Abstract
Importance
Previous studies have shown distinct associations between specific dietary fat and cardiovascular disease. However, evidence on specific dietary fats and mortality remains limited and inconsistent.
Objective
To examine the associations of specific dietary fats with total and cause-specific mortality in two large ongoing prospective cohort studies.
Design, setting, and participants
We investigated 83,349 women from the Nurses’ Health Study (1980-2012) and 42,884 men from the Health Professionals Follow-up Study (1986-2012) who were free from cardiovascular disease, cancer and diabetes at baseline. Dietary fat intake was assessed at baseline and updated every 2 to 4 years.
Main outcomes and measures
We performed systematic searches of the vital records of states and of the National Death Index, supplemented by reports from family members or postal authorities.
Results
We documented 33,304 deaths during 3,439,954 person-years of follow-up. After adjustment for known and suspected risk factors, dietary total fat, compared to total carbohydrate, was inversely associated with total mortality (P for trend <0.001). The hazard ratios (HRs) of total mortality comparing extreme quintiles of specific dietary fats was 1.08, (95% confidence interval (CI), 1.03-1.14) for saturated fat, 0.81 (95% CI, 0.78-0.84) for polyunsaturated fat, 0.89 (95% CI, 0.84-0.94) for monounsaturated fat and 1.13 (95% CI, 1.07-1.18) for trans fat (P for trend <0.001 for all). Replacing 5% of energy from saturated fats with equivalent energy from polyunsaturated fats and monounsaturated fats was associated with 27% (HR =0.73, 95% CI, 0.70-0.77) and 13% (HR =0.87, 95% CI, 0.82-0.93) estimated reductions in total mortality, respectively. HR of total mortality comparing extreme quintiles of n-6 polyunsaturated fat intake was 0.85 (95% CI, 0.81-0.89). Intake of n-6 polyunsaturated fat, especially linoleic acid, was inversely associated with mortality due to most major causes, while marine n-3 polyunsaturated fat intake was associated with a modestly lower total mortality (HR comparing extreme quintiles =0.96, 95% CI, 0.93-1.00).
Conclusions and relevance
Different types of dietary fats have divergent associations with total and cause-specific mortality. These findings support current dietary recommendations to replace saturated and trans fat with unsaturated fats.
Keywords: Fatty acids, Mortality, Cardiovascular diseases, Cancer, Saturated fat, Polyunsaturated fat, Diet
Introduction
Health effects of different types of dietary fat have been a long-standing research interest for decades. 1 Vast literature clearly indicates that specific types of dietary fat have distinct effects on cardiovascular disease (CVD) risk and replacing saturated fats with unsaturated fats and avoidance of trans fat is widely recommended. 1-4 However, a recent meta-analysis concluded that dietary polyunsaturated fatty acids (PUFAs) or saturated fatty acids (SFAs) had no significant relationships with risk of coronary heart disease (CHD), but this meta-analysis failed to specify the macronutrient to which saturated fat was compared; 5 by default this is largely refined starch and sugar in Western diets. 6,7 In addition, existing evidence, especially those from relatively small clinical trials, regarding the effects of n-3 PUFAs on total and cause-specific mortality remains inconsistent. 8,9 Health benefits of n-6 PUFA intake are still contentious; concern has been raised over its hypothesized pro-inflammatory and pro-thrombotic effects. 10 The evidence for industrially produced trans fatty acids (TFAs) and risk of CHD is well established, 11 although little data are available on TFA intake and mortality.
Numerous controlled metabolic trials have documented the effects of dietary fatty acids on blood lipids. 3,12 However, these fatty acids also influence other mechanistic pathways such as insulin resistance, endothelial function, electrophysiology, carcinogenesis, and systemic inflammation. 9,13,14 In addition to CVD, various dietary fatty acids have been associated with incidence of other major chronic diseases including type 2 diabetes, cancer, multiple sclerosis, and respiratory diseases in prospective cohort studies. 15-18 To address the role of dietary fats in overall health, it would be informative to analyze total and cause-specific mortality as outcomes. Therefore, we prospectively examined the associations of specific dietary fats with total and cause-specific mortality in two large ongoing prospective cohort studies, the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS). The many repeated dietary assessments in the cohorts provided a unique assessment of diet over multiple decades. Also, this allowed us to estimate the consequences of isocalorically replacing one type of fat with another, thus describing the choices we make on a daily basis and providing a basis for dietary guidelines.
Methods
Study population
The NHS is a prospective cohort study of 121,700 registered female nurses aged 30 to 55 years in 1976; 92,468 participants responded to the semi-quantitative food frequency questionnaire (SFFQ) in 1980. The HPFS is a prospective cohort of 51,529 male health professionals aged 40 to 75 years in 1986. The baseline of this analysis was defined as 1980 for the NHS and 1986 for the HPFS. The two cohorts have been followed via biennial mailed questionnaires that inquire about lifestyle risk factors and other exposures of interest, as well as newly diagnosed diseases. We also collected information on race, marital status and family history of major chronic diseases. The cumulative follow-up of the two cohorts exceeds 90% of potential person-time. The study was approved by the human research committees at the Harvard T.H. Chan School of Public Health and the Brigham and Women’s Hospital.
We excluded participants who had a history of diabetes, CVD or cancer, did not provide information on dietary fat intake, or reported implausible SFFQ data (total energy intake <800 or >4200 kcal/d for men and <600 or >3500 kcal/d for women), at baseline (Table S1). After exclusions, the analytical population consisted of 83,349 women and 42,884 men.
Dietary assessment
Dietary information was collected with SFFQs. 19 In each SFFQ, we asked how often, on average, the participant had consumed a specified portion size of each food over the preceding year. The number of listed foods was 61 in 1980 and was expanded to 116-150 in 1984 and thereafter; additional frequently used foods were reported in an open-ended section. We also collected detailed information on type of fat or oil used in food preparation and brand/type of margarines by the SFFQ. Fatty acid and other nutrient values were calculated based on the Harvard University Food Composition Database, 20 which is updated regularly using external publications and direct analysis of fatty acids in commonly used margarines and processed foods to take into account changes in manufacturing. We calculated average daily nutrient and total energy intakes by multiplying the frequency of consumption of each item by its nutrient content and summing across all foods, taking into account specific brand and type of margarines and types of fat used in food preparation. The assessment of specific types of fat has been validated by comparison with multiple weighed 1-week dietary records and with fatty acid measurements in adipose tissue and plasma. 19,21-23 The correlations between energy-adjusted intakes assessed by the 1986 questionnaire and the mean of diet records collected in 1980 and 1986, corrected for variation in the records, were 0.67 for total fat, 0.70 for SFAs, 0.69 for monounsaturated fatty acids (MUFAs), and 0.64 for PUFAs. 19 Correlations increased when the mean of three SFFQs (1980, 1984, and 1986) was used; for example, for SFAs the correlation was 0.95. 19 The correlation between dietary fatty acid intake assessed by the SFFQ and the composition of fatty acids in adipose tissue were 0.51 for TFAs, 0.35 for linoleic acid, and 0.48 for marine n-3 PUFAs in women, 21 and 0.29 for TFAs, 0.48 for linoleic acid, and 0.47 for eicosapentaenoic acid in men. Alcohol intake was also estimated from the SFFQs.
In the NHS, dietary questionnaires used in this analysis were completed in 1980, 1984, 1986, and then every four years for a total of nine. In the HPFS, dietary questionnaires were completed in 1986 and then every 4 years for a total of seven.
Ascertainment of death
We performed systematic searches of the vital records of states and of the National Death Index, supplemented by reports from family members or postal authorities. More than 98% of the deaths in each cohort were identified. 24 A physician reviewed death certificates and medical records to classify the cause of death according to the eighth and ninth revisions of the International Classification of Diseases (Table S2).
