Abstract
The aim of the present systematic review and meta‐analysis of observational studies was to gain further insight into the effects of adherence to Mediterranean Diet (MD) on overall cancer mortality, incidence of different types of cancer, and cancer mortality risk in cancer survivors. Literature search was performed using the electronic databases PubMed, and EMBASE until 2 July 2015. We included either cohort (for specific tumors only incidence cases were used) or case–control studies. Study specific risk ratios, hazard ratios, and odds ratios (RR/HR/OR) were pooled using a random effect model. The updated review process showed 23 observational studies that were not included in the previous meta‐analysis (total number of studies evaluated: 56 observational studies). An overall population of 1,784,404 subjects was included in the present update. The highest adherence score to an MD was significantly associated with a lower risk of all‐cause cancer mortality (RR: 0.87, 95% CI 0.81–0.93, I 2 = 84%), colorectal cancer (RR: 0.83, 95% CI 0.76–0.89, I 2 = 56%), breast cancer (RR: 0.93, 95% CI 0.87–0.99, I 2=15%), gastric cancer (RR: 0.73, 95% CI 0.55–0.97, I 2 = 66%), prostate cancer (RR: 0.96, 95% CI 0.92–1.00, I 2 = 0%), liver cancer (RR: 0.58, 95% CI 0.46–0.73, I 2 = 0%), head and neck cancer (RR: 0.40, 95% CI 0.24–0.66, I 2 = 90%), pancreatic cancer (RR: 0.48, 95% CI 0.35–0.66), and respiratory cancer (RR: 0.10, 95% CI 0.01–0.70). No significant association could be observed for esophageal/ovarian/endometrial/and bladder cancer, respectively. Among cancer survivors, the association between the adherence to the highest MD category and risk of cancer mortality, and cancer recurrence was not statistically significant. The updated meta‐analyses confirm a prominent and consistent inverse association provided by adherence to an MD in relation to cancer mortality and risk of several cancer types.
Keywords: Cancer, Mediterranean diet, meta‐analysis
Introduction
Dietary quality indexes (DASH pattern, and the Healthy Eating Index) are associated with reduced risk of chronic disease 1, 2. There is considerable evidence that the Mediterranean diet (MD) represents a dietary pattern suitable in the prevention of noncommunicable diseases 3. In March 2014 we published a meta‐analysis of observational studies investigating the effects of compliance with an MD on overall cancer risk (incidence and mortality) and different types of cancer 4. Adherence to the highest category of MD was associated with a significant lower risk of overall cancer mortality/incidence as well as the incidence of several cancer types, especially colorectal cancer, aerodigestive cancer (pharyngeal or esophageal cancer), and prostate cancer.
The number of cancer survivors in the United States and Europe is growing rapidly 5, 6. A few prospective cohort studies investigated the association between composition of diet and cancer survival, reporting inconsistent results 7. For example, several studies focused on the evaluation of the relationship between survival and nutrients rather than dietary patterns 7, 8. Due to the high number of studies that have been published since the release of the previous meta‐analysis, it seems reasonable update the original analysis. Due to the new types of cancer that have meanwhile been taken under consideration and because of the growing importance of cancer survivors, we decided not only to reexecute the original search but to expand the previous meta‐analysis including the effects of an MD diet in cancer survivors as an additional research question.
Methods
The systematic review protocol of the previous meta‐analysis is registered in PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp Identifier: CRD42013004382). The protocol has meanwhile been adapted to the updated version of this analysis.
Data sources and searches
Queries of literature were performed using the electronic databases PubMed (until 2 July 2015), and EMBASE (until 2 July 2015), with no restrictions to calendar date using the following search terms:
(“Mediterranean diet” OR “Mediterranean” OR “diet” OR “dietary pattern” OR “dietary score” OR “dietary adherence”) AND (“cancer” OR “neoplasm” OR “neoplastic disease” OR “survivors” OR “recurrence”) AND (“prospective” OR “follow‐ up” OR “cohort” OR “longitudinal”). Search terms added for this update are: “survivors”, “recurrence”, and “longitudinal”. The search strategy had no language restrictions.
Moreover, the reference lists from retrieved articles were checked to search for further relevant studies. Literature search was conducted independently by both authors, with disagreements resolved by consensus.
Study selection
Cohort studies and case–control studies investigating the association between MD and risk of cancer mortality, cancer types; cancer mortality, and cancer recurrence among cancer survivors were included in this update (for differences between the original analysis and the revised version with respect to grouping of clinical outcomes, see “Statistical analysis”).
The previously established statistical analysis plan was revised in order to pool data if outcomes were reported by at least two studies only, since in meta‐analyses published by the Cochrane collaboration, even single studies are presented and discussed in a systematic review context and the forest plots can still be helpful. Although this refers mainly to interventions or clinical trials, well‐designed prospective cohort studies provide important evidence with complementary strength and limitations as well, especially in the context of nutritional sciences 9.
In addition we expanded our meta‐analysis to include cancer survivors from cohort or case–control studies.
As only two studies of the previous meta‐analysis reported overall cancer incidence (with types of cancer not specified) 4 we focused on overall cancer mortality to increase transparency.
Data extraction and quality assessment
Data extraction and quality assessment was performed as already reported 4.
Definition: adherence to MD
Ten studies 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 used the MD score provided by Trichopoulou et al. 20, 10 studies 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 used the alternate MD score established by Fung et al. 31, and Whalen et al. 32 modified the score in relation to dairy foods, grains and starches, and alcohol intakes. Cottet et al. 33 decided to use principal component analysis, whereas Tognon et al. 34 and Xie et al. 35 modified the score by Trichopoulou (adding fruit juices and polyunsaturated fatty acids, focusing on whole grains, and excluding poultry). Two case–control studies 36, 37 applied the MD score established by Panagiotakos et al. 38.
Of the 56 observational studies only six studies excluded the alcohol component [14, 18, 39, 40, 41, 42] from the MD score. Four of these studies focused on risk of breast cancer.
For this meta‐analysis, the lowest adherence to MD category was compared with the highest MD category (according to the MD scores by Trichopoulou, Fung, or Panagiotakos; with the exception of four studies that used factor analysis or principal component analysis to define the MD score: [43] hazard ratio: per 1 standard deviation increase), [44] (odds ratio: fourth vs. first tertile), Cottet et al. (odds ratio: third vs. first tertile), Bessaoud et al. 2012 (odds ratio per increment of one standard error). The maximum ranges of the different MD scores are reported in Table 1.
