Abstract
Background
The purpose of this study was to investigate the potential association between socioeconomic status and ovarian reserve, anti-Mullerian hormone level, antral follicle count, and follicle stimulating hormone level in women of reproductive age.
Material/Methods
A total of 101 married women between 20–35 years of age who presented to the Department of Obstetrics and Gynecology, Health Research System In Vitro Fertilization (HRS IVF) Center between October 2014 and November 2015 and met the inclusion criteria were included in this study. The participants were divided into three socioeconomic groups using Kuppuswamy’s socioeconomic status scale. Thirty-one participants were assigned to the low socioeconomic status group, 37 to the middle socioeconomic status group, and 33 to the high socioeconomic status group. On days 3–6 of the menstrual cycle, 10 mL of blood was collected from the participants for follicle stimulating hormone and anti-Mullerian hormone measurements. Transvaginal ultrasonography was performed for both ovaries for the purpose of counting antral follicles measuring 2–10 mm in diameter.
Results
Both ovarian reserve parameters, namely anti-Mullerian hormone level and antral follicle count, exhibited a significant association with socioeconomic status (p=0.000 and p=0.000, respectively). The association between follicle stimulating hormone level and socioeconomic status was also significant (p=0.000).
Conclusions
A low socioeconomic status aggravated by sources of stress such as undernutrition and financial hardships affects ovarian reserve, which should be remembered in approaching infertile patients.
MeSH Keywords: Anti-Mullerian Hormone, Follicular Fluid, Social Class
Background
Three surveys on the overall prevalence of infertility published in the new millennium (2004, 2007, and 2012) have very different results: reporting prevalences between 48.5 million and 186 million. Secondary infertility or lack of conception after a previous pregnancy is the most common form of female infertility around the world [1], which corresponds to a global infertility rate of 8–12%.
Socioeconomic status (SES) is an important social determinant that can have profound impacts on reproductive health. In the last decades, populations of urban and rural regions have been subject to categorization by various means of measuring SES. A number of socioeconomic factors such as the woman’s educational status, income per capita, having a job, age at first marriage, life expectancy, and infant mortality rates have been reported to be associated with fertility rates by social scientists. Today, in a majority of IVF programs, selection of patients, stimulation regimens, and determination of prognosis are led by routine measurements of ovarian reserve [2].
Anti-Mullerian hormone (AMH), a dimeric glycoprotein, is a member of the transforming growth factor-β superfamily. Granulosa cells present in the ovary synthesize this hormone only when the fetal life ends or after the fetus is born. AMH testing is considered a significant novel tool for determination of ovarian reserve. On the other hand, later stage identification of antral follicle count (AFC) is believed to be more reliable for the said purpose. It has been shown that menopause at an early age is associated with reduced reproductive ability; however, this is of limited help to clinical practice if we do not make use of prospective markers for menopause.
Reduced estradiol and inhibin B levels in a small follicular cohort bring about increased pituitary follicle stimulating hormone (FSH) levels. This situation points to an increased early follicular phase FSH level. Causing ovarian follicles to grow rapidly, an elevated FSH level leads to an elevated estradiol level and shortens the follicular phase and the reproductive cycle [3].
AFC represents the number of ovarian follicles with a diameter of 2–10 mm visualized on transvaginal ultrasonography in the early follicular phase, i.e. days 2–5 of the menstrual cycle. This count exhibits a correlation with the amount of the remaining follicles as well as with the ovarian response to a controlled ovarian stimulation. In this respect, inter-cycle and inter-observer reliability of AFC has been shown [4,5]. A low AFC, representing a total of 3–6 antral follicles, exhibits an association with poor response to ovarian stimulation in a woman receiving IVF treatment; however, it is not necessarily a predictor of failure to conceive. A meta-analysis points to a mean number of 5.2 for an AFC to be considered low (2.11 standard deviation) [4]. Determination of ovarian volume necessitates ovarian measurements in three planes as well as utilization of the following formula for an ellipsoid: D1×D2×D3×0.52. Ultrasound software can provide automatic calculations of ovarian volume. Although ovarian volume exhibits a correlation with ovarian response during stimulation, it cannot be highly relied on as a predictor of failure to conceive [4]. Today, AFC and AMH are considered the most informative biomarkers of ovarian reserve [5]. Their improved performance is the result of significantly stronger correlation with non-growing follicles and primordial follicle counts [5]. Although it is still debatable which of the two biomarkers is superior for ovarian reserve appraisal, both predict poor response and cycle cancellation as well as excessive response and ovarian hyperstimulation syndrome development with equivalent levels of accuracy and clinical value [6,7]. Similarly, they also predict age at natural menopause, which is a related correlate of ovarian reserve [8,9].