Statistical analysis
The percentage energy intakes from total fat and specific fats were calculated as cumulative average up to the start of each two or four-year follow-up interval to best represent long-term dietary intake and dampen within-person variation. We categorized participants into quintiles of intake levels. Person-years of follow-up were calculated from baseline to the earliest of time of death, loss to follow-up or the end of follow-up. The end of follow-up was defined as June 2012 for the NHS and December 2012 for the HPFS. Proportional hazard Cox models were applied to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) of mortality comparing participants in each quintile to the lowest quintile. To quantify a linear trend, we assigned the median within each quintile and modeled this variable continuously; Wald test was used for statistical significance. In addition to including percentages of energy from total and specific fat as quintiles, we also included them as continuous terms in the multivariable models. For multivariable analyses, we built isocaloric substitution models which simultaneously included energy intake, the percentages of energy derived from protein and specific types of fat and other potentially confounding variables (see the footnote of Table 2). The coefficients from these models can be interpreted as the estimated effect of substituting a certain percentage of energy from fat for equivalent energy from carbohydrates. For repeatedly measured covariates, we included their updated values as time-varying variables in the model. To minimize missing covariates, we replaced missing data with the last valid values. The covariates had very few missing values after the replacement. In addition, we included missing indicators for the remaining missing covariates in the model. To evaluate the effect of substituting specific types of fat for saturated fat, we treated intakes as continuous variables and calculated the difference in coefficients. As we hypothesized that the effects of total and specific n-3 PUFAs might be more acute, we conducted an additional analysis using the most recent data on n-3 PUFAs at the beginning of each biennial follow-up. We conducted sensitivity analyses to test the robustness of our findings. To address concern that chronic disease occurrence in the years that precede diagnosis may influence dietary behavior, we conducted lagged analyses by excluding the first 4 years follow-up data and adding a 4-year lag period between dietary fat intake assessment and each follow-up period. To address the possibility that our findings may be explained by underlying overall dietary pattern, we further adjusted for overall dietary pattern score (the Alternate Healthy Eating Index-2010 score 25 minus component scores for fatty acids). To minimize the influence of hypertension and hypercholesterolemia on our results, we performed additional analysis by excluding participants who reported hypertension and hypercholesterolemia at baseline. An inverse-variance-weighted, fixed-effect meta-analysis was used to combine the results across cohorts. All analyses were performed using SAS software, version 9.2 (SAS Institute, North Carolina), at a two-tailed P value of 0.05.
Table 2.
Associations between total and specific types of fat intakes and total mortality (comparison is isocaloric substitution for total carbohydrate)
| Quintile of dietary fatty acid intake |
P trend | HR (95% CI) a | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Total fat | |||||||
| NHS | |||||||
| Median (% energy) | 25.4 | 30.0 | 33.2 | 36.6 | 42.2 | ||
| Deaths (n) | 5852 | 4625 | 4069 | 3386 | 2382 | ||
| Age-adjusted model | Ref. | 1.04 (1.00, 1.08) | 1.14 (1.10, 1.19) | 1.20 (1.15, 1.26) | 1.36 (1.29, 1.43) | <.001 | 1.10 (1.08, 1.11) |
| MV-adjusted model b | Ref. | 0.95 (0.92, 0.99) | 0.95 (0.91, 0.99) | 0.88 (0.84, 0.93) | 0.80 (0.76, 0.85) | <.001 | 0.94 (0.93, 0.96) |
| HPFS | |||||||
| Median (% energy) | 23.8 | 28.3 | 31.3 | 34.2 | 38.4 | ||
| Deaths (n) | 2698 | 2604 | 2647 | 2605 | 2436 | ||
| Age-adjusted model | Ref. | 1.02 (0.97, 1.08) | 1.10 (1.04, 1.16) | 1.16 (1.10, 1.23) | 1.21 (1.15, 1.28) | <.001 | 1.07 (1.05, 1.09) |
| MV-adjusted model | Ref. | 0.96 (0.91, 1.01) | 0.97 (0.91, 1.02) | 0.96 (0.91, 1.02) | 0.89 (0.84, 0.95) | 0.002 | 0.97 (0.95, 0.99) |
| Pooled c | |||||||
| Age-adjusted model | Ref. | 1.03 (1.00, 1.07) | 1.13 (1.09, 1.16) | 1.19 (1.15, 1.23) | 1.29 (1.24, 1.34) | <.001 | 1.09 (1.08, 1.10) |
| MV-adjusted model | Ref. | 0.95 (0.92, 0.99) | 0.96 (0.92, 0.99) | 0.91 (0.88, 0.95) | 0.84 (0.81, 0.88) | <.001 | 0.95 (0.94, 0.96) |
| Saturated fat | |||||||
| NHS | |||||||
| Median (% energy) | 8.2 | 10.2 | 11.8 | 13.5 | 16.5 | ||
| Deaths (n) | 5660 | 4729 | 4217 | 3376 | 2332 | ||
| Age-adjusted model | Ref. | 1.17 (1.13, 1.22) | 1.38 (1.32, 1.43) | 1.56 (1.50, 1.63) | 1.92 (1.82, 2.02) | <.001 | 1.49 (1.45, 1.54) |
| MV-adjusted model | Ref. | 1.05 (1.00, 1.09) | 1.11 (1.06, 1.17) | 1.12 (1.06, 1.18) | 1.07 (1.00, 1.15) | <.001 | 1.08 (1.04, 1.12) |
| HPFS | |||||||
| Median (% energy) | 7.1 | 9.0 | 10.2 | 11.5 | 13.5 | ||
| Deaths (n) | 2606 | 2662 | 2602 | 2548 | 2572 | ||
| Age-adjusted model | Ref. | 1.13 (1.07, 1.19) | 1.21 (1.15, 1.28) | 1.29 (1.22, 1.36) | 1.50 (1.42, 1.59) | <.001 | 1.36 (1.31, 1.42) |
| MV-adjusted model | Ref. | 1.03 (0.97, 1.09) | 1.05 (0.99, 1.13) | 1.04 (0.96, 1.12) | 1.09 (1.01, 1.18) | 0.02 | 1.07 (1.01, 1.14) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 1.16 (1.12, 1.19) | 1.32 (1.27, 1.36) | 1.45 (1.40, 1.50) | 1.71 (1.65, 1.78) | <.001 | 1.45 (1.42, 1.48) |
| MV-adjusted model | Ref. | 1.04 (1.00, 1.08) | 1.09 (1.05, 1.14) | 1.09 (1.04, 1.14) | 1.08 (1.03, 1.14) | <.001 | 1.08 (1.04, 1.11) |
| Unsaturated fat | |||||||
| NHS | |||||||
| Median (% energy) | 14.2 | 16.8 | 18.7 | 20.6 | 23.8 | ||
| Deaths (n) | 6024 | 4589 | 3864 | 3285 | 2552 | ||
| Age-adjusted model | Ref. | 0.98 (0.94, 1.01) | 1.00 (0.96, 1.04) | 1.01 (0.97, 1.05) | 1.03 (0.98, 1.08) | 0.19 | 1.02 (0.99, 1.04) |
| MV-adjusted model | Ref. | 0.88 (0.84, 0.91) | 0.82 (0.79, 0.86) | 0.78 (0.74, 0.82) | 0.73 (0.69, 0.77) | <.001 | 0.84 (0.81, 0.86) |
| HPFS | |||||||
| Median (% energy) | 13.7 | 16.3 | 18.0 | 19.7 | 22.3 | ||
| Deaths (n) | 2760 | 2666 | 2657 | 2488 | 2419 | ||
