 8 h consumption of the HFD and to the liver by the exogenous pathway via chylomicron remnants, but the cessation of feeding stops the flow of lipids from the gut so lipids transferred to the liver by chylomicron remnants are used as an energy source rather than stored. Support for this model comes from gene expression in the liver following TRF.
Expression of genes involved in lipid synthesis and storage are normalized to NC levels despite the consumption of the HFD, in particular. Cidec, an indicator of lipid storage, was reduced 8-fold from ALF levels with TRF, and is suppressed during fasting. Fgf21 is induced by both ALF and TRF regimes. This may seem paradoxical, but one of the roles of FGF21 is to stimulate β-oxidation that is critical during fasting, but is also needed when HFD is the major source of energy. In contrast to the changes in lipid metabolism, genes regulating glucose metabolism did not show consistent alterations in expression upon TRF. The expression data was generated from mice following a 6 h fast, however that may mask changes in glucoregulatory genes, so it will be interesting to measure expression on livers from mice sacrificed at different times during the 24 day.
In summary, we have shown that obese OVX female mice on a TRF regimen show metabolic improvements that appear to be a combination of those found in middle-aged male mice and younger male mice. A limitation of the study was the inability to accurately measure changes in the parametrial fat of the OVX females due to surgical interference and future studies with intact females will address this shortcoming.
This TRF study, as well as published mouse and human studies, support the translational potential of TRF regimens in modulating obesity-related disease risk [13, 23, 44, 45]. In fact, we have recently shown that restricting the majority of food intake to <11 hours per day was prospectively associated with reduced incidence of breast cancer recurrence [46], the first report of a clinical outcome associated with TRF. Evidence from mouse and human population studies supports testing the TRF regimen in a randomized controlled clinical trial. As an intervention, TRF is simple and feasible and therefore could be implemented in multiple contexts, at scale, with sustainability. TRF could be effective in lower socioeconomic populations that (1) have the greatest risk of metabolic dysregulation, obesity, and type 2 diabetes and (2) may struggle with access and compliance to multi-faceted, expensive, or time-consuming dietary interventions such as commercial weight loss programs. If randomized controlled clinical trials show that habitual TRF improves metabolic health in humans, this would be an important public health discovery to reduce the risk of type 2 diabetes and other chronic diseases.
5. Conclusions
Our results in obese OVX female mice support the findings in obese male mice that TRF is an effective strategy for improving the metabolic health of obese individuals. Whether TRF reverses hepatic steatosis and restores glucose tolerance or simply prevents its progression in our model, remains to be determined. Although further long-term animal studies are required to define the molecular basis for the beneficial effects of TRF on metabolism, animal studies on TRF correlate well with human studies [47, 48] and therefore hold considerable translational potential.
Supplementary Material
Acknowlegements
We thank volunteer undergraduate students Andrew Dao, Jay Kim, Jose Moreno, Angie Vo and David Zamro who assisted with changing the diets.
Funding
This work was supported by the National Cancer Institute Centers for Transdisciplinary Research on Energetics and Cancer (TREC) grant CA155435, National Cancer Institute grants CA196853 and CA023100, National Institute for Diabetes, Digestive and Kidney Disease grant DK063491, and Eunice Kennedy Shriver National Institute of Child Health and Human Development grant HD012303. The sponsor had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations
- ADF
alternate day fasting
- ALF
ad libitum feeding
- CLAMS
clinical laboratory animal monitoring system
- CR
calorie restriction
- Cry
crytochrome
- CT
circadian time
- GTT
glucose tolerance test
- HFD
high fat diet
- HOMR-IR
homeostatic model of insulin resistance
- IF
intermittent fasting
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NC
normal chow
- OCT
optimal cutting temperature compound
- OVX
ovariectomy
- Per2
period circadian clock 2
- RER
respiratory exchange ratio
- RT-PCR
real-time polymerase chain reaction
- TRF
time-restricted feeding
- ZT
Zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
LGE, DDS, RP and NW designed the research; HC, WC and LGE conducted the study; LGE, HC, DDS and NW analyzed and formatted the data; LGE, NW and DDS wrote the manuscript and LGE had primary responsibility for the final content; all authors critically reviewed and approved the final manuscript.
Conflicts of interest
None.