In recent years, the serum level of AMH has been considered a new marker of ovarian reserve. It is influenced by age and exhibits a declining trend until a woman reaches menopause. Studies have reported other factors than age may be linked with low serum levels of AMH, but the literature lacks definitive data proving this potential linkage. A low serum level of AMH is considered to be related to early menopause and reduced ovarian reserve. In cases of ovarian failure, AMH levels change prior to FSH levels.
Reports also link fertility transition to socioeconomic development. In 1993–94, 36% of the population in India lived below the poverty line and this rate dropped to 22% in 2005–2006, and this drop was linked to the socioeconomic development. In addition to a declining poverty rate, the fertility rate declined from 3.5 to 2.9 among Indians in the same time period [10,11]. In this respect, SES appears to be an important social determinant that can have a profound impact on reproductive health. Reports indicate that age at first marriage is higher and contraceptive use is more prevalent among educated women who are generally urban. Educated women act more autonomously compared to non-educated women, and educated women participate in decision-making, gain economic resources, and foster interactions with a broader social milieu. Thus, education has an influence on fertility [12–14]. Religion, caste, and region are other socioeconomic factors suggested to be influential on fertility. Kuppuswamy’s SES scale, which covers the three variables – occupation, education and household income – is a useful instrument for measuring SES of urban populations [10]. Using this scale, populations can be grouped into three socioeconomic levels as follows: persons scoring 10 or less are assigned to the low SES group, those scoring between 11 to 25 to the middle SES group, and those scoring more than 25 to the high SES group. There are only a limited number of studies placing emphasis on the social factors correlating with fertility [10,11,15,16].
FSH, AMH, and AFC are common markers used for determination of ovarian reserve in women of reproductive age. Such markers also prove helpful in deciding on the suitable treatment protocol and the first dose of gonadotropin in infertility treatments [17,18]. Despite the fact that both SES and ovarian reserve exert impact on fertility rates, the literature has yet to provide clear evidence of the association. The literature includes a few studies reporting on the impact of SES on markers of ovarian reserve. Ovarian reserve can be assessed by various methods, such as AFC, that requires the use of ultrasound [19]. Other methods, including FSH, and estrogen and inhibin B measurements may face some limitations such as specific timing of the measurement to the menstrual cycle [19,20]. On the other hand, AMH testing faces no such limitation and can be measured at any time of the menstrual cycle. In addition, as opposed to FSH and other markers, AMH serum concentration is not influenced by oral contraceptive use [21,22]. The purpose of this study was to investigate the potential association between SES and infertility using these three biomarkers to better understand unexplained infertility reported to affect 8–12% of all couples around the world.
Material and Methods
A total of 101 married women between 20–35 years of age who presented to the Department of Obstetrics and Gynecology, Health Research System In Vitro Fertilization (HRS IVF) Center between October 2014 and November 2015 and met the inclusion criteria were included in this study. Day 2–3 serum AMH levels were measured by sandwich enzyme immunoassay, and serum FSH levels were measured by solid-phase two-site chemiluminescent immunometric assay. In addition, assessment of ovarian volume and AFC was done using transvaginal ultrasonography. The modified Kuppuswamy’s SES Scale was used in this study. Kuppuswamy’s scale yields a composite score of three variables, namely education, occupation of the head of the family, and monthly household income. This score ranges from 3–29. Using this scale, populations can be divided into high, middle, and low SES groups.