Table 1.
General study characteristics of included studies (cohort and case–control studies)
| Author [Ref no.] | CountryCohort name | Outcome | PopulationFollow‐up (years) | Age at entry | Sex | Components of scoreScore range | Adjustment | Multivariate adjusted | Quality score (max. 9 points) [65]a |
|---|---|---|---|---|---|---|---|---|---|
| Buckland et al. 10 | EUEPIC | Bladder cancer | 477,31211 | 35–70 | M/W | 1. ↑ legumes; 2. ↑ cereals; 3. ↑ fruits/nuts; 4. ↑ vegetables; 5. ↑ fish; 6. ↑ MUFA:SFA ratio; 7. ↔ alcohol; 8. ↓ meat and poultry; 9. ↓ dairy productsMD score range: 0–18 | Energy intake, smoking status | HR: 0.84 (0.69, 1.03) for third (12–18) versus first tertile (0–6) | 8 |
| Cottet et al. 33 | FRAECP | Colorectal cancer incidence among cancer survivors | 4423 | 35–75 | M/W | Principal component analysis: ↑ olive oil, fruit, vegetables, legumes, lean meat, fish; ↓ coffee, meat, beer, fats, whole grain bread and vegetables, and delicatessen;MD score range: principal component analysis | Age, treatment group, presence of proximal adenomas at inclusion | OR: 0.61 (0.18, 2.07) principal component analysis for third versus first tertile | 5 |
| Cuenca‐García et al. 13 | USAACLS | Cancer mortality | 12,44911.6 | 20–84 | M/W | 1. ↑ legumes; 2. ↑ cereals; 3. ↑ fruits/nuts; 4. ↑ vegetables; 5. ↑ fish; 6. ↑ MUFA:SFA ratio; 7. ↔ alcohol; 8. ↓ meat and poultry; 9. ↓ dairy productsMD score range: 0–9 | Age, sex, energy intake, and baseline examination year, PA, cardiorespiratory fitness | HR: 1.63 (0.91, 2.92) for fourth versus first quartile | 8 |
| George et al. [] | USAWHIOS | Endometrial cancer | 84,41513,3 | 50–79 | W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meatsMD score range: 0–9 | Age, energy intake, ethnicity, education, MET‐h/week – PA, diabetes, postmenopausal HRT, oral contraceptive use, age at first birth, participant in Observational study, participant in HT trial, participant in DM trial, alcohol, and BMI | HR: 0.98 (0.82, 1.17) for fifth quintile versus first quintile | 8 |
| George et al. 24 | USAWHIOS | Cancer mortality | 63,80512.9 | 50–79 | W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meatsMD score range: 0–9 | Age, energy intake, ethnicity, educational level, marital status, smoking, PA, postmenopausal HRT, BMI, and diabetes status | HR: 0.80 (0.70, 0.92) for fifth quintile versus first quintile | 8 |
| Gnagnarella et al. 11 | ITACOSMOS | Lung cancer | 4,3365.7 | ≥50 | M/W | 1. ↑ legumes; 2. ↑ cereals; 3. ↑ fruits/nuts; 4. ↑ vegetables; 5. ↑ fish; 6. ↑ MUFA:SFA ratio; 7. ↔ alcohol; 8. ↓ meat and poultry; 9. ↓ dairy productsMD score range: 0–9 | Baseline risk probability and energy intake | HR: 0.10 (0.01, 0.77) for fifth quintile (8–9) versus first quintile (0–1) | 6 |
| Harmon et al. 29 | USAMultiethnic Cohort | Cancer mortality | 215,78213–18 | 45–75 | M/W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meatsMD score range: 0–9 | Age, BMI, diabetes, energy intake, ethnicity, education, marital status, smoking, PA, HRT, alcohol | HR: ♂ 0.81 (0.75, 0.89) ♀ 0.84 (0.76, 0.92) for fifth quintile (6–9) versus first quintile (0–2) | 9 |
| Fung et al. 27 | USANHS | Colorectal cancer mortality among cancer survivors | 120111.2 | 30–55 | W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meatsMD score range: 0–9 | Age, PA, BMI, weight change, cancer grade, chemotherapy, smoking status, energy intake, colon or rectal cancer, stage of disease, and date of colorectal cancer diagnosis | HR: 0.84 (0.50, 1.42) for fifth quintile (median 6) versus first quintile (median 2) | 7 |
| Kenfield et al. [28] | USAHPFS | Prostate Cancer mortality among cancer survivors | 51,52924 | 40–75 | M | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meatsMD score range: 0–9 | Age at diagnosis, time period, time since diagnosis to FFQ, energy intake, BMI, vigorous PA, smoking status, clinical stage, Gleason score, and treatment, race, height, history of diabetes, family history of prostate cancer, multivitamin use, supplement use did not change the effect estimates for lethal prostate cancer and were left out of the final model, parental history of myocardial infarction before age 60 years, blood pressure, and cholesterol | HR: 1.01 (0.75, 1.38) for third (≥6) versus first tertile (≤3) | 9 |
| Kim et al. 26 | USANHS | Breast cancer mortality among cancer survivors | 2729Diagnosis 1978–1998: follow‐up up trough 2004 | 30–55 | W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meatsMD score range: 0–9 | Age, time since diagnosis, alcohol intake, energy, multivitamin use, BMI, weight change, oral contraceptive use, age, smoking status, PA, stage, categories of treatment, age at first birth and parity, menopausal status and postmenopausal hormone use | HR: 1.15 (0.74, 1.77) for fifth quintile versus first quintile | 7 |
| Li et al. 22 | USANIH‐AARP | Head and neck cancer | 494,967≥10 | 50–70 | M/W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meatsMD score range: 0–9 | Age, race, smoking, alcohol intake, education, BMI, PA, usual activity, and total energy intake | HR: ♂ 0.80 (0.35, 1.01) ♀ 0.42 (0.24, 0.74) for the fifth (7–9) versus first quintile (0–2) | 9 |
