Abstract
Background
The aim of this study was to examine the association between serum expression of miRNA-155 and postoperative cognitive dysfunction (POCD) after laparoscopic surgery for colon cancer.
Material/Methods
We enrolled 110 patients scheduled to undergo colon tumor resection via laparotomy in Ningbo No. 2 Hospital from July 2013 to November 2015. The blood samples were collected from the participants 1 day before surgery. Multiple logistic regression analysis was used for the analysis of independent predictive biomarkers for POCD.
Results
On the 7th postoperative day, 29 of the 110 participants developed POCD, yielding a POCD incidence of 26.4%. Age, MMSE score, duration of surgery and anesthesia, serum levels of CRP, TNF-α, urea, creatinine, and miRNA-155 were highly associated with the occurrence of POCD. Serum expression of miRNA-155 was shown by multiple logistic regression analysis to be an independent predictive indicator for POCD after surgery (OR: 2.732; 95%CI 1.415–5.233; P=0.002).
Conclusions
The serum expression of miRNA-155 is an independent predictive factor for POCD after laparoscopic surgery for colon cancer.
MeSH Keywords: Colonic Neoplasms; Colorectal Surgery; Postoperative Complications; RNA, Ribosomal
Background
Colorectal cancer (CRC) is one of the most common cancers worldwide and ranks the second in cancer-related mortality [1]. Along with the development of diagnosis and treatment regimens, the survival rate of patients with CRC has significantly increased during the past few years. Multi-detector computed tomography (MDCT) and virtual colonoscopy with 64-row CT are both useful imaging methods for early detection of CRC and are tolerated well by patients [2,3]. The use of laparoscopic surgery for CRC is increasing rapidly, and some institutions regard laparoscopy as the standard procedure for surgical treatment of CRC. Postoperative cognitive dysfunction (POCD) is one of the most common postoperative complications in elderly patients, but the mechanism by which this occurs is unknown [4]. Recently, it was reported that the incidence of POCD for patients aged over 65 years who underwent general anesthesia can be as high as 25.8% at 1 week, and some of them will sustain long-term cognitive dysfunction or even develop dementia [5]2/22-rdf-syntax-ns#. Nonetheless, studies also showed that the incidence of POCD at 3 months after surgery was 12.7% [6]. It is suggested that cognitive dysfunction is caused by early postoperative nervous system damage, and the protective mechanism of the body can be simultaneously initiated for self-repair; therefore, the cognitive function in some of these patients could be significantly improved or restored [7]. It is expected that exploring the mechanism and early prediction of postoperative cognitive function will improve prognosis and therapy. MicroRNAs (miRNAs) were discovered in eukaryotic cells and play important roles in the transcription and translation of target genes [8]. MiRNAs also participate in the development of nervous system diseases, as described by recent studies [9]. For example, in the occurrence of Huntington’s disease, multiple miRNAs were found to be abnormally expressed and researchers have suggested that the miRNA expression levels have a close relationship with the occurrence and progression of Huntington’s disease [10]. Some other studies also suggested that miRNAs participate in the neural tube defects [11]x8fHx8fHx§5þf, nervous system development, neurobehavioral disorders [12], and nervous system damage repair [9]. As reported by recent studies, the levels of miRNAs in tissues and peripheral blood samples are very similar [13]. Because expressions of miRNAs from the peripheral blood samples are stable and convenient to detect, peripheral blood miRNAs have been commonly used as effective markers for the diagnosis and prognosis of various diseases, especially tumors [14]. Previous studies have reported that miRNA-155 is involved in regulating various physiological and pathological processes such as inflammation, immunity, cancer, cardiovascular diseases, and nervous system diseases [15,16]. However, less is known about miRNA-155 and POCD in patients with colon cancer after the surgery.