Inclusion criteria
Inclusion criteria included: 1) aged between 20–35 years; 2) infertile women who had regular, menstrual cycles of 21–45 days; 3) regular menstrual cycles (cycle length of 21–45 days duration); 4) no evidence of endocrine disorders (normal TSH, prolactin, testosterone, and androstanidione); 5) a BMI ranging from 18–28 kg/m2; 6) not on hormone therapy for previous 3 months; 7) no history of ovarian surgery; 8) adequate visualization of ovaries during transvaginal ultrasound scan; 9) ultrasonography was done on day 3 of menstrual cycle to measure ovarian volume and for antral follicles count.
Exclusion criteria
A detailed history, including age, occupation, number of previous pregnancies, educational status, and smoking status was taken for each participant. Participants meeting the following criteria were excluded from the study: 1) history of uterine or adnexal surgery, hysterectomy, oophorectomy, ovarian cystectomy, or fulguration; 2) endometriosis; 3) polycystic ovary syndrome; 3) body mass index ≥30 kg/m2; 4) serum prolactin levels >50 ng/mL; 5) irregular menstruation (interval: less than 21 days or more than 45 days); 6) endocrine diseases (thyroid disease, diabetes mellitus, or Cushing’s syndrome); 7) hormonal contraceptive use; 8) smoking; 9) pregnancy; and 10) lactation.
Study design
A total of 101 married women between 20–35 years of age who presented to the Department of Obstetrics and Gynecology, Health Research System In Vitro Fertilization (HRS IVF) Center between October 2014 and November 2015 and met the inclusion criteria were included in this observational cross-sectional study. Day 2–3 serum FSH and AMH levels were measured in all participants. All the study population had regular menstrual cycles (interval: 21–45 days). Ovarian volume assessment and AFC were carried out. Ovarian volume of each ovary was calculated by making measurements in three planes and using the following formula for an ellipsoid: D1×D2×D3×π/6. The volumes of the two ovaries were added together for determination of the total basal ovarian volume [23]. Total AFC represented the sum of the follicles found in both ovaries. The follicles measuring 2–10 mm in diameter were counted by transvaginal ultrasonography (TVUS) in the early follicular phase [23].
Measurements
Blood samples were taken from each subject by venipuncture on day 2–3 of menstruation for AMH and FSH measurements. Serum AMH levels were measured by enzyme immunoassay using an AMH/MIS EIA kit (Immunotech version; Beckman Coulter, Marseille, France), which is a two-immunological step sandwich type assay. Serum FSH levels were measured by solid-phase two-site chemiluminescent immunometric assay.
Statistical analysis
Parametric data were compared using Student’s t-test. Mann-Whitney U test was used for data that did not demonstrate a normal distribution and chi-square test was used for categorical data. Correlations were made by Spearman’s correlation test. All statistical analyses were performed by SPSS 15.0 (SPSS Inc., Chicago, IL, USA). A p value smaller than 0.05 was considered statistically significant.
Ethics statement
The study was conducted only after the local ethics committee approval had been obtained and each subject had signed an informed consent form.
Results
Records for 101 participants, who were divided into three SES groups, were analyzed and compared in terms of sociodemographic factors. The mean age of the participants was 28.40±5.27 years in the low SES group, 28.40±5.27 years in the middle SES group, and 28.15±4.84 years in the high SES group. The mean weight of the participants (kg) was 65.77±12.56 in the low SES group, 62.08±10.31 in the middle SES group, and 63.18±4.84 in the high SES group. The mean height of the participants (cm) was 1.62±0.049 in the low SES group, 1.61±0.056 in the middle SES group, and 1.64±0.076 in the high SES group. There were no significant differences between the three groups in age, weight, or height. The ovarian reserve parameters AMH and AFC exhibited a significant association with SES (p=0.000 and p=0.000, respectively). The association between FSH and SES was also significant (p=0.000), see Table 1 and Figures 1–3).
Table 1.