| Li et al. 22 | USANIH‐AARP | Hepatocellular carcinoma | 494,942≥10 | 50–70 | M/W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meatsMD score range: 0–9 | Age, sex, race, smoking, alcohol intake, education, BMI, diabetes, usual activity throughout the day, PA, and total energy intake | HR: 0.62 (0.47, 0.84) for the fifth (6–9) versus first quintile (0–2) | 9 |
| Lopez‐Garcia et al. 21 | USANHSHPFS | Cancer mortality | 17,4155.8–7.7 | M: 40–75W: 30–55 | M/W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meatsMD score range: 0–9 | Age, smoking status, BMI, PA, parental history of myocardial infarction before age 65 year, menopausal status and use of HT in women, multivitamin use, and medication use | HR: ♂ 0.88 (0.63, 1.21) ♀ 0.80 (0.48, 1.33) for the fifth versus first quintile | 7 |
| Reedy et al. 25 | USANIH‐AARP | Cancer mortality | 492,82315 | 50–70 | M/W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meatsMD score range: 0–9 | Age, race/ethnicity, education, marital status, PA, smoking, energy intake, BMI, diabetes, and alcohol | HR: ♂ 0.80 (0.77, 0.83) ♀ 0.79 (0.74, 0.84) for the fifth (6–9) versus first quintile (0–2) | 9 |
| Tognon et al. 34 | SWEVIP | Respiratory cancer | 77,1519 | 30–60 | M/W | 1. ↑ vegetables and potatoes; 2. ↑ fruit and juices; 3. ↑ whole grain cereals; 4. ↑ fish and fish products; 5 ↑ ratio of MUFA+PUFA to SFA; 6. ↔ alcohol intakes; 7. ↓ meat and meat products; 8. ↓ dairy productsMD score range: 0–8 | Energy intake, age, obesity, smoking status, education, and PA | HR: 0.93 (0.83, 1.04) for cut‐off (>4) versus (≤4) | 8 |
| Vormund et al. [15] | SUIMONICANRP 1A | Cancer mortality | 17,86121.4 | 25–74 | M/W | 1. ↑ legumes; 2. ↑ cereals; 3. ↑ fruits/nuts; 4. ↑ vegetables; 5. ↑ fish; 6. ↑ MUFA:SFA ratio; 7. ↔ alcohol; 8. ↓ meat and poultry; 9. ↓ dairy productsMD score range: 0–9 | Age, sex and survey wave, marital status, smoking, BMI, region and nationality | HR: 0.97 (0.95, 1.01) per 1‐point increase | 7 |
| Xie et al. 35 | USANHS | Ovarian cancer | 82,94824 | 30–55 | W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ cereal fiber; 4. ↑ fruits; 5. ↑ nuts; 6. ↑ vegetables; 7. ↑ fish; 8. ↑ MUFA:SFA ratio; 9. ↔ alcohol; 10. ↓ red and processed meatsMD score range: 0–10 | Age, total energy intake, family history of ovarian cancer, tubal ligation, BMI, parity, number of additional pregnancies, oral contraceptive use duration, smoking, menopausal status, type and duration of PMH use, age at menarche, hysterectomy, unilateral oophorectomy, lactose intake, caffeine intake, PA | HR: 0.91 (0.71, 1.18) for the fifth (≥5.5) versus first quintile (≤2.6) | 8 |
| Author [Ref no.] | Country | Outcome | Cases/controls | Age | Sex | Components of score | Adjustment | Multiple adjusted | Quality score (max. 9 points) |
|---|---|---|---|---|---|---|---|---|---|
| Castello et al. 18 | SPA | Breast cancer | 1017/1107 | n.d | W | 1. ↑ legumes; 2. ↑ whole grain products; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ vegetables; 6. ↑ fish; 7. ↑ MUFA:SFA ratio; 8. ↓ red and processed meatsMD score range: 0–8 | Energy intake, alcohol consumption, BMI, PA, smoking, education, history of breast disease other than cancer, family history of BC, age at menarche, age at first delivery and menopausal status | OR: 0.74 (0.46, 1.18) for fourth (7–8) versus first quartile (0–2) | 8 |
| Dalvi et al. 17 | USA | Endometrial cancer | 647/633 | 35–79 | W | 1. ↑ legumes; 2. ↑ cereals; 3. ↑ fruits/nuts; 4. ↑ vegetables; 5. ↑ fish; 6. ↑ MUFA:SFA ratio; 7. ↔ alcohol; 8. ↓ meat and poultry; 9. ↓ dairy productsMD score range: 0–9 | Age, race/ethnicity, age at menarche, OC use, parity, energy intake, PA, menopausal status, HRT, and BMI | OR: 0.92 (0.59, 1.43) for fifth versus first quintile | 7 |
| Filomeno et al. 12 | ITA/SUI | Oral and pharyngeal | 768/2078 | 22–79 | M/W | 1. ↑ legumes; 2. ↑ cereals; 3. ↑ fruits/nuts; 4. ↑ vegetables; 5. ↑ fish; 6. ↑ MUFA:SFA ratio; 7. ↔ alcohol; 8. ↓ meat and poultry; 9. ↓ dairy productsMD score range: 0–9 | Sex, age, study center, year of interview, education, tobacco smoking, body mass index, and total energy intake | OR: 0.20 (0.14, 0.28) for fifth (6–9) versus first quintile (0–2) | 6 |
| Filomeno et al. 19 | ITA/SUI | Endometrial cancer | 1411/3368 | 22–79 | M/W | 1. ↑ legumes; 2. ↑ cereals; 3. ↑ fruits/nuts; 4. ↑ vegetables; 5. ↑ fish; 6. ↑ MUFA:SFA ratio; 7. ↔ alcohol; 8. ↓ meat and poultry; 9. ↓ dairy productsMD score range: 0–9 | Age, study center, year of interview, education, tobacco smoking, body mass index, age at menopause, age at menarche, parity, oral contraceptive use, hormone replacement therapy use, history of hypertension, diabetes and total energy intake | OR: 0.43 (0.34, 0.56) for fifth (7–9) versus first quintile (0–3) | 6 |
| Grosso et al. 36 | ITA | Colorectal cancer | 338/676 | 65.3 | M/W | 1. ↑ nonrefined cereals; 2. ↑ potatoes; 3. ↑ fruits; 4. ↑ vegetables; 5. ↑ legumes; 6. ↑ fish; 7. ↑ olive oil; 8. ↓ red and processed meats; 9. ↓ poultry; 10. ↓ full‐fat dairy products; 11. ↔ alcoholMD score range: 0–55 | Age and sex | OR: 0.46 (0.28, 0.75) for third versus first tertile | 6 |