| Age-adjusted model | Ref. | 0.99 (0.94, 1.04) | 1.04 (0.99, 1.10) | 1.04 (0.98, 1.09) | 1.04 (0.98, 1.09) | 0.07 | 1.02 (0.99, 1.06) |
| MV-adjusted model | Ref. | 0.90 (0.85, 0.96) | 0.89 (0.84, 0.95) | 0.84 (0.79, 0.90) | 0.79 (0.74, 0.85) | <.001 | 0.87 (0.84, 0.91) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 0.98 (0.95, 1.01) | 1.02 (0.98, 1.05) | 1.02 (0.99, 1.05) | 1.03 (0.99, 1.07) | 0.03 | 1.02 (1.00, 1.04) |
| MV-adjusted model | Ref. | 0.89 (0.86, 0.92) | 0.85 (0.82, 0.88) | 0.80 (0.77, 0.83) | 0.76 (0.72, 0.79) | <.001 | 0.85 (0.83, 0.87) |
| Polyunsaturated fat | |||||||
| NHS | |||||||
| Median (% energy) | 4.2 | 5.0 | 5.6 | 6.3 | 7.5 | ||
| Deaths (n) | 4423 | 4380 | 3997 | 3829 | 3685 | ||
| Age-adjusted model | Ref. | 0.92 (0.88, 0.96) | 0.84 (0.80, 0.87) | 0.79 (0.76, 0.83) | 0.69 (0.66, 0.72) | <.001 | 0.58 (0.54, 0.62) |
| MV-adjusted model | Ref. | 1.01 (0.97, 1.05) | 0.93 (0.88, 0.97) | 0.89 (0.85, 0.94) | 0.82 (0.78, 0.86) | <.001 | 0.74 (0.69, 0.79) |
| HPFS | |||||||
| Median (% energy) | 4.4 | 5.2 | 5.8 | 6.5 | 7.7 | ||
| Deaths (n) | 2872 | 2633 | 2513 | 2545 | 2427 | ||
| Age-adjusted model | Ref. | 0.90 (0.85, 0.94) | 0.87 (0.82, 0.92) | 0.84 (0.80, 0.89) | 0.78 (0.74, 0.83) | <.001 | 0.69 (0.64, 0.75) |
| MV-adjusted model | Ref. | 0.91 (0.86, 0.96) | 0.87 (0.82, 0.93) | 0.84 (0.79, 0.89) | 0.80 (0.75, 0.85) | <.001 | 0.71 (0.65, 0.79) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 0.91 (0.88, 0.94) | 0.85 (0.82, 0.88) | 0.81 (0.78, 0.84) | 0.73 (0.70, 0.75) | <.001 | 0.62 (0.59, 0.65) |
| MV-adjusted model | Ref. | 0.97 (0.94, 1.00) | 0.91 (0.87, 0.94) | 0.87 (0.84, 0.91) | 0.81 (0.78, 0.84) | <.001 | 0.73 (0.69, 0.77) |
| Monounsaturated fat | |||||||
| NHS | |||||||
| Median (% energy) | 9.4 | 11.4 | 12.8 | 14.4 | 17.2 | ||
| Deaths (n) | 6241 | 4769 | 3789 | 3191 | 2324 | ||
| Age-adjusted model | Ref. | 1.07 (1.03, 1.12) | 1.12 (1.07, 1.16) | 1.18 (1.13, 1.24) | 1.28 (1.22, 1.35) | <.001 | 1.17 (1.14, 1.21) |
| MV-adjusted model | Ref. | 0.95 (0.91, 0.99) | 0.91 (0.87, 0.96) | 0.90 (0.85, 0.96) | 0.86 (0.80, 0.92) | <.001 | 0.88 (0.84, 0.92) |
| HPFS | |||||||
| Median (% energy) | 8.9 | 10.8 | 12.1 | 13.3 | 15.3 | ||
| Deaths (n) | 2748 | 2637 | 2622 | 2598 | 2385 | ||
| Age-adjusted model | Ref. | 1.00 (0.95, 1.06) | 1.07 (1.02, 1.13) | 1.15 (1.09, 1.21) | 1.15 (1.08, 1.21) | <.001 | 1.14 (1.09, 1.18) |
| MV-adjusted model | Ref. | 0.95 (0.90, 1.01) | 0.97 (0.90, 1.04) | 0.99 (0.92, 1.07) | 0.93 (0.85, 1.02) | 0.18 | 0.95 (0.89, 1.02) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 1.05 (1.02, 1.08) | 1.10 (1.06, 1.14) | 1.17 (1.13, 1.21) | 1.22 (1.17, 1.26) | <.001 | 1.16 (1.13, 1.19) |
| MV-adjusted model | Ref. | 0.95 (0.92, 0.99) | 0.93 (0.89, 0.97) | 0.93 (0.89, 0.98) | 0.89 (0.84, 0.94) | <.001 | 0.90 (0.87, 0.94) |
| trans fat | |||||||
| NHS | |||||||
| Median (% energy) | 0.9 | 1.2 | 1.5 | 1.9 | 2.5 | ||
| Deaths (n) | 5747 | 5158 | 4268 | 3099 | 2042 | ||
| Age-adjusted model | Ref. | 1.40 (1.35, 1.45) | 1.68 (1.61, 1.75) | 1.85 (1.76, 1.94) | 2.02 (1.91, 2.14) | <.001 | 2.50 (2.35, 2.66) |
| MV-adjusted model | Ref. | 1.12 (1.08, 1.17) | 1.17 (1.11, 1.22) | 1.13 (1.06, 1.19) | 1.06 (0.99, 1.14) | 0.06 | 1.08 (1.00, 1.17) |
| HPFS | |||||||
| Median (% energy) | 0.7 | 1.0 | 1.2 | 1.4 | 1.9 | ||
| Deaths (n) | 2511 | 2642 | 2683 | 2698 | 2456 | ||
| Age-adjusted model | Ref. | 1.14 (1.08, 1.20) | 1.23 (1.16, 1.30) | 1.37 (1.30, 1.45) | 1.47 (1.39, 1.56) | <.001 | 1.95 (1.78, 2.13) |
| MV-adjusted model | Ref. | 1.08 (1.02, 1.15) | 1.11 (1.04, 1.18) | 1.19 (1.11, 1.27) | 1.19 (1.11, 1.28) | <.001 | 1.34 (1.20, 1.50) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 1.31 (1.27, 1.35) | 1.49 (1.44, 1.54) | 1.63 (1.57, 1.69) | 1.73 (1.66, 1.80) | <.001 | 2.31 (2.20, 2.43) |
| MV-adjusted model | Ref. | 1.11 (1.07, 1.15) | 1.14 (1.10, 1.19) | 1.15 (1.10, 1.20) | 1.13 (1.07, 1.18) | <.001 | 1.16 (1.09, 1.24) |
Abbreviations: HR, hazard ratio; CI, confidence interval; MV, multivariable; NHS, Nurses’ Health Study; HPFS, Health Professional Follow-up Study.
Hazard ratios (95% confidence interval) of total mortality for substituting 5% of energy from saturated fatty acids, unsaturated fatty acids, polyunsaturated fatty acids, and monounsaturated fatty acids, and 2% of energy from trans fatty acids, for the same energy from carbohydrate.
Multivariable-adjusted model adjusted for age (in month), Caucasian (yes vs. no), marital status (with spouse, yes or no), body-mass index (<23, 23-24.9, 25-29.9, 30-34.9, ≥35 kg/m2), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 hours of metabolic equivalent tasks per week), smoking status (never, past, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d), alcohol consumption (women: 0, 0.1-4.9, 5.0-14.9, ≥15 g/d; men: 0, 0.1-4.9, 5.0-29.9, ≥30 g/d), multivitamin use (yes vs. no), vitamin E supplementation use (yes vs. no), current aspirin use (yes vs. no), family history of myocardial infarction (yes vs. no), family history of diabetes (yes vs. no), family history of cancer (yes vs. no), history of hypertension (yes vs. no), history of hypercholesterolemia (yes vs. no), intakes of total energy, dietary cholesterol and percentage of energy intake from dietary protein(quintiles), and menopausal status and hormone use in women (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users). All models, except total fat, also included percentages of energy intake from remaining fatty acids (saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids and trans fatty acids, all in quintiles).
Results for NHS and HPFS from the multivariate model were combined using the fixed-effects model.
Results
Population characteristics
During 32 years of follow-up in the NHS (2,464,852 person-years), we documented 20,314 deaths; during 26 years of follow-up in the HPFS (975,102 person-years), we documented 12,990 deaths. At baseline, participants with higher SFA and MUFA intakes had higher levels of BMI, total energy intake, and dietary cholesterol intake, but were less likely to be physically active, use multivitamin and vitamin E supplementations, and report histories of hypercholesterolemia and hypertension (Table 1). The prevalence of current smoking was higher among men with higher SFA and MUFA intakes. Women with higher intakes of SFA, PUFA and MUFA were generally older.
Table 1.