Comparison of ovarian reserve markers in different socioeconomic groups.
| LSES (31) Mean ± standart deviation | MSES (37) Mean ± standart deviation | HSES (33) Mean ± standart deviation | P value | |
|---|---|---|---|---|
| Age (years) | 28.40±5.27 | 28.40±5.27 | 28.14±4.84 | β: 0.180 α: 0.108 μ: 0.835 |
| Weight (kg) | 65.77±12.56 | 62.08±10.31 | 63.18±4.84 | β: 0.188 α: 0.367 μ: 0.656 |
| Height (cm) | 1.62±0.049 | 1.61±0.056 | 1.64±0.076 | β: 0.478 α: 0.261 μ: 0.086 |
| FSH (IU/L) | 13.56±9.70 | 4.59±2.35 | 4.66±2.75 | β: 0.000 α: 0.000 μ: 0.906 |
| AMH (ng/ml) | 0.92±1.02 | 2.92±1.00 | 3.16±0.70 | β: 0.000 α: 0.000 μ: 0.257 |
| AFC (mm) | 5.03±2.84 | 12.45±4.38 | 11.57±3.60 | β: 0.000 α: 0.000 μ: 0.364 |
Student’s t-test and Mann-Whitney U test were used for statistical analysis. p<0.05 was considered statistically significant. LSES – low socioeconomic status; MSES – middle socioeconomic status; HSES – high socioeconomic status, AMH – anti-Müllerian hormone; FSH – follicle stimulating hormone; AFC – Antral follicle count. Comparison between LSES(3) and MSES(2) – β; Comparison between LSES(3) and HSES(1) – α; Comparison between MSES(2) and HSES(1) – μ.
Figure 1.
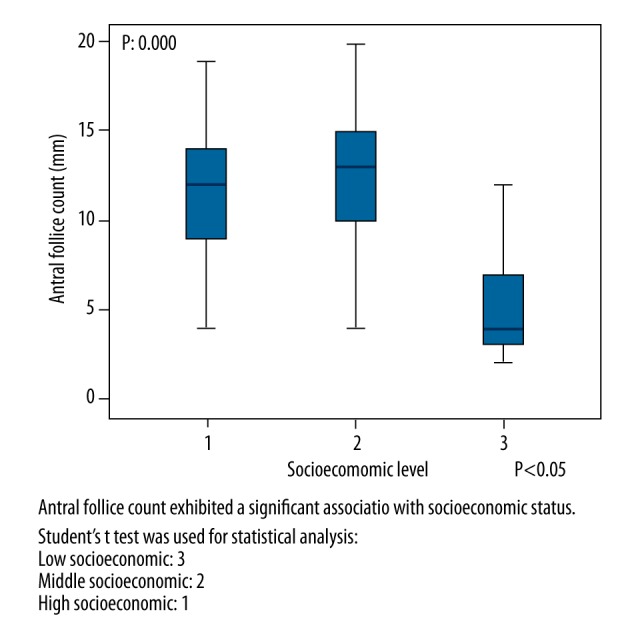
Comparison of Antral follicle count and socioeconomic level.
Figure 2.
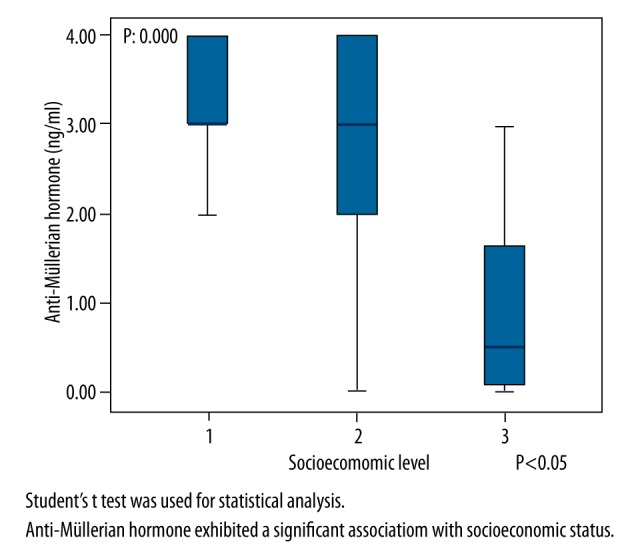
Comparison of Anti-mullerian hormone and socioeconomic levels.
Figure 3.