| Mourouti et al. 37 | GRE | Breast cancer | 250/250 | 56 | W | 1. ↑ nonrefined cereals; 2. ↑ potatoes; 3. ↑ fruits; 4. ↑ vegetables; 5. ↑ legumes; 6. ↑ fish; 7. ↑ olive oil; 8. ↓ red and processed meats; 9. ↓ poultry; 10. ↓ full‐fat dairy products; 11. ↔ alcoholMD score range: 0–55 | Age, educational status, BMI, current smoking, PA, family history of breast cancer, age of menarche, age of menopause, and use of HRT | OR: 0.91 (0.86, 0.97) per 1/55 units | 6 |
| Pot et al. 14 | UK | Breast cancer | 610/1891 | 57 | W | 1. ↑ legumes; 2. ↑ cereals; 3. ↑ fruits/nuts; 4. ↑ vegetables; 5. ↑ fish; 6. ↑ MUFA:SFA ratio; 7. ↓ meat and poultry; 8. ↓ dairy productsMD score range: 0–8 | Age, parity, use of HRT, weight, height, PA, menopausal status, family history of breast cancer, breast feeding and education level | OR: 1.04 (0.70, 1.53) for third versus first tertile | 7 |
| Turati et al. 16 | ITA/GRE | Hepatocellular carcinoma | 518/772 | <85 | M/W | 1. ↑ legumes; 2. ↑ cereals; 3. ↑ fruits/nuts; 4. ↑ vegetables; 5. ↑ fish; 6. ↑ MUFA:SFA ratio; 7. ↔ alcohol; 8. ↓ meat and poultry; 9. ↓ dairy productsMD score range: 0‐9 | Center, age, sex, education, BMI, smoking, diabetes, nonalcohol energy intake, and HBsAg, and/or anti‐HCV positivity | OR: 0.51 (0.34, 0.75) for third (≥5) versus first tertile (0–3) | 6 |
| Whalen et al. 32 | USA | Colorectal cancer | 564/1202 | 30–74 | M/W | 1. ↑ vegetables; 2. ↑ fruits; 3. ↑ lean meats; 4. ↑ nuts; 5. ↑ fish; 6. ↑ MUFA:SFA ratio; 7. ↓ red and processed meat; 8. ↓ sodium; 9. ↔ dairy products; 10. ↔ grains and starches; 11. ↔ alcoholMD score range: 11–55 | Age, sex, family history of colon cancer in a first‐degree relative, regular use of nonsteroidal anti‐inflammatory drugs, BMI, PA, total energy intake, and use of HRT | OR: 0.77 (0.53, 1.11) for fifth versus first quintile | 7 |
↑, high intake; ↓, low intake; ↔, moderate intake; ACLS, aerobics center longitudinal study; BMI, body mass index; CRC, colorectal cancer; COSMOS, Continuous observation of smoking subjects; EPIC, European prospective investigation into cancer and nutrition; HPFS, health professional follow‐up study; HR, hazard ratio; HRT, hormone replacement therapy; MUFA, monounsaturated fat; NRP 1A, national research program 1 A; NHS, Nurses’ Health Study; OR, odds ratio; PA, physical activity; PUFA, polyunsaturated fat; RR, risk ratio; SFA, saturated fat; VIP, Västerbotten Intervention Program; WHIOS, Women's Health Imitative Observational Study.
Newcatle Ottawa Scale: Selection (max. 4 points), Comparability (max. 1 point), Exposure (max. 3 points).
Statistical analysis
The meta‐analysis was performed by combining the multivariable adjusted RRs, HR or ORs of the highest compared with the lowest MD adherence category based on random effects model using Der Simonian–Laird method, which incorporated both within and between study variability. To evaluate the weighting of each study, the standard error for the logarithm HR/RR/OR of each study was calculated and regarded as the estimated variance of the logarithm HR/RR/OR, using an inverse variance method [45]. Studies were grouped according to the different clinical outcomes (overall risk of cancer mortality, risk of colorectal cancer/breast cancer/prostate cancer/gastric cancer/head and neck cancer (pharynx, larynx, oral cavity)/esophageal cancer/pancreatic cancer/liver cancer/ovarian cancer/endometrial cancer/respiratory cancer/bladder cancer). The outcome “aerodigestive cancer” used in the original meta‐analysis was replaced by more detailed categories (i.e., head and neck and esophageal). We expanded our previous meta‐analysis investigating the effects (outcomes: cancer‐specific mortality, and cancer recurrence) of adherence to MD in cancer survivors. Subgroup analysis was performed for cohort studies, and case–control studies. Furthermore, subgroup analyses were performed for pre versus postmenopausal status (breast cancer), and breast cancer subtypes (e.g., ER+/PR+ and HER2−, ER+, ER−, HER2+, HER2−). Furthermore, sensitivity analyses were performed for outcomes presented by at least five studies (no merging of cohort and case–control studies was done in the sensitivity analysis) taking into account country of origin, follow‐up time, and quality of studies. Moreover, to investigate possible sources of heterogeneity across studies, we performed a meta‐regression analysis to investigate the effects of various characteristics of studies on the study estimates of RRs. All analyses were conducted using the Review Manager by the Cochrane Collaboration (version 5.3) and Stata 12.0 (Stata‐Corp, College Station, TX).
Results
Literature search and study characteristics
The detailed steps of the updated meta‐analysis article search (Fig. S1) and selection process are given as an adapted PRISMA flow diagram [46].
Taken together, 23 additional observational studies (14 cohort studies 10, 11, 13, 15, 21, 22, 23, 24, 25, 26, 27, 29, 30, 35, and nine case–control studies 12, 14, 16, 17, 18, 19, 32, 36, 37) were identified that were not included in the previous meta‐analysis. Two studies were included in the original version of this systematic review, data of these were extracted for the update in a modified form: the cohort by Kenfield et al. 28 provided data on cancer survivors previously not synthesized, and the results by Cottet et al. 33 on cancer recurrence were placed in a new context (i.e., cancer survivors).