Age-adjusted characteristics of men and women across quintiles of fatty acids intake at baseline
| Saturated fat |
Polyunsaturated fat |
Monounsaturated fat |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Nurses' Health Study (1980) | |||||||||
| Fatty acid intake, % energy | 8.0 | 11.9 | 17.9 | 3.8 | 5.6 | 7.9 | 8.9 | 12.9 | 18.7 |
|
| |||||||||
| Age, y | 48.2 | 46.5 | 45.9 | 47.2 | 45.8 | 45.1 | 47.9 | 46.3 | 45.8 |
| BMI, kg/m2 | 23.6 | 24.2 | 24.4 | 24.2 | 24.4 | 24.3 | 23.9 | 24.2 | 24.4 |
| Alcohol, g/day | 10.0 | 8.0 | 5.4 | 8.3 | 5.4 | 4.5 | 9.4 | 7.6 | 5.4 |
| Total energy intake, kcal/d | 1380 | 1467 | 1631 | 1553 | 1591 | 1552 | 1410 | 1495 | 1633 |
| Protein, % energy | 18.1 | 18.6 | 19.4 | 19.5 | 19.0 | 18.1 | 19.2 | 18.6 | 19.4 |
| Carbohydrate, % energy | 53.6 | 45.8 | 34.7 | 40.4 | 38.1 | 37.3 | 51.4 | 44.6 | 34.0 |
| Cholesterol, mg/day | 218 | 275 | 360 | 322 | 337 | 308 | 246 | 290 | 360 |
| Physical activity, MET-h/wk | 17.9 | 15.5 | 13.1 | 15.2 | 13.5 | 12.2 | 18.5 | 14.9 | 12.8 |
| Current smoking, % | 26.7 | 26.6 | 29.7 | 30.0 | 27.1 | 29.4 | 26.5 | 27.4 | 29.5 |
| Premenopausal, % | 55.2 | 55.9 | 56.3 | 55.8 | 56.2 | 56.5 | 55.0 | 56.4 | 56.1 |
| Current menopausal hormone use, % | 8.4 | 8.2 | 8.3 | 8.5 | 8.5 | 7.9 | 8.6 | 8.3 | 8.4 |
| Multivitamin use, % | 65.4 | 59.8 | 58.3 | 59.9 | 57.6 | 58.4 | 64.2 | 58.5 | 57.9 |
| Aspirin use, % | 42.3 | 46.4 | 47.4 | 45.7 | 47.4 | 47.9 | 43.1 | 46.8 | 47.3 |
| Vitamin E use, % | 86.3 | 82.7 | 80.0 | 81.1 | 81.4 | 82.3 | 85.6 | 81.7 | 80.5 |
| Family history, % | |||||||||
| Myocardial infarction | 8.4 | 8.9 | 8.8 | 8.6 | 9.3 | 8.8 | 8.5 | 8.7 | 8.9 |
| Diabetes | 27.3 | 28.1 | 28.5 | 27.9 | 28.5 | 28.2 | 27.6 | 26.3 | 28.9 |
| Cancer | 15.1 | 13.9 | 14.0 | 14.0 | 14.3 | 13.9 | 14.6 | 14.2 | 14.1 |
| Hypercholesterolemia, % | 8.3 | 5.6 | 4.5 | 5.2 | 5.3 | 5.9 | 7.0 | 5.0 | 4.8 |
| Hypertension, % | 17.0 | 16.3 | 14.9 | 16.5 | 15.5 | 14.0 | 17.3 | 15.6 | 15.1 |
|
| |||||||||
| Health Professionals Follow-up Study (1986) | |||||||||
| Fatty acid intake, % energy | 6.8 | 10.3 | 14.2 | 4.1 | 5.8 | 8.1 | 8.4 | 12.1 | 15.7 |
|
| |||||||||
| Age, y | 54.2 | 53.1 | 52.5 | 53.6 | 52.8 | 53.1 | 53.7 | 52.8 | 53.1 |
| BMI, kg/m2 | 24.5 | 25.4 | 26.0 | 25.3 | 25.5 | 25.5 | 24.8 | 25.5 | 26.0 |
| Alcohol, g/day | 14.5 | 12.4 | 8.7 | 14.7 | 11.1 | 9.0 | 14.5 | 12.0 | 8.1 |
| Total energy intake, kcal/d | 1878 | 1986 | 2068 | 1983 | 2008 | 1978 | 1873 | 2000 | 2088 |
| Protein, % energy | 18.2 | 18.4 | 18.8 | 18.1 | 18.7 | 18.5 | 18.4 | 18.4 | 18.8 |
| Carbohydrate, % energy | 55.6 | 47.7 | 41.4 | 50.4 | 46.6 | 43.4 | 54.9 | 46.9 | 40.0 |
| Cholesterol, mg/day | 212 | 287 | 366 | 286 | 311 | 298 | 226 | 303 | 362 |
| Physical activity, MET-h/wk | 29.4 | 21.5 | 17.1 | 22.5 | 21.2 | 20.4 | 27.7 | 21.1 | 16.7 |
| Current smoking, % | 5.6 | 8.4 | 13.7 | 11.3 | 9.1 | 8.7 | 6.7 | 9.7 | 13.2 |
| Multivitamin use, % | 69.7 | 63.1 | 57.6 | 62.4 | 61.8 | 62.5 | 68.5 | 61.7 | 58.6 |
| Aspirin use, % | 26.5 | 26.7 | 26.1 | 26.5 | 26.6 | 27.0 | 26.7 | 26.4 | 26.7 |
| Vitamin E use, % | 30.2 | 21.0 | 16.1 | 20.4 | 19.4 | 22.3 | 27.2 | 20.0 | 16.8 |
| Family history, % | |||||||||
| Myocardial infarction | 36.1 | 31.0 | 29.7 | 31.6 | 31.2 | 32.6 | 34.8 | 31.6 | 30.8 |
| Diabetes | 19.6 | 19.6 | 20.8 | 19.6 | 20.7 | 20.9 | 20.3 | 19.8 | 20.5 |
| Cancer | 32.7 | 34.2 | 33.9 | 32.7 | 35.1 | 35.1 | 33.6 | 34.6 | 34.4 |
| Hypercholesterolemia, % | 17.2 | 10.1 | 7.0 | 9.4 | 10.1 | 11.6 | 14.3 | 9.6 | 8.2 |
| Hypertension, % | 21.3 | 20.0 | 18.2 | 20.0 | 19.2 | 19.4 | 20.9 | 20.0 | 18.3 |
Values are means for continuous variables and percentages for categorical variables. All variables except age were age standardized.
Total mortality
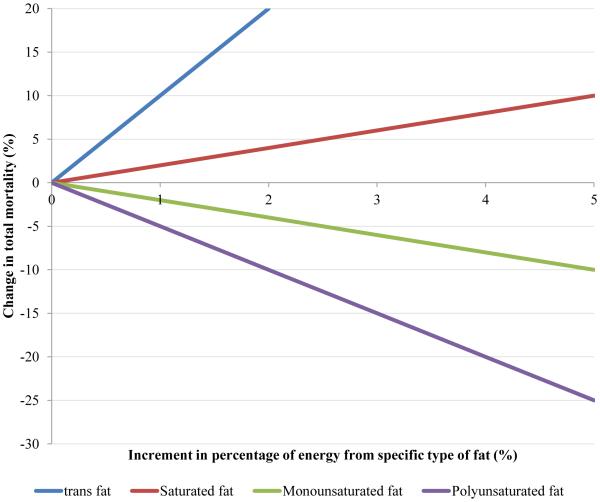
Although total fat intake was positively associated with total mortality in age-adjusted models, an inverse association became apparent after adjusting for other potential confounding variables (P for trend <0.001, Table 2). When substituted for total carbohydrate, a higher intake of SFA was associated with a slightly higher total mortality (HR comparing extreme quintiles =1.08, 95% CI, 1.03-1.14, P for trend <0.001). For TFA intake, a significant positive association with total mortality was observed (HR comparing extreme quintiles =1.13, 95% CI, 1.07-1.18, P for trend <0.001). Intakes of PUFA and MUFA were inversely associated with total mortality (Figure 1); HRs comparing extreme quintiles were 0.81 (95% CI, 0.78-0.84) for PUFA and 0.89 (95% CI, 0.84-0.94) for MUFA (P for trend <0.001 for both). The inverse association between total PUFA and mortality was mainly driven by linoleic acid as illustrated by their high correlation (correlation coefficient =0.99, Table S3). The corresponding HR for linoleic acid intake was 0.82 (95% CI, 0.79-0.86, P for trend <0.001, Table 3). Intake of total n-3 PUFA was associated with modestly lower total mortality, which was mainly driven by the inverse association of marine n-3 PUFAs (docosahexaenoic acid and eicosapentaenoic acid) with total mortality. HRs comparing extreme quintiles were 0.95 (95% CI, 0.91-0.99, P for trend =0.03) for total n-3 PUFAs and 0.96 (95% CI, 0.93-1.00, P for trend =0.002) for marine n-3 PUFAs. The inverse associations became stronger in continuous analyses and when recent intakes of total and marine n-3 PUFAs were used (Table S14); HRs comparing extreme quintiles were 0.91 (95% CI, 0.87-0.95) and 0.90 (95% CI, 0.87-0.93, P for trend <0.001 for both) for recent intakes of total and marine n-3 PUFAs, respectively. The n-6/n-3 ratio was not significantly associated with total mortality (P for trend =0.29). The associations for total and specific types of fat remained largely unchanged in sensitivity analyses (Tables S15-17).
Figure 1. Change in total mortality associated with increases in the percentage of energy from specific types of fat.
Figure was based on multivariable HRs of total mortality associated with replacing the percentage of energy from total carbohydrate by the same energy form specific types of fat (P for trend <0.001 for all). Model was adjusted for age (in month), Caucasian (yes vs. no), marital status (with spouse, yes or no), body-mass index (<23, 23-24.9, 25-29.9, 30-34.9, ≥35 kg/m2), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 hours of metabolic equivalent tasks per week), smoking status (never, past, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d), alcohol consumption (women: 0, 0.1-4.9, 5.0-14.9, ≥15 g/d; men: 0, 0.1-4.9, 5.0-29.9, ≥30 g/d), multivitamin use (yes vs. no), vitamin E supplementation use (yes vs. no), current aspirin use (yes vs. no), family history of myocardial infarction (yes vs. no), family history of diabetes (yes vs. no), family history of cancer (yes vs. no), history of hypertension (yes vs. no), history of hypercholesterolemia (yes vs. no), intakes of total energy, dietary cholesterol and percentage of energy intake from dietary protein(quintiles), and in women, menopausal status, hormone use (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users) and percentage of energy from remaining specific types of fat (saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids and trans fatty acids, all modeled as continuous variables).
Results for NHS and HPFS from the multivariate model were combined using the fixed-effects model.
Table 3.