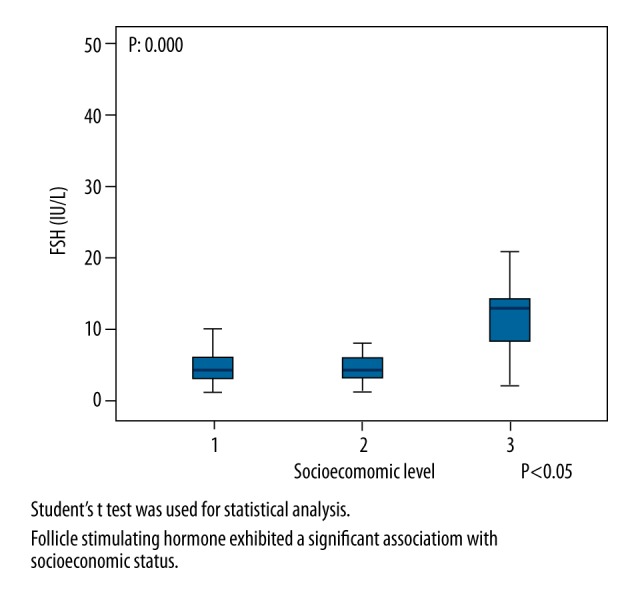
Comparison of FSH and socioeconomic levels.
SES exhibited a significant positive correlation with AFC and AMH level (r=+0.524 and p=0.000; r =+0.659 and p=0.000, respectively) as well as a significant negative correlation with FSH level (r=−0.498 and p=0.000)
Discussion
The purpose of this study was to investigate the potential association between SES and infertility to better understand unexplained infertility reported to affect 8–12% of all couples around the world. This study revealed a significant association between the available clinical markers of ovarian reserve and SES. Both ovarian reserve parameters AMH and AFC were found to be significantly associated with SES. The other clinical parameter FSH also showed a significant association with SES.
In recent years, serum AMH has been considered a new marker of ovarian reserve. It is expressed in developing pre-antral follicles and small antral follicles of the ovary. It demonstrates the recruited ovarian follicular pool. AMH has some advantages as a test, including its low inter- and intra-cycle variability and the declining trend it exhibits in direct proportion to advancing age. AMH, FSH, and AFC were reported to indicate a reduced ovarian reserve in animals representing a wide spectrum of ages up to 86 weeks. Furthermore, the period of maternal undernutrition has been reported to be a concomitant of increased maternal testosterone. Serum AMH level is diminished gradually in direct proportion to advancing age and can no longer be detected after a woman reaches menopause. Education and family income are considered the most important determinants of fertility. In this respect, composition of the mother’s diet impacts the development of fetal reproductive system.
Social factors and environmental contaminant exposure after birth and during adulthood should be taken into account as factors that might have an impact on age at natural menopause (ANM). Considering the fact that ANM is very low among people living in poor communities, economic hardships are very likely to be associated with reduced ANM. This reduced ANM might partly be due to insufficient deposition of fat as a natural result of undernutrition. The exact mechanism remains unclear; however, a link to nutrition is highly likely. The suggestion that the influence of diet in early life marks the initial ovarian reserve, which is diminished as people age, should be made subject to further research. However, as yet there is no clear explanation for the very low ANM observed among people living in some poor communities, although it will most likely involve the influence of nutrition on ovarian reserve.
Today, there are two markers widely considered helpful in prediction of ovarian reserve in women of reproductive age, namely AMH and FSH. Given the fact that serum FSH levels are increased in poor responders and in the late periods of reproduction, we were unable to provide a precise prediction of poor ovarian reserve in young women through serum FSH measurements only. Reduced AMH and increased FSH levels point to decreasing ovarian reserve. Serum FSH together with serum estradiol (E2) checked on day 3 or 4, will most likely give better results compared to FSH alone. However, E2 was not among the parameters included in our study. On the other hand, ultrasonographic markers, including AFC and ovarian volume, are subject to the limitation of inter-observer variability in addition to limitations in serologic testing. In addition, ultrasonographic examination should be performed in the early period of menstruation.
This study revealed a significant association between SES and ovarian reserve, which was measured by available clinical markers, namely FSH, AMH, and AFC, which showed significant associations with SES. The National Health Family Survey-3 points to education and family income as the most important determinants of fertility rates in India. Furthermore, the problem of the unmet need for family planning has been reported to be highly pressing in communities with a low SES [16,24].