Material and Methods
Patients
This study was approved by the Medical Institutional Ethics Committee of Zhejiang province. Patients scheduled to undergo colon tumor resection via laparotomy in Ningbo No. 2 hospital from July 2013 to November 2015 were enrolled in this study. The inclusion criteria were age 60–75 years old, laparoscopic surgery for colon cancer under spinal anesthesia, American Society of Anesthesiologists (ASA) grade 2 and 3, and patient consent. Exclusion criteria were cognitive impairment characterized by MMSE<24, history of dementia, disease of the central nervous system, history of surgery within 6 months, low patient compliance, inability to read, serious hearing or vision loss, poor comprehension of the Chinese language, and recent cerebrovascular accident (<6 months).
Methods
Cytokines measurements
All the blood samples in this study were collected from patients who provided signed informed consent. The obtained blood samples were collected in anticoagulant tubes on the day before surgery and were centrifuged at 820 rpm at 4°C for 10 min. The supernatant was stored at −70°C for further analysis. Inflammatory cytokines, including C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), were measured by using enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions, and the ELISA kits purchased from R&D Systems (Minneapolis, MN).
RNA isolation and quantitative real-time PCR
TRIzol reagent was used for the isolation of total RNA from serum samples and reverse transcription reactions using miRcute miRNA First-strand cDNA Synthesis kits following the instructions of the manufacturer. The kits used in this study were all purchased from Invitrogen Co. Ltd. (Carlsbad, CA). Real-time PCR was then conducted and miRNA expression levels were calculated (relative to β-actin) using the 2−ΔΔCt method.
Assessment of POCD
To evaluate the neuropsychological testing, the Cambridge Neuropsychological Test Automated Battery (CANTAB, CANTAB Cognition, Cambridge, UK), a touchscreen computer system, was used in our study [17]. The CANTAB test includes a spatial recognition memory test, a pattern recognition memory test, a choice reaction time test, a motor screening Test, the visual verbal learning test, and the Stroop color-word interference test in 3 parallel versions in random order. Patients were required to complete the CANTAB test on the day before surgery, and at 7 days and at 3 months after the surgery. To eliminate the possible interference factors, all tests were carried out in a quiet room at the same time of day and with no other people present except for the patient and investigator. To define POCD, the calculation of reliable change index (RCI) according to the recommendations of Rasmussen et al. was used: POCD was defined when the RCI score was <−1.96 at least on 2 tests or when the combined Z score was <−1.96 [18].
Statistical analysis
SPSS 19.0 (SPSS, Inc.) was used for statistical analysis. Data are all presented as number (n) and percentage (%), or mean ± standard error (SEM), as appropriate. The chi-square test and Mann-Whitney U test were used for the statistical analysis. Multiple logistic regression analysis was used for the analysis of independent predictive biomarkers for POCD. All statistical tests were 2-tailed and P<0.05 was accepted as statistically significant.
Results
POCD and various clinicopathological variables
During the inclusion period a total of 165 participants met the inclusion criteria and were asked to participate. Of these 165 participants, 55 were excluded: 41 refused informed consent, 12 had information missing, and 2 had their surgery canceled. On the 7th postoperative day, 29 of the 110 participants developed POCD, yielding a POCD incidence of 26.4%. The indicators of general characteristics and intraoperative conditions were with not significantly different between patients with and without POCD. To measure the possible predicative indicators for POCD, blood samples were collected from the 110 participants before the surgery. As shown in Table 1, significant preoperative differences were found between patients with and without POCD in age (P=0.004), MMSE score (P=0.038), duration of surgery (P=0.034), and anesthesia (P=0.029). The potential serum indexes CRP, TNF-α, urea, and creatinine were also significantly higher in patients with POCD on the 7th postoperative day (P<0.05). As shown in the scatter plots of Figure 1, patients who developed POCD had a significant higher expression of serum miRNA-155 (P<0.01). Previous studies have revealed that serum CRP and urinary trypsin inhibitor are involved in postoperative cognitive dysfunction, especially in elderly patients [19]. Elevated plasma concentrations of TNF-α, IL-6, and S-100β protein also contributed to the occurrence of POCD as biomarkers of systemic inflammation [20]. Therefore, we chose to measure these cytokines (including CRP, TNF-α, IL-6, urea, and creatinine) in our study as possible predicative factors or confounding factors for POCD according to the results reported by previous studies [21].