References
- 1.Arthur FK, Adu-Frimpong M, Osei-Yeboah J, Mensah FO, Owusu L. The prevalence of metabolic syndrome and its predominant components among pre-and postmenopausal Ghanaian women. BMC research notes. 2013;6:446. doi: 10.1186/1756-0500-6-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heianza Y, Arase Y, Kodama S, Hsieh SD, Tsuji H, Saito K, et al. Effect of postmenopausal status and age at menopause on type 2 diabetes and prediabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 17 (TOPICS 17). Diabetes Care. 2013;36:4007–14. doi: 10.2337/dc13-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morling JR, Balkau B, Wild SH. Diabetes in women: a life-course approach. Menopause international. 2013;19:87–95. doi: 10.1177/1754045313487719. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Xu M, Hu Z, Shrestha UK. Prevalence of nonalcoholic fatty liver disease and its metabolic risk factors in women of different ages and body mass index. Menopause. 2015;22:667–73. doi: 10.1097/GME.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues MH, Bruno AS, Nahas-Neto J, Santos ME, Nahas EA. Nonalcoholic fatty liver disease and metabolic syndrome in postmenopausal women. Gynecol Endocrinol. 2014;30:325–9. doi: 10.3109/09513590.2013.875992. [DOI] [PubMed] [Google Scholar]
- 6.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiologic reviews. 2014;36:114–36. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett-Connor E. The Rancho Bernardo Study: 40 years studying why women have less heart disease than men and how diabetes modifies women's usual cardiac protection. Glob Heart. 2013:8. doi: 10.1016/j.gheart.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet. 2012;3:268. doi: 10.3389/fgene.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitale C, Miceli M, Rosano GM. Gender-specific characteristics of atherosclerosis in menopausal women: risk factors, clinical course and strategies for prevention. Climacteric. 2007;10(Suppl 2):16–20. doi: 10.1080/13697130701602712. [DOI] [PubMed] [Google Scholar]
- 10.Kalyani RR, Lazo M, Ouyang P, Turkbey E, Chevalier K, Brancati F, et al. Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care. 2014;37:830–8. doi: 10.2337/dc13-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meynet O, Ricci JE. Caloric restriction and cancer: molecular mechanisms and clinical implications. Trends Mol Med. 2014;20:419–27. doi: 10.1016/j.molmed.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164:302–11. doi: 10.1016/j.trsl.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Patterson RE, Laughlin GA, LaCroix AZ, Hartman SJ, Natarajan L, Senger CM, et al. Intermittent Fasting and Human Metabolic Health. J Acad Nutr Diet. 2015;115:1203–12. doi: 10.1016/j.jand.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138–43. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama NN, Denmon A, Uchio EM, Jordan M, Mercola D, Zi X. When Anti-Aging Studies Meet Cancer Chemoprevention: Can Anti-Aging Agent Kill Two Birds with One Blow? Curr Pharmacol Rep. 2015;1:420–33. doi: 10.1007/s40495-015-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. doi: 10.1093/ajcn/86.1.7. [DOI] [PubMed] [Google Scholar]
- 17.Seimon RV, Roekenes JA, Zibellini J, Zhu B, Gibson AA, Hills AP, et al. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol. 2015 doi: 10.1016/j.mce.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Varady KA, Allister CA, Roohk DJ, Hellerstein MK. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J Nutr Biochem. 2010;21:188–95. doi: 10.1016/j.jnutbio.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Baumeier C, Kaiser D, Heeren J, Scheja L, John C, Weise C, et al. Caloric restriction and intermittent fasting alter hepatic lipid droplet proteome and diacylglycerol species and prevent diabetes in NZO mice. Biochim Biophys Acta. 2015;1851:566–76. doi: 10.1016/j.bbalip.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 20.McCaffree J. What you should know about calorie restriction. J Am Diet Assoc. 2004;104:1524, 6. doi: 10.1016/j.jada.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Sofer S, Stark AH, Madar Z. Nutrition targeting by food timing: time-related dietary approaches to combat obesity and metabolic syndrome. Adv Nutr. 2015;6:214–23. doi: 10.3945/an.114.007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wegman MP, Guo MH, Bennion DM, Shankar MN, Chrzanowski SM, Goldberg LA, et al. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res. 2015;18:162–72. doi: 10.1089/rej.2014.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–60. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–78. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:12662–7. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvas JM, Vukolic A, Yepuri G, Xiong Y, Popp K, Schmutz I, et al. Period2 gene mutant mice show compromised insulin-mediated endothelial nitric oxide release and altered glucose homeostasis. Front Physiol. 2012;3:337. doi: 10.3389/fphys.2012.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 28.Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347:1265–9. doi: 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundaram S, Yan L. Time-restricted feeding reduces adiposity in mice fed a high-fat diet. Nutr Res. 2016;36:603–11. doi: 10.1016/j.nutres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Kamada Y, Kiso S, Yoshida Y, Chatani N, Kizu T, Hamano M, et al. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1031–43. doi: 10.1152/ajpgi.00211.2011. [DOI] [PubMed] [Google Scholar]
- 34.Brown JE, Mosley M, Aldred S. Intermittent fasting: a dietary intervention for prevention of diabetes and cardiovascular disease? The British Journal of Diabetes & Vascular Disease. 2013;13:68–72. [Google Scholar]
- 35.Skaznik-Wikiel ME, Polotsky AJ. The health pros and cons of continuous versus intermittent calorie restriction: more questions than answers. Maturitas. 2014;79:275–8. doi: 10.1016/j.maturitas.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hevener A, Reichart D, Janez A, Olefsky J. Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes. 2002;51:1907–12. doi: 10.2337/diabetes.51.6.1907. [DOI] [PubMed] [Google Scholar]
- 38.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–17. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 39.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–8. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab. 2010;298:E304–19. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duncan MJ, Smith JT, Narbaiza J, Mueez F, Bustle LB, Qureshi S, et al. Restricting feeding to the active phase in middle-aged mice attenuates adverse metabolic effects of a high-fat diet. Physiol Behav. 2016;167:1–9. doi: 10.1016/j.physbeh.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 43.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 44.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93:107–35. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marinac CR, Natarajan L, Sears DD, Gallo LC, Hartman SJ, Arredondo E, et al. Prolonged Nightly Fasting and Breast Cancer Risk: Findings from NHANES (2009-2010). Cancer Epidemiol Biomarkers Prev. 2015;24:783–9. doi: 10.1158/1055-9965.EPI-14-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marinac CR, Nelson SH, Breen CI, Hartman SJ, Natarajan L, Pierce JP, et al. Prolonged Nightly Fasting and Breast Cancer Prognosis. JAMA Oncol. 2016;2:1049–55. doi: 10.1001/jamaoncol.2016.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moran-Ramos S, Baez-Ruiz A, Buijs RM, Escobar C. When to eat? The influence of circadian rhythms on metabolic health: are animal studies providing the evidence? Nutr Res Rev. 2016:1–14. doi: 10.1017/S095442241600010X. [DOI] [PubMed] [Google Scholar]
- 48.Longo VD, Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016;23:1048–59. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.