This study investigated the socioeconomic factors that might potentially influence fertility with a particular emphasis on economic aspects such as job, income, and educational status. Of these factors, increased educational status, which leads to higher participation in economic life, have been found to be highly impactful on declining fertility rates. There are studies pointing to fewer juvenile members in families where the mother is a participant of economic life. The fact that people living in communities that enjoy a higher SES have easier access to health care as well as better control over their reproductive health in comparison to people living in communities with a lower SES might provide a fairly good explanation for the lower fertility rates observed in communities with a higher SES. Another explanation might involve a higher mean age at marriage and raised awareness of family planning among people living in communities with a higher SES [25].
Research in this area increasingly became prominent in the first decades of the new millennium, which is marked by the increasingly pressing problem of low birth rates. Kwon and Kim [26] reported a lower birth rate among the middle-income families compared to high- and low-income families. High-income families were reported not to be very concerned about bringing up children. Whereas low-income families may have to make a gambling-like choice regarding children to reach a better socioeconomic status. Lee [27] suggested an association between greater job experience among better-educated youth and lower marriage rates. There are several other suggestions put forward to explain this matter; however, factors impacting low birth rates require further investigation. This study led to the consideration of a link between high SES and good ovarian reserve as measured by the available markers.
The composition of the mother’s diet has been reported to impact the development of the fetal reproductive system [29]. The Developmental Origins of Health and Disease (DOHAD) hypothesis mainly focuses on nutritional availability to the fetus and whether or not the mother’s poor diet affects the ovarian reserve. In a broader sense, circulating hormones in pregnancy may impact ovarian reserve. However, exposure to social factors and environmental contaminants also should be considered [28]. Prolonged exposure after birth and during adulthood may also have an impact on ANM.
Changes in cellular proliferation observed in the ovaries of the fetus are potentially regulated by a number of factors that can be linked to the mother’s diet [30]. In line with the DOHAD hypothesis, whether or not the regulatory mechanisms influences the mother’s diet on the initial ovarian reserve in early life (maybe at critical phases of the pregnancy) has yet to be clarified considering the potential prolonged effects during adulthood. The question “How does undernutrition experienced during pregnancy impact on the ovarian reserve of an adult?” has yet to be answered. In this respect, a previous study involving sheep reported a lower ovulation rate in adulthood as a long-term impact [31]; AMH, FSH, and AFC indicated a reduced ovarian reserve in animals representing a wide spectrum of ages up to 86 weeks.
ANM can be employed as a proxy measure to investigate the potential affects of the socioeconomic factors on ovarian reserve. A global literature survey of ANM [32] identified particular ages (median 50–53 years) for Europe and North America, another study found insignificant regional differences between southern and northeastern USA) [33]. On the other hand, women living in poorer Asian and Latin American regions that cannot enjoy a middle or high SES tend to exhibit a remarkably reduced ANM. However, even an extraordinary dietary exposure does not seem to cause a big change in ANM compared to the reduction experienced by women living in poor communities in Asia and Latin America. It is also reasonable to consider that events in pre-natal, early stages of post-natal life, and adulthood combine to act as mediators of the effects of prolonged exposure to poor nutrition. In this respect, the positive effect of fat depositions might be through the delayed ANM and potentially led by estrone causing extra-estrogenic activities [34,35]. On the other hand, it is important to remember that some studies found no association between BMI and ANM [36]. Indeed, fat- and protein-rich diet might lead to an increased ANM [37]. Given that, the reduced ANM experienced in poorer communities might be partly due to insufficient deposition of fat as a natural result of undernutrition. This suggestion is consistent with the results of a UK cohort study that found underweight women generally received hormone replacement therapy at an earlier age compared to normal weight women, which may point to a reduced ANM [36]. It also fits with Frisch’s suggestion (1987) that a low body fat concentration may result in blocked menstrual cycling, both delaying menarche and bringing ANM down. However, studies involving people within single populations did not indicate a general inverse correlation between the age of menarche and that of menopause [38].