Associations between dietary n-6 and n-3 polyunsaturated fatty acid intake and total mortality (comparison is isocaloric substitution for carbohydrate)
| Quintile of dietary fatty acid intake |
P trend | HR (95% CI) a | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| n-6 polyunsaturated fatty acid | |||||||
| Total n-6 polyunsaturated fatty acids | |||||||
| NHS | |||||||
| Median (% energy) | 3.4 | 4.3 | 4.9 | 5.5 | 6.7 | ||
| Deaths (n) | 4124 | 4346 | 4158 | 3897 | 3789 | ||
| Age-adjusted model | Ref. | 0.89 (0.85, 0.92) | 0.83 (0.79, 0.87) | 0.77 (0.74, 0.81) | 0.72 (0.69, 0.76) | <.001 | 0.82 (0.80, 0.84) |
| MV-adjusted model b | Ref. | 0.99 (0.95, 1.04) | 0.94 (0.90, 0.99) | 0.90 (0.85, 0.95) | 0.88 (0.83, 0.93) | <.001 | 0.92 (0.89, 0.95) |
| HPFS | |||||||
| Median (% energy) | 3.7 | 4.5 | 5.1 | 5.8 | 6.9 | ||
| Deaths (n) | 2874 | 2679 | 2622 | 2477 | 2338 | ||
| Age-adjusted model | Ref. | 0.90 (0.85, 0.95) | 0.89 (0.85, 0.94) | 0.84 (0.80, 0.89) | 0.80 (0.76, 0.85) | <.001 | 0.88 (0.85, 0.91) |
| MV-adjusted model | Ref. | 0.91 (0.86, 0.96) | 0.92 (0.87, 0.97) | 0.85 (0.79, 0.90) | 0.81 (0.75, 0.87) | <.001 | 0.88 (0.84, 0.92) |
| Pooled c | |||||||
| Age-adjusted model | Ref. | 0.89 (0.86, 0.92) | 0.85 (0.83, 0.88) | 0.80 (0.77, 0.83) | 0.75 (0.73, 0.78) | <.001 | 0.84 (0.82, 0.86) |
| MV-adjusted model | Ref. | 0.96 (0.93, 0.99) | 0.93 (0.90, 0.97) | 0.88 (0.84, 0.92) | 0.85 (0.81, 0.89) | <.001 | 0.90 (0.88, 0.93) |
| Linoleic acid | |||||||
| NHS | |||||||
| Median (% energy) | 3.3 | 4.2 | 4.8 | 5.4 | 6.5 | ||
| Deaths (n) | 4165 | 4358 | 4148 | 3896 | 3747 | ||
| Age-adjusted model | Ref. | 0.89 (0.85, 0.93) | 0.83 (0.80, 0.87) | 0.78 (0.74, 0.81) | 0.70 (0.67, 0.74) | <.001 | 0.80 (0.78, 0.82) |
| MV-adjusted model | Ref. | 0.98 (0.94, 1.03) | 0.93 (0.89, 0.98) | 0.89 (0.84, 0.94) | 0.84 (0.79, 0.89) | <.001 | 0.89 (0.86, 0.92) |
| HPFS | |||||||
| Median (% energy) | 3.6 | 4.4 | 5.0 | 5.6 | 6.7 | ||
| Deaths (n) | 2910 | 2670 | 2569 | 2508 | 2333 | ||
| Age-adjusted model | Ref. | 0.92 (0.88, 0.97) | 0.90 (0.85, 0.95) | 0.85 (0.81, 0.90) | 0.79 (0.75, 0.83) | <.001 | 0.86 (0.83, 0.89) |
| MV-adjusted model | Ref. | 0.94 (0.89, 1.00) | 0.90 (0.85, 0.96) | 0.86 (0.81, 0.92) | 0.80 (0.74, 0.86) | <.001 | 0.86 (0.82, 0.90) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 0.90 (0.87, 0.93) | 0.86 (0.83, 0.89) | 0.81 (0.78, 0.84) | 0.74 (0.71, 0.76) | <.001 | 0.82 (0.81, 0.84) |
| MV-adjusted model | Ref. | 0.97 (0.93, 1.00) | 0.92 (0.89, 0.96) | 0.88 (0.84, 0.91) | 0.82 (0.79, 0.86) | <.001 | 0.88 (0.86, 0.91) |
| Arachidonic acid | |||||||
| NHS | |||||||
| Median (% energy) | 0.05 | 0.06 | 0.07 | 0.09 | 0.11 | ||
| Deaths (n) | 5809 | 4329 | 3648 | 3419 | 3109 | ||
| Age-adjusted model | Ref. | 0.99 (0.95, 1.03) | 0.96 (0.92, 1.00) | 0.97 (0.93, 1.02) | 0.94 (0.90, 0.99) | 0.01 | 0.77 (0.63, 0.94) |
| MV-adjusted model | Ref. | 0.99 (0.95, 1.03) | 0.94 (0.89, 0.98) | 0.94 (0.89, 0.99) | 0.90 (0.85, 0.96) | <.001 | 0.61 (0.46, 0.81) |
| HPFS | |||||||
| Median (% energy) | 0.05 | 0.06 | 0.07 | 0.09 | 0.11 | ||
| Deaths (n) | 2644 | 2595 | 2519 | 2546 | 2686 | ||
| Age-adjusted model | Ref. | 1.03 (0.97, 1.08) | 1.01 (0.95, 1.06) | 1.04 (0.99, 1.10) | 1.02 (0.96, 1.08) | 0.45 | 1.08 (0.85, 1.38) |
| MV-adjusted model | Ref. | 1.00 (0.94, 1.06) | 0.94 (0.88, 1.00) | 0.96 (0.90, 1.03) | 0.89 (0.82, 0.96) | 0.005 | 0.55 (0.38, 0.78) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 1.00 (0.97, 1.04) | 0.98 (0.94, 1.01) | 1.00 (0.97, 1.03) | 0.97 (0.94, 1.01) | 0.14 | 0.89 (0.76, 1.03) |
| MV-adjusted model | Ref. | 0.99 (0.96, 1.03) | 0.94 (0.90, 0.97) | 0.94 (0.91, 0.98) | 0.90 (0.85, 0.94) | <.001 | 0.58 (0.47, 0.73) |
|
| |||||||
| n-3 polyunsaturated fatty acid | |||||||
| Total n-3 polyunsaturated fatty acids | |||||||
| NHS | |||||||
| Median (% energy) | 0.48 | 0.57 | 0.63 | 0.72 | 0.88 | ||
| Deaths (n) | 4132 | 3786 | 3875 | 4090 | 4431 | ||
| Age-adjusted model | Ref. | 0.90 (0.86, 0.94) | 0.87 (0.83, 0.91) | 0.82 (0.78, 0.86) | 0.63 (0.60, 0.66) | <.001 | 0.71 (0.69, 0.74) |
| MV-adjusted model | Ref. | 1.00 (0.95, 1.04) | 1.01 (0.96, 1.06) | 1.03 (0.98, 1.08) | 0.95 (0.90, 1.00) | 0.04 | 0.96 (0.93, 1.00) |
| HPFS | |||||||
| Median (% energy) | 0.46 | 0.57 | 0.65 | 0.75 | 0.94 | ||
| Deaths (n) | 2441 | 2548 | 2645 | 2644 | 2712 | ||
| Age-adjusted model | Ref. | 0.87 (0.83, 0.93) | 0.85 (0.80, 0.90) | 0.82 (0.78, 0.87) | 0.72 (0.68, 0.77) | <.001 | 0.83 (0.80, 0.86) |
| MV-adjusted model | Ref. | 0.98 (0.92, 1.04) | 0.99 (0.94, 1.05) | 1.00 (0.94, 1.06) | 0.96 (0.90, 1.02) | 0.26 | 0.97 (0.94, 1.01) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 0.89 (0.86, 0.92) | 0.86 (0.83, 0.89) | 0.82 (0.79, 0.85) | 0.66 (0.64, 0.69) | <.001 | 0.77 (0.75, 0.78) |
| MV-adjusted model | Ref. | 0.99 (0.96, 1.03) | 1.00 (0.97, 1.04) | 1.02 (0.98, 1.06) | 0.95 (0.91, 0.99) | 0.03 | 0.97 (0.94, 0.99) |
| α-linolenic acid | |||||||
| NHS | |||||||
| Median (% energy) | 0.41 | 0.48 | 0.53 | 0.59 | 0.70 | ||
| Deaths (n) | 4274 | 3800 | 3831 | 3839 | 4570 | ||
| Age-adjusted model | Ref. | 0.95 (0.91, 0.99) | 0.97 (0.93, 1.01) | 0.91 (0.87, 0.95) | 0.75 (0.72, 0.78) | <.001 | 0.75 (0.72, 0.78) |
| MV-adjusted model | Ref. | 1.00 (0.95, 1.04) | 1.05 (1.00, 1.10) | 1.02 (0.97, 1.07) | 0.98 (0.93, 1.04) | 0.49 | 0.98 (0.93, 1.04) |
| HPFS | |||||||
| Median (% energy) | 0.38 | 0.45 | 0.50 | 0.56 | 0.68 | ||
| Deaths (n) | 2398 | 2431 | 2544 | 2700 | 2917 | ||
| Age-adjusted model | Ref. | 0.97 (0.91, 1.02) | 0.95 (0.90, 1.01) | 0.95 (0.90, 1.00) | 0.85 (0.80, 0.90) | <.001 | 0.86 (0.81, 0.90) |
| MV-adjusted model | Ref. | 1.00 (0.94, 1.06) | 1.02 (0.96, 1.08) | 1.04 (0.98, 1.10) | 1.01 (0.94, 1.08) | 0.67 | 0.99 (0.93, 1.05) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 0.96 (0.92, 0.99) | 0.96 (0.93, 1.00) | 0.93 (0.90, 0.96) | 0.79 (0.76, 0.81) | <.001 | 0.79 (0.77, 0.82) |
| MV-adjusted model | Ref. | 1.00 (0.96, 1.03) | 1.04 (1.00, 1.08) | 1.03 (0.99, 1.07) | 0.99 (0.95, 1.03) | 0.80 | 0.98 (0.94, 1.02) |
| Marine n-3 fats (DHA+EPA) | |||||||
| NHS | |||||||
| Median (% energy) | 0.03 | 0.05 | 0.08 | 0.12 | 0.21 | ||
| Deaths (n) | 3725 | 4123 | 4247 | 4019 | 4200 | ||