Table 1.
Preoperative characteristics of patients with and without POCD.
| POCD (n=29) | Non-POCD (n=81) | P-value | |
|---|---|---|---|
| Age (year) | 71.8±6.5 | 67.4±7.0 | 0.004* |
| Gender | |||
| Male | 13 (44.8%) | 30 (37.0%) | |
| Female | 16 (55.2%) | 51 (63.0%) | 0.461 |
| ASA physical status | |||
| II | 12 (41.4%) | 42 (51.9%) | |
| III | 17 (58.6%) | 39 (48.1%) | 0.333 |
| BMI (kg/m2) | 22.2±2.7 | 22.1±3.3 | 0.884 |
| MMSE (score) | 27.5±1.7 | 28.5±1.6 | 0.038* |
| Charlson Comorbidity Index | 1.4±0.7 | 1.5±0.5 | 0.410 |
| Duration of surgery (min) | 167.2±30.3 | 153.8±28.2 | 0.034* |
| Duration of anesthesia (min) | 190.2±41.4 | 172.3±35.9 | 0.029* |
| Estimated blood loss (ml) | 311.3±100.4 | 324.1±135.7 | 0.644 |
| Duration of hospital stay (days) | 11.3±3.8 | 10.9±4.1 | 0.647 |
| TNM stage | |||
| I, II | 17 | 43 | |
| III, IV | 12 | 38 | 0.608 |
| Tumor metastasis | |||
| Yes | 9 | 20 | |
| No | 20 | 61 | 0.506 |
| Anaesthetic | |||
| Propofol (TIVA) | 10 (34.5%) | 34 (42.0%) | |
| Volatile anaesthesia | 19 (65.5%) | 47 (58.0%) | 0.480 |
| Drugs | |||
| Fentanyl | 20 (69.0%) | 53 (65.4%) | |
| Remifentanil | 9 (31.0%) | 28 (34.6%) | 0.730 |
| CRP (mg/L) | 14.3±5.5 | 12.1±4.8 | 0.044* |
| IL-6 (pg/mL) | 19.1±10.8 | 18.5±8.4 | 0.761 |
| TNF-α (nmol/L) | 9.1±2.4 | 7.8±2.0 | 0.005* |
| Creatinine (mmol/L) | 98.3±22.1 | 84.4±26.1 | 0.012* |
| Urea (mmol/L) | 7.8±2.1 | 6.3±2.9 | 0.012* |
| Relative miRNA-155 expression | 1.78±0.67 | 1.13±0.53 | 0.001* |
ASA – American Society of Anesthesiologists; MMSE – mini-mental state examination; BMI – body mass index; TIVA – total intravenous anesthesia; POCD – postoperative cognitive dysfunction; CRP – C-reactive protein; IL-6 – interleukin-6; TNF-α – tumor necrosis factor-α. P-values were calculated by Chi-square test or Mann-Whitney U-test.
P value <0.05.
Figure 1.
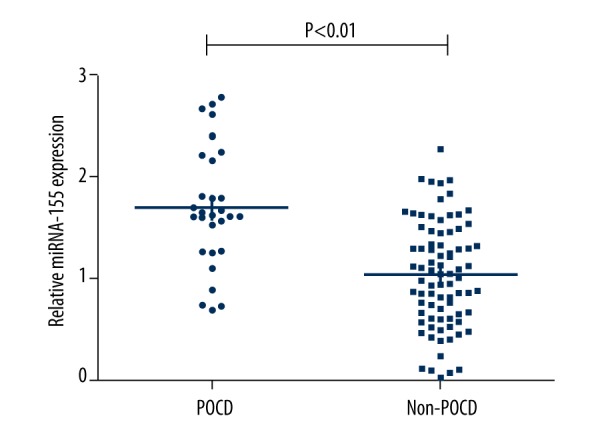
Scatter plots of preoperative relative serum expression of miRNA-155 in patients with and without post-operative cognitive dysfunction (POCD).