The present study had some limitations. First, the number of participants included in this study was relatively low. Second, this was a single-center study. In this respect, multi-center studies with higher numbers of participants might be useful to shed brighter light on this area of research.
Conclusions
We are of the opinion that ovarian reserve is influenced by socioeconomic status. A low socioeconomic status aggravated by sources of stress, such as undernutrition and financial hardships, affects the ovarian reserve which should be remembered in approaching infertile patients. The results of the current study showed that serum AMH levels were strongly related with AFC levels; this relationship was more significant than other ovarian reserve parameters. Measuring AMH levels in combination with AFC may improve the assessment of ovarian reserve for evaluating fertility potential and monitoring infertility treatment. The idea that nutritional influences in early life set initial levels of ovarian reserve (which later decay as adult life proceeds) is a concept which requires further investigation. However, the much earlier age of natural menopause experienced by many women in certain poorer should be considered.
Footnotes
Source of support: Departmental sources
References
- 1.Mascarenhas MN, Flaxman SR, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: Mechanisms and clinical consequences. Endocrine Reviews. 2009;30:465–93. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 3.Committee opinion no. 618: Ovarian reserve testing. Committee on Gynecologic Practice Obstet Gynecol. 2015;125(1):268–73. doi: 10.1097/01.AOG.0000459864.68372.ec. [DOI] [PubMed] [Google Scholar]
- 4.Hendriks DJ, Mol BW, Bancsi LF, et al. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005;83:291–301. doi: 10.1016/j.fertnstert.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Broer SL, Van Disseldorp J, Broeze KA, et al. IMPORT study group. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update. 2013;19:26–36. doi: 10.1093/humupd/dms041. [DOI] [PubMed] [Google Scholar]
- 6.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–75. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reprod Biomed Online. 2015;31:486–96. doi: 10.1016/j.rbmo.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Wellons MF, Bates GW, Schreiner PJ, et al. Antral follicle count predicts natural menopause in a population-based sample: The Coronary Artery Risk Development in Young Adults Women’s Study. Menopause. 2013;20:825–30. doi: 10.1097/GME.0b013e31827f06c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C, Yuan Z, Yao J, et al. AMH and AMHR2 genetic variants in Chinese women with primary ovarian insufficiency and normal age at natural menopause. Reprod Biomed Online. 2014;29:311–18. doi: 10.1016/j.rbmo.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Mohanty SK, Ram F. IIPS Working Paper No. 5. 2011. Pattern of poverty reduction and fertility transition in India. [Google Scholar]
- 11.Sujatha DS, Reddy GB. Women’s education, autonomy and fertility behavior. Asia-Pac J Soc Sci. 2009;1:35–50. [Google Scholar]
- 12.Rodolfo AB, Lee R. Overview of determinants of fertility in developing countries. New York, London: Academic Press; 1983. Determinants of fertility in developing countries. II; pp. 757–87. [Google Scholar]
- 13.Simmons GB. Research on the determinants of fertility. In: Farooq G, Simmons GB, editors. Fertility in developing countries: An economic perspective on research and policy ıssues. London: MacMillan; 1985. pp. 67–108. [Google Scholar]
- 14.Alia A. Women and Fertility in Bangladesh. New Delhi, London, California: Sage Publications; 1991. [Google Scholar]
- 15.Kumar N, Gupta N, Kishore J. Kuppuswamy’s socioeconomic scale: Updating income ranges for the year 2012. Indian J Public Health. 2012;56:103–4. doi: 10.4103/0019-557X.96988. [DOI] [PubMed] [Google Scholar]
- 16.National Family Health Survey (NHFS-3) [Last accessed on 2013 May 20]. Available from: http://www.nfhsindia.org.