General study characteristics are summarized in Table 1. Overall, 35 cohort studies including 1,703,579 subjects (incidence cases; bladder: 1425; breast 15,832; colorectal: 8935; endometrial: 1392; esophageal: 848; gastric: 1382; head and neck: 1868; liver: 509; prostate: 29,806; ovarian: 696; respiratory: 124), and 21 case–control studies with 80,825 subjects met the objectives and were included in the updated meta‐analysis (Supplemental References). The total number of subjects in the included studies was 1,784,404.
One study resulted to be an updated analysis of cancer mortality outcome of a cohort already included in the previous meta‐analysis, so only the most updated study was added to this final analysis 25.
Main outcomes
Documentations of the different clinical outcomes are distributed as follows: overall risk of cancer mortality was evaluated in 11 cohorts, breast cancer risk in four cohorts and eight case–control studies, colorectal cancer risk in three cohorts and four case–control studies, prostate cancer risk in three cohorts and one case–control study, gastric cancer risk in two cohorts and one case–control study, head and neck cancer in one cohort study and three case–control studies; endometrial cancer in one cohort and two case–control studies, liver cancer, pancreatic cancer, and esophageal cancer in one cohort study and one case–control study, ovarian cancer, bladder cancer, respiratory cancer, in one cohort study, pancreatic cancer in one case–control study, cancer mortality among cancer survivors in three cohort studies, and cancer recurrence among cancer survivors in one cohort study, and cancer‐specific mortality in one cohort study.
Using a random effects model, we found that the highest adherence score to an MD was significantly associated with a lower risk of overall cancer mortality (risk ratio 0.87, 95% confidence interval (CI) 0.81–0.93) (Fig. 1). Among cancer survivors, the association between the adherence to the highest MD category and risk of cancer mortality (RR: 1.01, 95% CI 0.81–1.26), and cancer recurrence (RR: 0.61, 95% CI 0.18–2.07) was not statistically significant (Fig. 2).
Figure 1.
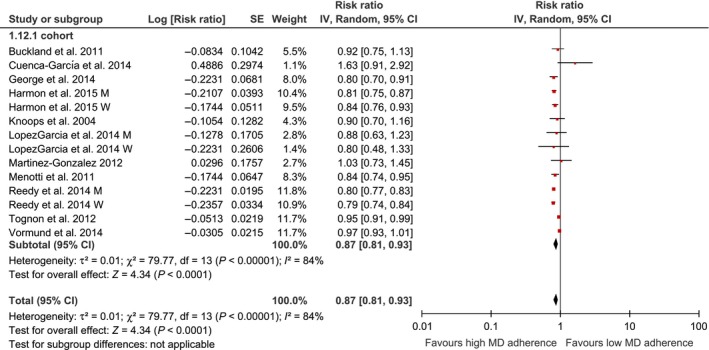
Forest plot showing pooled risk ratios (RRs) with 95% CI for overall cancer mortality risk for eleven cohort studies. I2, Inconsistency; MD, Mediterranean Diet; SE, standard error; tau, estimate between study variance.
Figure 2.
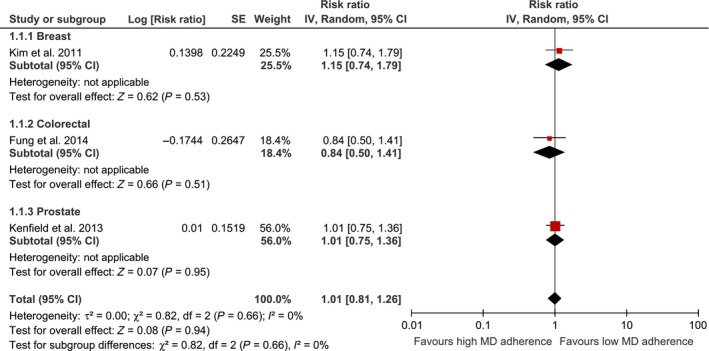
Forest plot showing pooled risk ratios (RRs) with 95% CI for risk of cancer mortality among cancer survivors for three cohort studies. I2, Inconsistency; MD, Mediterranean Diet; SE, standard error; tau, estimate between study variance.
With respect to incidence of different types of cancer, enumerative data are summarized in Table 2 and the corresponding forest plots are given as Figures S2–S13.
Table 2.
Risk ratio/odds ratio associated with the highest adherence to Mediterranean dietary pattern
| Outcome | No of studies | Study type | Risk ratio/odds ratio | 95% CI | I² (%) |
|---|---|---|---|---|---|
| Cancer mortality | 11 | Cohort | 0.87 | 0.81–0.93 | 84 |
| Colorectal cancer | 7 | Combined | 0.83 | 0.76–0.89 | 56 |
| 3 | Cohort | 0.84 | 0.75–0.94 | 56 | |
| 4 | Case–control | 0.79 | 0.67–0.93 | 65 | |
| Breast cancer | 12 | Combined | 0.93 | 0.87–0.99 | 15 |
| 4 | Cohort | 0.99 | 0.89–1.12 | 33 | |
| 8 | Case–control | 0.90 | 0.85–0.95 | 0 | |
| Prostate cancer | 4 | Combined | 0.96 | 0.92–1.00 | 0 |
| 3 | Cohort | 0.96 | 0.92–1.00 | 0 | |
| 1 | Case–control | 1.03 | 0.81–1.31 | n.a | |
| Gastric cancer | 3 | Combined | 0.73 | 0.55–0.97 | 66 |
| 2 | Cohort | 0.82 | 0.61–1.10 | 49 | |
| 1 | Case–control | 0.57 | 0.45–0.72 | n.a | |
| Liver cancer | 2 | Combined | 0.58 | 0.46–0.73 | 0 |
| 1 | Cohort | 0.62 | 0.47–0.82 | n.a | |
| 1 | Case–control | 0.51 | 0.34–0.77 | n.a | |
| Esophageal cancer | 2 | Combined | 0.49 | 0.22–1.09 | 83 |
| 1 | Cohort | 0.68 | 0.34–1.36 | n.a | |
| 1 | Case–control | 0.26 | 0.13–0.52 | n.a | |
| Head and neck cancer | 4 | Combined | 0.40 | 0.24–0.66 | 90 |
| 1 | Cohort | 0.61 | 0.33–1.14 | n.a | |
| 3 | Case–control | 0.32 | 0.19–0.55 | 83 | |
| Endometrial cancer | 3 | Combined | 0.72 | 0.40–1.31 | 94 |
| 1 | Cohort | 0.98 | 0.82–1.17 | n.a | |
| 2 | Case–control | 0.61 | 0.29–1.29 | 89 | |
| Respiratory cancer | 1 | Cohort | 0.10 | 0.10–0.70 | n.a |
| Bladder cancer | 1 | Cohort | 0.84 | 0.69–1.02 | n.a |
| Pancreatic cancer | 1 | Case–control | 0.48 | 0.35–0.66 | n.a |
| Mortality among cancer survivors | 3 | Cohort | 1.01 | 0.81–1.26 | 0 |
| Recurrence among cancer survivors | 1 | Cohort | 0.61 | 0.18–2.07 | n.a |
n.a, not applicable.