POCD and predicative factors
Table 1 shows that age, MMSE score, duration of surgery and anesthesia, and serum levels of CRP, TNF-α, urea, creatinine, and miRNA-155 were highly associated with the occurrence of POCD after colon surgery via laparotomy. The multiple logistic regression analysis used for the analysis of independent predictive biomarkers for POCD shows that elevated serum expression of miRNA-155 was an independent predictive indicator for POCD after surgery (OR: 2.732; 95%CI 1.415–5.233; P=0.002) (Table 2).
Table 2.
Multiple logistic regression analysis for POCD after laparoscopic surgery for colon cancer.
| Parameter | POCD | ||
|---|---|---|---|
| OR | 95%CI | P value | |
| Age | 1.837 | 0.972–3.352 | 0.068 |
| MMSE | 2.313 | 0.685–7.251 | 0.147 |
| Duration of surgery | 2.486 | 0.784–7.894 | 0.119 |
| Duration of anesthesia | 3.873 | 0.857–18.153 | 0.081 |
| CRP | 2.271 | 0.824–6.235 | 0.124 |
| TNF-α | 2.051 | 0.808–5.210 | 0.131 |
| Creatinine | 1.421 | 0.232–9.143 | 0.703 |
| Urea | 2.219 | 0.205–21.551 | 0.513 |
| Relative miRNA-155 expression | 2.732 | 1.415–5.233 | 0.002* |
MMSE – mini-mental state examination; CRP – C-reactive protein; TNF-α – tumor necrosis factor-α; POCD – postoperative cognitive dysfunction; CI – confidence interval; OR – odds ratio.
P value<0.05.
Discussion
POCD has been reported to be a common complication after surgery, but the mechanism by which this occurred was unknown [22]. Direct brain insults such as hypotension, hypoxia, and infarcts, as well as aberrant stress responses induced by infection, surgical trauma, and anxiety, were suggested as 2 major categories of etiologies for POCD [23,24]. These risk factors mentioned above are common in abdominal surgeries, leading to the higher incidence of POCD after surgery, especially in elderly patients [25]. This study aimed to investigate the potential non-invasive biomarkers for POCD after colon tumor resection via laparotomy. The results showed that individuals who developed POCD by the 7th postoperative day had higher preoperative age and MMSE score, longer duration of surgery and anesthesia, and higher levels of CRP, TNF-α, urea, and creatinine. These findings suggest that there was greater cellular immune activation and cognitive function changes in participants at risk of POCD, even before the surgery. However, as shown in the multiple logistic regression analysis, these factors were not independent predictors for POCD after the surgery. In contrast to our results, other studies suggested that age, level of education, and anesthesia duration might be independent risk factors in the pathogenesis of POCD [26,27]. The different results were probably due to the differences in sample size, operation type, and age of participants.