- 17.Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of Antimullerian hormone in prediction of outcome after IVF: Comparison with the Antral follicle count. Fertil Steril. 2009;91:705–14. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 18.La Marca A, Broekmans FJ, Volpe A, et al. ESHRE Special Interest Group for Reproductive Endocrinology – AMH Round Table. Anti-Mullerian hormone (AMH): What do we still need to know? Hum Reprod. 2009;24:2264–75. doi: 10.1093/humrep/dep210. [DOI] [PubMed] [Google Scholar]
- 19.Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol. 2011;9 doi: 10.1186/1477-7827-9-23. article 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis NA, Houghton LA, Jones G, et al. The level of serum anti-mullerian hormone correlates with vitamin d status in men and women but not in boys. J Clin Endocrinol Metab. 2012;97(7):2450–55. doi: 10.1210/jc.2012-1213. [DOI] [PubMed] [Google Scholar]
- 21.Sills ES, Alper MM, Walsh APH. Ovarian reserve screening in infertility: Practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol. 2009;146(1):30–36. doi: 10.1016/j.ejogrb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Hagen CP, Aksglaede L, Sørensen K, et al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 turner syndrome patients. J Clin Endocrinol Metab. 2010;95(11):5003–10. doi: 10.1210/jc.2010-0930. [DOI] [PubMed] [Google Scholar]
- 23.Kwee J, Elting ME, Schats R, et al. Ovarian volume and antral follicle count for the prediction of low and hyper responders with in vitro fertilization. Reprod Biol Endocrinol. 2007;5:9. doi: 10.1186/1477-7827-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ram U. IIPS Research Brief No. 5. 2008. Childlessness and its consequences in India: Levels, patterns and differentials. [Google Scholar]
- 25.Himabindu Y, Gopinathan KK, Pandey AK, Sriharibabu M. Correlation of age and Antimullerian hormone in assisted reproductive technology program outcome. Indian J Physiol Pharmacol. 2013;57:9–15. [PubMed] [Google Scholar]
- 26.Kwon TH, Kim DS. Understanding of the population. Seoul: Seoul National University Press; 1990. pp. 195–99. [Google Scholar]
- 27.Lee SS. Studies of causes of low fertility and comprehensive measures. Seoul: Presidential Committee on Ageing Society and Population Policy; 2005. pp. 135–48. [Google Scholar]
- 28.Mark-Kappeler CJ, Hoyer PB, Devine PJ. Xenobiotic effects on ovarian preantral follicles. Biol Reprod Biol Reprod. 2011;85:871–83. doi: 10.1095/biolreprod.111.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhind SM. Effects of maternal nutrition on fetal and neonatal reproductive development and function. Anim Reprod Sci. 2004;82:169–81. doi: 10.1016/j.anireprosci.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Lea RG, Andrade LP, Rae MT, et al. Effects of maternal undernutrition during early pregnancy on apoptosis regulators in the ovine fetal ovary. Reproduction. 2006;131:113–24. doi: 10.1530/rep.1.00844. [DOI] [PubMed] [Google Scholar]
- 31.Rae MT, Kyle CE, Miller DW, et al. The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim Reprod Sci. 2002;72:63–71. doi: 10.1016/s0378-4320(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 32.Palacios S, Henderson VW, Siseles N, et al. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric. 2010;13:419–28. doi: 10.3109/13697137.2010.507886. [DOI] [PubMed] [Google Scholar]
- 33.McKnight KK, Wellons MF, Sites CK, et al. Racial and regional differences in age at menopause in the United States: findings from the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study. Am J Obst Gynecol. 2011;205:353.e1–8. doi: 10.1016/j.ajog.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akahoshi M, Soda M, Nakashima E, et al. The effects of body mass index on age at menopause. Int J Obes. 2002;26:961–68. doi: 10.1038/sj.ijo.0802039. [DOI] [PubMed] [Google Scholar]
- 35.Palmer JR, Rosenberg L, Wise LA, et al. Onset of natural menopause in African American women. Am J Public Health. 2003;93:299–306. doi: 10.2105/ajph.93.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy R, Mishra GD, Kuh D. Body mass index trajectories and age at menopause in a British birth cohort. Maturitas. 2008;59:304–14. doi: 10.1016/j.maturitas.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagel G, Altenburg HP, Nieters A, et al. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas. 2005;52:337–47. doi: 10.1016/j.maturitas.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 38.van Noord PAH, Dubas JS, Dorland M, et al. Age at natural menopause in a population-based screening cohort: The role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68:95–102. doi: 10.1016/s0015-0282(97)81482-3. [DOI] [PubMed] [Google Scholar]