One cohort study investigated the effects of adherence to MD on cancer‐specific mortality. Tognon et al. observed an inverse association between higher adherence to MD and pancreatic cancer mortality, whereas no significant correlation could be detected for breast, colorectal, gastric, prostate, and respiratory cancer mortality, respectively 34.
Sensitivity analyses
Sensitivity analysis was performed for breast cancer comparing pre versus postmenopausal women. There was a trend for high adherence to MD to be associated with a lower risk of breast cancer in postmenopausal women (RR: 0.95, 95% CI 0.88–1.02) (Fig. S14). Additional sensitivity analyses regarding breast cancer types classified by receptor status yielded significant results comparing the highest versus lowest adherence category to MD only for the ER−/PR+ type (RR: 0.71, 95% CI 0.56–0.89) (Fig. S15).
Publication bias
The Egger's linear regression tests provided no evidence for a publication bias for overall cancer mortality (P = 0.983), and breast cancer (P = 0.976), but for colorectal cancer (P = 0.096), following comparison of the highest versus lowest adherence to MD category. Funnel plots were only generated when ~10 studies were available for a comparison. The funnel plots for risk of overall cancer mortality as well as risk of breast and colorectal cancer indicate moderate asymmetry, suggesting that publication bias cannot be completely excluded as a factor of influence on the present meta‐analysis (Figs. S16–S18).
Meta‐regression
To investigate the effects of various study characteristics on the study estimates of the RRs (if at least 5 studies were available), we conducted a meta‐regression analysis (only for cohort studies, since discrepancies compared to case–control are too excessive) by grouping studies according to specific characteristics, that is, sample size, age of the patients, and length of follow‐up. There was a significant inverse association between sample size (P < 0.05) and years of age (P < 0.05) and risk of cancer mortality, respectively (Figs. S19–S20).
Discussion
In this updated systematic review and meta‐analysis of observational studies investigating the association between adherence to MD and risk of cancer, findings were pooled from >1.7 million subjects. The main results suggest that adherence to the highest category of an MD is associated with a significant lower risk of overall cancer mortality (by approximately 13%) as well as incidence of colorectal cancer (by 15%), breast cancer (by 7%, no significant lower risk could be observed for cohort studies), gastric cancer (by 27%, no significant lower risk could be observed for cohort studies), prostate cancer (by 4%), liver cancer (by 42%), and head and neck cancer (by 60%, no significant lower risk could be observed for cohort studies). No significant lower risk could be demonstrated with respect to incidence of bladder, ovarian, endometrial, and esophageal cancer. Adherence to an MD has previously been reported to be effective in the primary and secondary prevention of a number of chronic noncommunicable diseases, such as cardiovascular diseases [47], neurodegenerative diseases [48], type 2 diabetes mellitus [49], and neoplastic diseases [50, 51]. We were able to demonstrate an inverse association of MD with respect to overall risk of cancer mortality/incidence and risk of incidence of specific types of cancer in a recently published systematic review 4. We decided to update (and expand) this analysis to synthesize further and additional evidence for a potential inverse association of an MD. Further evidence was granted by the large number of additional observational studies enrolled in this update (+23), resulting in a total of ~1.8 Mio patients.
There are some notable differences when comparing the results of the updated analysis with the first version of 2014 3. In the original analysis, overall cancer mortality data were synthesized together with overall cancer incidence, whenever no information on the specific site of neoplasm was given (yielding a 10% lower risk following adherence to an MD). For reasons of transparency, we decided to focus only on overall cancer mortality in the update. With respect to types of cancer, the positive effects of an MD on colorectal carcinoma demonstrated in the first analysis could be confirmed by the inclusion of new studies. Additional evidence could be found with respect to distinct types of cancer such as liver cancer, or head and neck cancer, which were either not depicted in the original analysis due to lack of corresponding studies or had to be rearranged to fit into the adapted classification of cancer types used for the update. Furthermore, an inverse association could be observed for breast cancer and gastric cancer risk (taking into account the exclusion of Tognon et al., who reported only on cancer‐specific mortality cases). Although it was not always possible to strictly adhere to the cancer categories given in the GLOBOCAN database of the International Agency for the Research on Cancer (http://globocan.iarc.fr), we tried to synthesize the data on various cancer sites by staying as close as possible in line with these definitions. Thus, the cancer type “aerodigestive cancer” used in the original analysis to summarize esophageal/pharyngeal neoplasms has been differentiated into esophageal cancer and head‐and‐neck cancer (laryngeal, pharyngeal, oral cavity). Some data must be interpreted with caution, since the number of observations dealing with these types of cancer are still low (e.g., liver cancer). Likewise, the effects of an MD on breast cancer will remain a matter of debate. The inverse association of an MD dietary pattern after pooling only case–control studies was present in the original analysis and is now further substantiated by three additional studies. However, pooling cohort studies (which are characterized by a higher level of evidence) did not confirm these results (there are no additional cohorts in this update). For future studies, it might be interesting to differentiate between post and premenopausal breast cancer or even to classify breast cancer according to receptor type.