| Age-adjusted model | Ref. | 0.98 (0.94, 1.03) | 0.93 (0.89, 0.97) | 0.83 (0.80, 0.87) | 0.68 (0.65, 0.72) | <.001 | 0.52 (0.48, 0.55) |
| MV-adjusted model | Ref. | 1.06 (1.02, 1.11) | 1.05 (1.01, 1.10) | 1.03 (0.98, 1.08) | 0.96 (0.91, 1.01) | <.001 | 0.87 (0.81, 0.94) |
| HPFS | |||||||
| Median (% energy) | 0.04 | 0.08 | 0.12 | 0.18 | 0.31 | ||
| Deaths (n) | 2558 | 2655 | 2635 | 2490 | 2652 | ||
| Age-adjusted model | Ref. | 0.97 (0.91, 1.02) | 0.97 (0.91, 1.02) | 0.90 (0.85, 0.95) | 0.82 (0.78, 0.87) | <.001 | 0.80 (0.76, 0.85) |
| MV-adjusted model | Ref. | 1.00 (0.95, 1.06) | 1.05 (1.00, 1.12) | 1.03 (0.97, 1.09) | 0.98 (0.92, 1.04) | 0.28 | 0.97 (0.91, 1.04) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 0.98 (0.94, 1.01) | 0.94 (0.91, 0.98) | 0.86 (0.83, 0.89) | 0.74 (0.71, 0.76) | <.001 | 0.67 (0.64, 0.70) |
| MV-adjusted model | Ref. | 1.04 (1.00, 1.07) | 1.05 (1.02, 1.09) | 1.03 (0.99, 1.07) | 0.96 (0.93, 1.00) | 0.002 | 0.93 (0.89, 0.98) |
|
| |||||||
| n-6/n-3 ratio | |||||||
| NHS | |||||||
| Median | 5.5 | 6.7 | 7.6 | 8.4 | 9.9 | ||
| Deaths (n) | 4070 | 4316 | 4275 | 4147 | 3506 | ||
| Age-adjusted model | Ref. | 1.14 (1.09, 1.19) | 1.18 (1.13, 1.23) | 1.22 (1.16, 1.27) | 1.23 (1.18, 1.29) | <.001 | 1.05 (1.04, 1.06) |
| MV-adjusted model | Ref. | 1.06 (1.02, 1.11) | 1.05 (1.01, 1.10) | 1.06 (1.01, 1.11) | 1.02 (0.97, 1.07) | 0.55 | 1.00 (0.99, 1.01) |
| HPFS | |||||||
| Median | 5.5 | 6.9 | 7.9 | 8.9 | 10.8 | ||
| Deaths (n) | 2968 | 2782 | 2642 | 2432 | 2166 | ||
| Age-adjusted model | Ref. | 1.07 (1.02, 1.13) | 1.11 (1.05, 1.17) | 1.13 (1.07, 1.19) | 1.11 (1.05, 1.18) | <.001 | 1.02 (1.01, 1.03) |
| MV-adjusted model | Ref. | 1.00 (0.95, 1.06) | 0.97 (0.92, 1.03) | 0.96 (0.90, 1.02) | 0.94 (0.88, 1.00) | 0.03 | 0.99 (0.98, 1.00) |
| Pooled | |||||||
| Age-adjusted model | Ref. | 1.11 (1.07, 1.15) | 1.15 (1.11, 1.19) | 1.18 (1.14, 1.22) | 1.18 (1.14, 1.23) | <.001 | 1.03 (1.03, 1.04) |
| MV-adjusted model | Ref. | 1.04 (1.01, 1.08) | 1.02 (0.99, 1.06) | 1.02 (0.98, 1.06) | 0.99 (0.95, 1.03) | 0.29 | 1.00 (0.99, 1.00) |
Abbreviations: HR, hazard ratio; CI, confidence interval; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MV, multivariable; NHS, Nurses’ Health Study; HPFS, Health Professional Follow-up Study.
Hazard ratios (95% confidence interval) of total mortality for substituting 2% of energy from total n-6 polyunsaturated fatty acids and linoleic acid, and 0.3% of energy from total n-3 polyunsaturated fatty acids, arachidonic acid, and α-linolenic acid and marine n-3 fats, for the same energy from carbohydrate. For n-6/n-3 ration, the Hazard ratios (95% confidence interval) of total mortality were calculated for 1-unit increment.
Multivariable-adjusted model adjusted for age (in month), Caucasian (yes vs. no), marital status (with spouse, yes or no), body-mass index (<23, 23-24.9, 25-29.9, 30-34.9, ≥35 kg/m2), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 hours of metabolic equivalent tasks per week), smoking status (never, past, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d), alcohol consumption (women: 0, 0.1-4.9, 5.0-14.9, ≥15 g/d; men: 0, 0.1-4.9, 5.0-29.9, ≥30 g/d), multivitamin use (yes vs. no), vitamin E supplementation use (yes vs. no), current aspirin use (yes vs. no), family history of myocardial infarction (yes vs. no), family history of diabetes (yes vs. no), family history of cancer (yes vs. no), history of hypertension (yes vs. no), history of hypercholesterolemia (yes vs. no), intakes of total energy, dietary cholesterol and percentage of energy intake from dietary protein(quintiles), and menopausal status and hormone use in women (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users). All models also included percentages of energy intake from remaining fatty acids (saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, n-6 polyunsaturated fatty acids, n-3 polyunsaturated fatty acids, linoleic acid, arachidonic acid, α-linolenic acid and marine n-3 fats, all in quintiles).
Results for NHS and HPFS from the multivariate model were combined using the fixed-effects model.
Cause-specific mortality
SFA intake, when substituted for total carbohydrates, was not significantly associated with CVD mortality (P for trend across quintiles =0.17, Table S5), while TFA intake was associated a 20% higher CVD mortality across quintiles (HR =1.20, 95% CI, 1.08-1.33, P for trend <0.001). PUFA intake was inversely associated with CVD mortality (P for trend <0.001). We observed an inverse association, primarily in women, between MUFA intake and CVD mortality (P for trend =0.01). Among specific PUFAs, linoleic acid intake was most strongly related to lower risk of CVD mortality (P for trend <0.001, Table S6).
Dietary intake of SFA, when substituted for total carbohydrate, was associated with slightly higher cancer mortality (HR comparing extreme quintiles =1.07, 95% CI, 0.98-1.17, P for trend =0.02), whereas PUFA intake, especially linoleic acid intake, was associated with modestly lower cancer mortality (HR comparing extreme quintiles of PUFA intake =0.93, 95% CI, 0.87-0.99, P for trend =0.02, Tables S7 and S8). Other major subclasses of dietary fat generally were not associated with cancer mortality except that α-linolenic acid (ALA) intake was associated with a slightly elevated risk of cancer mortality (Tables S7 and S8). However, this association was not significant when recent ALA intake was analyzed (Table S14). We observed inverse associations of PUFA and MUFA intakes and strong positive associations of TFA intake with both neurodegenerative (Table S9) and respiratory disease mortality (Table S11). Higher SFA intake was associated with a substantial increase of mortality due to respiratory disease (HR comparing extreme quintiles =1.56, 95% CI, 1.30-1.87, P for trend <0.001). Among major PUFAs, n-3 PUFA intake, primarily α-linolenic acid, was inversely associated with neurodegenerative disease mortality (Table S10). Marine n-3 PUFA intake was inversely associated with respiratory disease mortality (Table S12). Sensitivity analyses minimally changed these results for cause-specific mortality (Tables S15-17).