Neurobehavioral disorders associated with N-methyl-D-aspartate receptor were reported to be associated with the abnormal expression of miR-219 [12]. Patients with Alzheimer’s disease were found to have significantly abnormal expression of miR-124 [28]. All these studies suggest that miRNAs have a very close correlation with neural tube defects, neurobehavioral disorders, and nervous system damage repair [9]. In the present study, we detected the expressions of miRNA-155 in circulating peripheral blood by the analyses of quantitative real-time PCR. The patients who developed POCD on the 7th postoperative day had significantly up-regulated miRNA-155 expression in the peripheral blood before the surgery in comparison with those who did not develop POCD, as supported by quantitative real-time PCR analysis. These results strongly suggest a close correlation between serum expression of miRNA-155 and the occurrence of POCD. As reported by previous studies, miRNA-155 is specifically expressed in hematopoietic cells, endothelial cells, smooth muscle cells, and cells involved in vascular remodeling, including T cells, B cells, monocytes, and granulocytes [29,30]. MiRNA-155 can also promote tissue inflammation by regulating the development of inflammatory T cells, and the inhibition of miRNA-155 is accompanied by reduced inflammation. Therefore, some researchers suggested miRNA-155 as a promising therapeutic target in proinflammatory conditions [31]. MiRNA-155 is also significantly associated with endothelial and vascular function through the modulation of endothelial nitric oxide synthase (eNOS) expression, nitric oxide (NO) production, and regulation of vascular inflammation [32,33]. Previous literature reports and some in vivo investigations suggested that miR-155 inhibition can broadly influence vascular function and brain tissue remodeling, in addition to its specific effect on endothelial tight junctions [34]. Multivariate logistic regression analysis was used in this study to examine the potential role of miRNA-155 to predict POCD. The results revealed that serum expression of miRNA-155 was an independent predictive factor for POCD, but the mechanism involved remains unclear and requires further research. It is unfortunate that some other confounding factors, including depth of anesthesia [35], pre-surgically impaired mental status, alcohol abuse [36], hypoxemia, or hypotension [37] were not taken into consideration. The depth of anesthesia during elective surgical procedures of intermediate duration was a significant factor for postoperative cognitive function [35].
Conclusions
This current study is the first to demonstrate that evaluation of preoperative serum miRNA-155 might be a promising clinical tool for predication of POCD after laparoscopic surgery for colon cancer.
Study limitations
The sample size of this study was relatively small and the mechanisms involved remain unclear. Long-term follow-up and monitoring the incidence of POCD would improve the precision of results.
Footnotes
Competing interests
All the authors declare that they have no competing interests.
Source of support: This work was supported by a grant from the Medical and Health Science and Technology Plan Project of Zhejiang Province (No. 2014KYB232)
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Zaleska-Dorobisz U, Lasecki M, Nienartowicz E, et al. Value of virtual colonoscopy with 64 row CT in evaluation of colorectal cancer. Pol J Radiol. 2014;79:337–43. doi: 10.12659/PJR.890621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai CC, Wu MN. Frequent ischemic stroke as first manifestation of occult colon cancer: A rare case. Am J Case Rep. 2015;16:723–27. doi: 10.12659/AJCR.895130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora SS, Gooch JL, Garcia PS. Postoperative cognitive dysfunction, Alzheimer’s disease, and anesthesia. Int J Neurosci. 2014;124(4):236–42. doi: 10.3109/00207454.2013.833919. [DOI] [PubMed] [Google Scholar]
- 5.Steinmetz J, Christensen KB, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548–55. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 6.Engelhard K, Werner C. [Postoperative cognitive dysfunction in geriatric patients]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2008;43(9):606–14. doi: 10.1055/s-0028-1090023. [in German] [DOI] [PubMed] [Google Scholar]
- 7.Yu X, Liu S, Li J, et al. MicroRNA-572 improves early post-operative cognitive dysfunction by down-regulating neural cell adhesion molecule 1. PloS One. 2015;10(2):e0118511. doi: 10.1371/journal.pone.0118511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murchison EP, Hannon GJ. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol. 2004;16(3):223–29. doi: 10.1016/j.ceb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Wang WY, Mao YW, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466(7310):1105–9. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee ST, Chu K, Im WS, et al. Altered microRNA regulation in Huntington’s disease models. Exp Neurol. 2011;227(1):172–79. doi: 10.1016/j.expneurol.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Gu H, Li H, Zhang L, et al. Diagnostic role of microRNA expression profile in the serum of pregnant women with fetuses with neural tube defects. J Neurochem. 2012;122(3):641–49. doi: 10.1111/j.1471-4159.2012.07812.x. [DOI] [PubMed] [Google Scholar]