Expanded evidence was expected by synthesizing data on cancer survivors provided by prospective cohort studies. However, we could not find a significant correlation between adherence to an MD and risk of cancer mortality and cancer recurrence. This might be explained by a number of reasons. Cancer survivors are defined as patients with diagnosed cancer, from the time of discovery until death [52]. Fortunately, technical progress in diagnosis and therapy have ensured that the number of cancer survivors is constantly increasing. Thus, it is of paramount importance to establish evidence‐based recommendations for these individuals with respect to lifestyle management. At the same time, the increasing number of cancer survivors results in an increasing heterogeneity of these patients making a one‐size‐fits‐all kind of guideline highly unlikely. For example, different states of diseases are associated with different recommendations (treatment, short‐term or long‐term recovery, stable disease). A number of studies provide evidence that nutrition is an important influencing factor either for tumor progression, recurrence, or survival with most of these reports investigating macronutrient composition or specific nutrients rather than dietary patterns 6, 7. However, there are some studies dealing with the effects of food groups in cancer survivors. A diet rich in fruits, vegetables, whole grains, and fish while at the same time low in red or processed meat as well as refined carbohydrates was associated with decreased mortality rates in breast and colorectal cancer survivors [53, 54, 55]. Most of these food groups fit well within the characteristics of an MD. When breaking down an MD diet into its composing food groups, the item of red wine requires critical balancing. Although consumption of red wine is not expressly requested, a maximum score for adherence to an MD is associated with moderate intake of alcohol in most studies. Alcohol consumption is regarded to be a risk factor in the development of primary tumors of the mouth, pharynx, larynx, esophagus, liver, breast, and colorectal [56]. This will also apply for cancer survivors [57, 58]. In breast cancer survivors, consumption alcohol has been reported to increase mortality risk [59, 60]. On the other hand, a beneficial effect on survival time has been demonstrated in other studies as well [61, 62]. Thus, it is hard to give formulaic advice on red wine (alcohol) consumption for cancer survivors. Most health professionals recommend to avoid any amount of alcohol during cancer treatment, and it is conceivable to modify an MD dietary pattern vie exclusion of red wine either permanently or temporarily.
With respect to protective food groups and their mechanisms of action, an MD is rich in various dietary factors which may affect neoplastic diseases and their outcome in a beneficial manner. New data providing some insight in this topic have recently been published. The results of the largest MD trial, the PREDIMED study including 7447 subjects, showed that the highest category of nuts intake (>3 servings/week) was associated with a 40% risk reduction in cancer mortality when compared to the lowest category [63], whereas the differences observed between consumers of extra virgin olive oil did not attain statistical significance [64]. Insofar, this summarizes the results of a single study and it would be premature to exclude oleic acid or the phenolic content of extra virgin olive oil as a benefactor of an MD.
Although the larger number of studies and patients increased the strength of the updated review, it still has limitations as well. For some cancer sites such as liver cancer, the number of studies is still rather small. In addition, case–control studies may have deficits with respect to measurement and recall bias, while cohort studies have limits regarding validity and reliability of nutritional assessment. In general, incidence and progress of cancer are multifactorial and therefore not only affected by single conditions such as nutrition. In this respect, an MD is a heterogeneous pattern rather than a defined diet. However, the majority of studies in the updated review made use of the MD score established by Trichopoulou et al. 18 or Fung et al. 31 thereby ensuring some degree of homogeneity.
In conclusion, the present systematic review and meta‐analysis provided further evidence that adherence to an MD is associated with lower risk of overall cancer mortality as well as incidence of colorectal, breast, gastric, prostate, liver, and head and neck cancer. If we take into account the number of observations reporting a beneficial effect of an MD in the protection against other chronic diseases as well, it seems reasonable to promote an MD dietary pattern.
Conflict of Interest
None declared.
Supporting information
Figure S1. Updated flow chart for meta‐analysis article selection process.
Figure S2. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of colorectal cancer for three cohort studies, and four case–control studies.
Figure S3. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of breast cancer for four cohort studies and eight case–control studies.
Figure S4. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of prostate cancer for three cohort studies and one case–control study.
Figure S5. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of gastric cancer for two cohort studies and one case–control study.
Figure S6. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of esophageal cancer for one cohort study and one case–control study.
Figure S7. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of endometrial cancer for one cohort study, and two case–control studies.
Figure S8. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of respiratory cancer for two cohort studies.
Figure S9. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of bladder for one cohort study.
Figure S10. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of pancreatic cancer for one cohort study and one case–control study.
Figure S11. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of liver cancer for one cohort study and one case–control study.
Figure S12. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of head and neck cancer for one cohort study and three case–control studies.
Figure S13. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of ovarian cancer for one cohort study and three case–control studies.
Figure S14. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of breast cancer for pre versus postmenopausal women.
Figure S15. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of breast cancer for breast cancer types.
Figure S16. Funnel plot showing study precision against the relative risk effect estimate with 95% CIs for cancer mortality. SE, standard error.
Figure S17. Funnel plot showing study precision against the relative risk effect estimate with 95% CIs for colorectal cancer. SE, standard error.
Figure S18. Funnel plot showing study precision against the relative risk effect estimate with 95% CIs for breast cancer. SE, standard error.
Figure S19. Bubble plot showing the association between sample size and cancer mortality (P = 0.045).
Figure S20. Bubble plot showing the association between years of age and cancer mortality (P = 0.000).
Acknowledgement
This article was supported by the Open Access Publishing Fund of the University of Vienna.
Cancer Medicine 2015; 4(12): 1933–1947
References
- 1. Schwingshackl, L. , and Hoffmann G.. 2015. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: a systematic review and meta‐analysis of cohort studies. J. Acad. Nutr. Diet. 115:780–800. [DOI] [PubMed] [Google Scholar]
- 2. Scientific Report of the 2015 Dietary Guidelines Advisory Committee . 2015. http://www.health.gov/dietaryguidelines/2015-scientific-report/PDFs/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf accessed 15 April 2015.