Fat substitution analysis
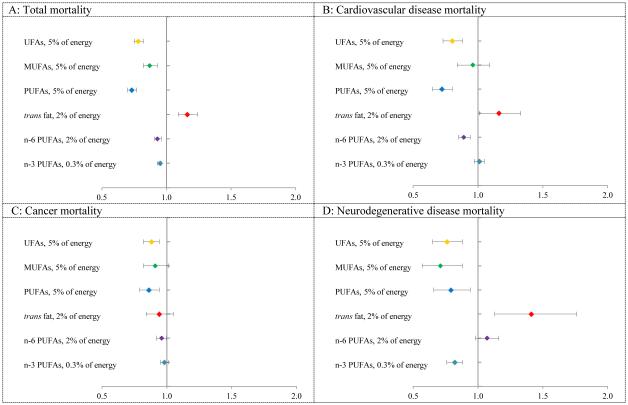
Figure 2 shows that replacing 5% of energy from SFAs with the same energy from PUFAs and MUFAs was associated with 27% (HR =0.73, 95% CI, 0.70-0.77, Table S13) and 13% (HR =0.87, 95% CI, 0.82-0.93, Table S13) estimated reduction in total mortality, respectively. Replacing SFAs with the same energy from PUFAs was associated with lower risk of mortality due to CVD, cancer and neurodegenerative disease. Replacement of 5% of energy from SFAs with 5% of energy from MUFAs was associated with 29% estimated reduction in neurodegenerative disease mortality (HR =0.71, 95% CI, 0.57-0.88, Table S13).
Figure 2. Multivariable HRs of mortality by isocaloric substitution of specific types of fatty acid for saturated fatty acids.
Abbreviations: UFA, unsaturated fatty acid; MUFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids.
Model was adjusted for age (in month), Caucasian (yes vs. no), marital status (with spouse, yes or no), body-mass index (<23, 23-24.9, 25-29.9, 30-34.9, ≥35 kg/m2), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 hours of metabolic equivalent tasks per week), smoking status (never, past, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d), alcohol consumption (women: 0, 0.1-4.9, 5.0-14.9, ≥15 g/d; men: 0, 0.1-4.9, 5.0-29.9, ≥30 g/d), multivitamin use (yes vs. no), vitamin E supplementation use (yes vs. no), current aspirin use (yes vs. no), family history of myocardial infarction (yes vs. no), family history of diabetes (yes vs. no), family history of cancer (yes vs. no), history of hypertension (yes vs. no), history of hypercholesterolemia (yes vs. no), intakes of total energy, dietary cholesterol and percentage of energy intake from dietary protein(quintiles), and in women, menopausal status, hormone use (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users) and percentage of energy from remaining fatty acids (saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, n-6 PUFAs, n-3 PUFAs, linoleic acid, arachidonic acid, α-linolenic acid and marine n-3 fats, all modeled as continuous variables).
Results for NHS and HPFS from the multivariate model were combined using the fixed-effects model.
Discussion
In two large cohorts with many repeated measures of diet and long duration of follow-up, we found that higher intakes of PUFA and MUFA were associated with lower mortality, whereas higher intakes of SFA and TFA were associated with increased mortality. Although the modest positive association between SFA intake and mortality suggests small health benefits of replacing SFAs by total carbohydrate, replacing SFAs with MUFAs and/or PUFAs was associated with a significantly lower risk of total mortality and cause-specific mortality due to several major chronic diseases. Dietary intake of total fat, compared to total carbohydrate, was inversely associated with total mortality. However, the association between total fat intake and mortality largely depends on specific types of fat. Intake of linoleic acid, the most abundant n-6 PUFA, showed strong inverse associations with total and most cause-specific mortality, without any evidence of detrimental effects. Interestingly, a higher ratio of n-6 to n-3 PUFAs was not associated with increased mortality, but with a slightly lower total, CVD, and cancer mortality.
Previous data on the associations between different types of n-6 PUFA intake and total mortality have been limited. Concordant with our findings, two prospective cohort studies using circulating biomarkers 26,27 found a lower total mortality associated with higher concentrations of n-6 PUFA in blood. Evidence from clinical trials also supported a protective effect of soybean oil with a higher ratio of n-6 to n-3 PUFAs on CHD risk. 28,29 Contrary to our findings, a recent re-analysis of the Sydney Diet Heart Study, a secondary prevention trial, reported a significant increase in total mortality in participants assigned to higher n-6 PUFA intervention. 30 However, this study was very small (n =221), of short duration (39 months), only among individuals with existing CVD, and the intervention likely reduced n-3 PUFA intake. In addition, the results may have been confounded by trans fat in the special margarines used for intervention that were high in linoleic acid 6.
We observed a modest inverse association between marine n-3 PUFA intake and total mortality. Previously, prospective cohorts in generally healthy populations yielded mixed results 31-35, while most RCTs found non-significant effects of fish oil supplementation on total mortality 9,36. The significant inverse association between intake of n-3 PUFAs and death due to neurodegenerative diseases has not, to our knowledge, been previously reported.
We found a significant inverse association between MUFA intake and total mortality. In contrast, previous studies generally reported non-significant or even positive associations with MUFA 2,37,38 This discordance might be due to the strong correlations between MUFA and SFA because animal fats are major sources of both types of fats in most Western diets, and between MUFA and TFA because partial hydrogenation produces both. In our two cohorts the correlation between MUFA and SFA has decreased during the follow-up and the major food sources of MUFA have shifted from animal-sourced to plant-sourced foods over time (Table S18), thus we had greater power to differentiate the association for MUFA with mortality. Consistent with our analysis, the major source of MUFA in Mediterranean populations, olive oil, has been associated with a substantially lower total mortality, 37 Important benefits of MUFA from plant sources have also been supported by the PREDIMED trial, in which the addition of olive oil and nuts, also high in MUFA, reduced incidence of cardiovascular disease and diabetes 39,40.
Compared to overall carbohydrates, higher SFA intake was associated with a slight increase in total mortality, but not significantly associated with CVD mortality. The lack of the association with CVD is expected because the major sources of carbohydrate in typical Western diet are highly processed foods with large amounts of refined starch and sugar, providing a high glycemic load that can increase cardiovascular disease risk independent of SFA. 7,25,41,42 In our substitution analyses, replacing SFA with unsaturated fatty acids was associated with substantially lower risk of total and CVD mortality. These findings were generally consistent with evidence from previous studies. 2,43-45 However, a recent meta-analysis concluded that specific types of fat including saturated, monounsaturated or polyunsaturated fats had no significant impact on risk of CHD. 5 Another meta-analysis also reported nonsignificant associations of SFA intake with total and CVD mortality and CHD incidence. 46 However, most studies included in these meta-analyses did not explicitly model the effects of macronutrient substitution and did not specify the comparison source of energy for the type of fat under scrutiny, which limits the interpretations of these findings. 6,43 Our analyses provide strong evidence that using PUFAs and/or MUFAs as the replacement nutrients for SFAs can confer substantial health benefits, while replacing SFAs with carbohydrates has little impact on CVD mortality. However, the effects of replacement by carbohydrates may depend in part on the quality of carbohydrates.
Data on specific types of dietary fat and non-CVD mortality are sparse. We observed no major effects of most types of dietary fat on cancer mortality, although a modest inverse association with linoleic acid was observed. Our findings of an inverse association between higher intake of PUFAs, primarily α-linolenic acid, and lower mortality due to neurodegenerative diseases are consistent with limited evidence on incidence of major neurodegenerative diseases, including Alzheimer disease 47, amyotrophic lateral sclerosis 15, and Parkinson's disease 48 in prospective cohorts. Finally, we observed a positive association between saturated and trans fat intake and respiratory disease mortality, but an inverse association with PUFAs. These findings are novel and therefore require confirmation in further studies.
Our results have several limitations. First, reverse causation is a possible explanation for our findings, because people with chronic disease and poor health might change their habitual diet. However, we excluded participants with known major chronic diseases at baseline. Also, it is likely that those concerned about a serious illness would change toward a diet generally perceived to be healthier, which would not explain our findings. In addition, our findings remain largely unchanged when we excluded the first 4 years of follow-up or added a 4 year lag period between dietary assessment and each follow-up period. Secondly, because our study was observational in nature, causality cannot be established. However, our results were largely consistent with those from results from existing observational studies and randomized clinical trials on diet and CVD-related outcomes. Thirdly, even though we adjusted for many potential confounders, residual confounding could not be ruled out. Fourthly, measurement errors are inevitable in estimates of food and nutrient intakes. However, our adjustment for energy intake and use of prospectively collected, cumulatively averaged intake using many repeated dietary assessments reduced the impact of measurement errors. 19 The strengths of the current study included a large sample size, high follow-up, and repeated assessments of dietary and lifestyle variables during a long-time period.