- 12.Kocerha J, Faghihi MA, Lopez-Toledano MA, et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci USA. 2009;106(9):3507–12. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zen K, Zhang CY. Circulating microRNAs: A novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev. 2012;32(2):326–48. doi: 10.1002/med.20215. [DOI] [PubMed] [Google Scholar]
- 14.Guo F, Tian J, Lin Y, et al. Serum microRNA-92 expression in patients with ovarian epithelial carcinoma. J Int Med Res. 2013;41(5):1456–61. doi: 10.1177/0300060513487652. [DOI] [PubMed] [Google Scholar]
- 15.Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: A typical multifunctional microRNA. Biochim Biophys Acta. 2009;1792(6):497–505. doi: 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell RM, Kahn D, Gibson WS, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33(4):607–19. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radtke FM, Franck M, Lendner J, et al. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110(Suppl 1):i98–105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen LS, Larsen K, Houx P, et al. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001;45(3):275–89. doi: 10.1034/j.1399-6576.2001.045003275.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YH, Guo XH, Zhang QM, et al. Serum CRP and urinary trypsin inhibitor implicate postoperative cognitive dysfunction especially in elderly patients. Int J Neurosci. 2015;125(7):501–6. doi: 10.3109/00207454.2014.949341. [DOI] [PubMed] [Google Scholar]
- 20.Qiao Y, Feng H, Zhao T, et al. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: The influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015;15:154. doi: 10.1186/s12871-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R, Chen J, Wu G. Variable lung protective mechanical ventilation decreases incidence of postoperative delirium and cognitive dysfunction during open abdominal surgery. Int J Clin Exp Med. 2015;8(11):21208–14. [PMC free article] [PubMed] [Google Scholar]
- 22.Gottesman RF, Grega MA, Bailey MM, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67(3):338–44. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tully PJ, Baker RA. The reliable change index for assessment of cognitive dysfunction after coronary artery bypass graft surgery. Ann Thorac Surg. 2013;96(4):1529. doi: 10.1016/j.athoracsur.2013.03.072. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado JR. Neuropathogenesis of delirium: Review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190–222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, He R, Chen S, Qu Y. Effect of dexmedetomidine on early postoperative cognitive dysfunction and peri-operative inflammation in elderly patients undergoing laparoscopic cholecystectomy. Exp Ther Med. 2015;10(5):1635–42. doi: 10.3892/etm.2015.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care. 2011;17(4):376–81. doi: 10.1097/MCC.0b013e328348bece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudson AE, Hemmings HC., Jr Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107(1):30–37. doi: 10.1093/bja/aer122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Wang ZH, Wu YY, et al. Melatonin attenuates scopolamine-induced memory/synaptic disorder by rescuing EPACs/miR-124/Egr1 pathway. Mol Neurobiol. 2013;47(1):373–81. doi: 10.1007/s12035-012-8355-9. [DOI] [PubMed] [Google Scholar]
- 29.Landgraf P, Rusu M, Sheridan R, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–14. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murugaiyan G, Beynon V, Mittal A, et al. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2011;187(5):2213–21. doi: 10.4049/jimmunol.1003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurowska-Stolarska M, Alivernini S, Ballantine LE, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci USA. 2011;108(27):11193–98. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun HX, Zeng DY, Li RT, et al. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60(6):1407–14. doi: 10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 33.Weber M, Kim S, Patterson N, et al. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am J Physiol Heart Circ Physiol. 2014;306(8):H1192–203. doi: 10.1152/ajpheart.00521.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caballero-Garrido E, Pena-Philippides JC, Lordkipanidze T, et al. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J Neurosci. 2015;35(36):12446–64. doi: 10.1523/JNEUROSCI.1641-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farag E, Chelune GJ, Schubert A, Mascha EJ. Is depth of anesthesia, as assessed by the Bispectral Index, related to postoperative cognitive dysfunction and recovery? Anesth Analg. 2006;103(3):633–40. doi: 10.1213/01.ane.0000228870.48028.b5. [DOI] [PubMed] [Google Scholar]
- 36.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134–39. [PubMed] [Google Scholar]
- 37.Rosenberg J, Kehlet H. Postoperative mental confusion – association with postoperative hypoxemia. Surgery. 1993;114(1):76–81. [PubMed] [Google Scholar]