In summary, we found that different types of dietary fats have divergent associations with total and cause-specific mortality. Replacement of saturated fats with unsaturated fats can confer substantial health benefits and should continue to be a key message in dietary recommendations. These findings also support the elimination of partially hydrogenated of vegetable oils, the primary source of trans fatty acids.
Supplementary Material
Acknowledgement
Funding/Support: This study was supported by research grants UM1 CA186107, P01 CA87969, R01 HL034594, R01 HL088521, UM1 CA167552, R01 HL35464, R01 HL60712 and P30 DK46200 from the National Institutes of Health.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Author contributions:
Willett and Hu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of the study: Wang, Willett, and Hu
Collection, management, analysis, and interpretation of the data: All authors
Preparation, review, or approval of the manuscript: All authors
Decision to submit the manuscript for publication: All authors
Statistical analysis: Wang and Hu
Conflict of interest disclosure: Hu has received research support from California Walnut Commission and Metagenics.
Additional Contributions: We are indebted to the participants in the Nurses’ Health Study and the Health Professionals Follow-up Study for their continuing outstanding level of cooperation; to the state cancer registries for their kind help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY; to the staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions; to Drs. Alberto Ascherio and Kathryn C. Fitzgerald for their helpful comments; to Lisa Li for her valuable programming assistance. The authors assume full responsibility for analyses and interpretation of these data.
Reference
- 1.Willett WC. Dietary fats and coronary heart disease. J Intern Med. 2012 Jul;272(1):13–24. doi: 10.1111/j.1365-2796.2012.02553.x. [DOI] [PubMed] [Google Scholar]
- 2.Jakobsen MU, O'Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009 May;89(5):1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997 Nov 20;337(21):1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 4.Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010 Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010, to the Secretary of Agriculture and the Secretary of Health and Human Services. Agricultural Research Service. 2010 [Google Scholar]
- 5.Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014 Mar 18;160(6):398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 6.Willett WC, Stampfer MJ, Sacks FM. Association of dietary, circulating, and supplement fatty acids with coronary risk. Ann Intern Med. 2014 Sep 16;161(6):453. doi: 10.7326/L14-5018. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Hruby A, Bernstein AM, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol. 2015;66(14):1538–1548. doi: 10.1016/j.jacc.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris WS. Are n-3 fatty acids still cardioprotective? Curr Opin Clin Nutr Metab Care. 2013 Mar;16(2):141–149. doi: 10.1097/MCO.0b013e32835bf380. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011 Nov 8;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 10.Harris WS, Shearer GC. Omega-6 Fatty Acids and Cardiovascular Disease: Friend or Foe? Circulation. 2014 Aug 26; doi: 10.1161/CIRCULATIONAHA.114.012534. [DOI] [PubMed] [Google Scholar]
- 11.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006 Apr 13;354(15):1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 12.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003 May;77(5):1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 13.Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. 2010 Oct;45(10):893–905. doi: 10.1007/s11745-010-3393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004 Jun;79(6):935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald KC, O'Reilly EJ, Falcone GJ, et al. Dietary omega-3 polyunsaturated fatty acid intake and risk for amyotrophic lateral sclerosis. JAMA Neurol. 2014 Sep;71(9):1102–1110. doi: 10.1001/jamaneurol.2014.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu JH, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012 Jun;107(Suppl 2):S214–227. doi: 10.1017/S0007114512001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giovannucci E, Rimm EB, Colditz GA, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993 Oct 6;85(19):1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 18.Astorg P. Dietary N-6 and N-3 polyunsaturated fatty acids and prostate cancer risk: a review of epidemiological and experimental evidence. Cancer Causes Control. 2004 May;15(4):367–386. doi: 10.1023/B:CACO.0000027498.94238.a3. [DOI] [PubMed] [Google Scholar]
- 19.Willett W. Nutritional epidemiology. Third Vol. 40. Oxford University Press; 2013. Chapter 5: Food Frequency Methods and Chapter 6: Reproducibility and Validity of Food-Frequency Questionnaires. [Google Scholar]
- 20.Harvard TH, Chan School of Public Health Harvard University Food Composition Table. https://regepi.bwh.harvard.edu/health/nutrition/. Accessed March 8th, 2015.
- 21.London SJ, Sacks FM, Caesar J, Stampfer MJ, Siguel E, Willett WC. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr. 1991 Aug;54(2):340–345. doi: 10.1093/ajcn/54.2.340. [DOI] [PubMed] [Google Scholar]
- 22.Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992 Feb 15;135(4):418–427. doi: 10.1093/oxfordjournals.aje.a116302. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007 Jul;86(1):74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- 24.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994 Dec 1;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71(6):1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 26.Wu JH, Lemaitre RN, King IB, et al. Circulating omega-6 polyunsaturated Fatty acids and total and cause-specific mortality: the cardiovascular health study. Circulation. 2014 Oct 7;130(15):1245–1253. doi: 10.1161/CIRCULATIONAHA.114.011590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warensjo E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr. 2008 Jul;88(1):203–209. doi: 10.1093/ajcn/88.1.203. [DOI] [PubMed] [Google Scholar]
- 28.Controlled trial of soya-bean oil in myocardial infarction. Lancet. 1968 Sep 28;2(7570):693–699. [PubMed] [Google Scholar]
- 29.Leren P. The Oslo diet-heart study. Eleven-year report. Circulation. 1970 Nov;42(5):935–942. doi: 10.1161/01.cir.42.5.935. [DOI] [PubMed] [Google Scholar]
- 30.Ramsden CE, Zamora D, Leelarthaepin B, et al. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. Bmj. 2013;346:e8707. doi: 10.1136/bmj.e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folsom AR, Demissie Z. Fish intake, marine omega-3 fatty acids, and mortality in a cohort of postmenopausal women. Am J Epidemiol. 2004 Nov 15;160(10):1005–1010. doi: 10.1093/aje/kwh307. [DOI] [PubMed] [Google Scholar]
- 32.Virtanen JK, Voutilainen S, Rissanen TH, et al. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol. 2005 Jan;25(1):228–233. doi: 10.1161/01.ATV.0000150040.20950.61. [DOI] [PubMed] [Google Scholar]
- 33.Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001 Nov 1;154(9):809–816. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- 34.Bell GA, Kantor ED, Lampe JW, Kristal AR, Heckbert SR, White E. Intake of long-chain omega-3 fatty acids from diet and supplements in relation to mortality. Am J Epidemiol. 2014 Mar 15;179(6):710–720. doi: 10.1093/aje/kwt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozaffarian D, Lemaitre RN, King IB, et al. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med. 2013 Apr 2;158(7):515–525. doi: 10.7326/0003-4819-158-7-201304020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. Jama. 2012 Sep 12;308(10):1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 37.Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014;13:154. doi: 10.1186/1476-511X-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Virtanen JK, Mursu J, Tuomainen TP, Voutilainen S. Dietary fatty acids and risk of coronary heart disease in men: the Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler Thromb Vasc Biol. 2014 Dec;34(12):2679–2687. doi: 10.1161/ATVBAHA.114.304082. [DOI] [PubMed] [Google Scholar]
- 39.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013 Apr 4;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 40.Salas-Salvado J, Bullo M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med. 2014 Jan 7;160(1):1–10. doi: 10.7326/M13-1725. [DOI] [PubMed] [Google Scholar]
- 41.Hu FB. Are refined carbohydrates worse than saturated fat? Am J Clin Nutr. 2010 Jun;91(6):1541–1542. doi: 10.3945/ajcn.2010.29622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jakobsen MU, Dethlefsen C, Joensen AM, et al. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr. 2010 Jun;91(6):1764–1768. doi: 10.3945/ajcn.2009.29099. [DOI] [PubMed] [Google Scholar]
- 43.Farvid MS, Ding M, Pan A, et al. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulation. 2014 Aug 26; doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiuve SE, Rimm EB, Sandhu RK, et al. Dietary fat quality and risk of sudden cardiac death in women. Am J Clin Nutr. 2012 Sep;96(3):498–507. doi: 10.3945/ajcn.112.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010 Mar;7(3):e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. Bmj. 2015;351:h3978. doi: 10.1136/bmj.h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otaegui-Arrazola A, Amiano P, Elbusto A, Urdaneta E, Martínez-Lage P. Diet, cognition, and Alzheimer’s disease: food for thought. Eur J Nutr. 2014;53(1):1–23. doi: 10.1007/s00394-013-0561-3. 2014/02/01. [DOI] [PubMed] [Google Scholar]
- 48.de Lau LM, Bornebroek M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology. 2005 Jun 28;64(12):2040–2045. doi: 10.1212/01.WNL.0000166038.67153.9F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.