- 3. Sofi, F. , Macchi C., Abbate R., Gensini G. F., and Casini A.. 2013. Mediterranean diet and health status: an updated meta‐analysis and a proposal for a literature‐based adherence score. Public Health Nutr. 17:2769–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwingshackl, L. , and Hoffmann G.. 2014. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta‐analysis of observational studies. Int. J. Cancer 135:1884–1897. [DOI] [PubMed] [Google Scholar]
- 5. DeSantis, C. E. , Lin C. C., Mariotto A. B., Siegel R. L., Stein K. D., Kramer J. L., et al. 2014. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 64:252–271. [DOI] [PubMed] [Google Scholar]
- 6. Rowland, J. H. , Kent E. E., Forsythe L. P., Loge J. H., Hjorth L., Glaser A., et al. 2013. Cancer survivorship research in Europe and the United States: where have we been, where are we going, and what can we learn from each other? Cancer 119(Suppl. 11):2094–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rock, C. L. , Doyle C., Demark‐Wahnefried W., Meyerhardt J., Courneya K. S., Schwartz A. L., et al. 2012. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 62:243–274. [DOI] [PubMed] [Google Scholar]
- 8. Jones, L. W. , and Demark‐Wahnefried W.. 2006. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol. 7:1017–1026. [DOI] [PubMed] [Google Scholar]
- 9. Balk, E. M. , Horsley T. A., Newberry S. J., Lichtenstein A. H., Yetley E. A., Schachter H. M., et al. 2007. A collaborative effort to apply the evidence‐based review process to the field of nutrition: challenges, benefits, and lessons learned. Am. J. Clin. Nutr. 85:1448–1456. [DOI] [PubMed] [Google Scholar]
- 10. Buckland, G. , Ros M. M., Roswall N., Bueno‐de‐Mesquita H. B., Travier N., Tjonneland A., et al. 2014. Adherence to the Mediterranean diet and risk of bladder cancer in the EPIC cohort study. Int. J. Cancer 134:2504–2511. [DOI] [PubMed] [Google Scholar]
- 11. Gnagnarella, P. , Maisonneuve P., Bellomi M., Rampinelli C., Bertolotti R., Spaggiari L., et al. 2013. Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann. Oncol. 24:2606–2611. [DOI] [PubMed] [Google Scholar]
- 12. Filomeno, M. , Bosetti C., Garavello W., Levi F., Galeone C., Negri E., et al. 2014. The role of a Mediterranean diet on the risk of oral and pharyngeal cancer. Br. J. Cancer 111:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuenca‐Garcia, M. , Artero E. G., Sui X., Lee D. C., Hebert J. R., and Blair S. N.. 2014. Dietary indices, cardiovascular risk factors and mortality in middle‐aged adults: findings from the Aerobics Center Longitudinal Study. Ann. Epidemiol. 24:e2. [DOI] [PubMed] [Google Scholar]
- 14. Pot, G. K. , Stephen A. M., Dahm C. C., Key T. J., Cairns B. J., Burley V. J., et al. 2014. Dietary patterns derived with multiple methods from food diaries and breast cancer risk in the UK Dietary Cohort Consortium. Eur. J. Clin. Nutr. 68:1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vormund, K. , Braun J., Rohrmann S., Bopp M., Ballmer P., and Faeh D.. 2015. Mediterranean diet and mortality in Switzerland: an alpine paradox? Eur. J. Nutr. 54:139–148. [DOI] [PubMed] [Google Scholar]
- 16. Turati, F. , Trichopoulos D., Polesel J., Bravi F., Rossi M., Talamini R., et al. 2014. Mediterranean diet and hepatocellular carcinoma. J. Hepatol. 60:606–611. [DOI] [PubMed] [Google Scholar]
- 17. Dalvi, T. B. , Canchola A. J., and Horn‐Ross P. L.. 2007. Dietary patterns, Mediterranean diet, and endometrial cancer risk. Cancer Causes Control 18:957–966. [DOI] [PubMed] [Google Scholar]
- 18. Castello, A. , Pollan M., Buijsse B., Ruiz A., Casas A. M., Baena‐Canada J. M., et al. 2014. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case‐control EpiGEICAM study. Br. J. Cancer 111:1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Filomeno, M. , Bosetti C., Bidoli E., Levi F., Serraino D., Montella M., et al. 2015. Mediterranean diet and risk of endometrial cancer: a pooled analysis of three italian case‐control studies. Br. J. Cancer 112:1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trichopoulou, A. , Costacou T., Bamia C., and Trichopoulos D.. 2003. Adherence to a Mediterranean diet and survival in a Greek population. N Engl. J. Med. 348:2599–2608. [DOI] [PubMed] [Google Scholar]
- 21. Lopez‐Garcia, E. , Rodriguez‐Artalejo F., Li T. Y., Fung T. T., Li S., Willett W. C., et al. 2014. The Mediterranean‐style dietary pattern and mortality among men and women with cardiovascular disease. Am. J. Clin. Nutr. 99:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li, W. Q. , Park Y., Wu J. W., Goldstein A. M., Taylor P. R., Hollenbeck A. R., et al. 2014. Index‐based dietary patterns and risk of head and neck cancer in a large prospective study Am. J. Clin. Nutr. 99:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li, W. Q. , Park Y., McGlynn K. A., Hollenbeck A. R., Taylor P. R., Goldstein A. M., et al. 2014. Index‐based dietary patterns and risk of incident hepatocellular carcinoma and mortality from chronic liver disease in a prospective study. Hepatology 60:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. George, S. M. , Ballard‐Barbash R., Manson J. E., J. Reedy , Shikany J. M., Subar A. F., et al. 2014. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. Am. J. Epidemiol. 180:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reedy, J. , Krebs‐Smith S. M., Miller P. E., Liese A. D., Kahle L. L., Park Y., et al. 2014. Higher diet quality is associated with decreased risk of all‐cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 144:881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim, E. H. , Willett W. C., Fung T., Rosner B., and Holmes M. D.. 2011. Diet quality indices and postmenopausal breast cancer survival. Nutr. Cancer 63:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fung, T. T. , Kashambwa R., Sato K., Chiuve S. E., Fuchs C. S., Wu K., et al. 2014. Post diagnosis diet quality and colorectal cancer survival in women. PLoS ONE 9:e115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kenfield, S. A. , Dupre N., Richman E. L., Stampfer M. J., Chan J. M., and Giovannucci E. L.. 2013. Mediterranean diet and prostate cancer risk and mortality in the health professionals follow‐up study. Eur. Urol. 65:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harmon, B. E. , Boushey C. J., Shvetsov Y. B., Ettienne R., Reedy J., Wilkens L. R., et al. 2015. Associations of key diet‐quality indexes with mortality in the Multiethnic Cohort: the dietary patterns methods project. Am. J. Clin. Nutr. 101:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. George, S. M. , Ballard‐Barbash R., Shikany J. M., Crane T. E., and Neuhouser M. L.. 2015. A prospective analysis of diet quality and endometrial cancer among 84,415 postmenopausal women in The Women's Health Initiative. Ann. Epidemiol. Available http://dx.doi.org/10.1016/j.annepidem.2015.05.009 (accessed 5 June 2015), ISSN 1047‐2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fung, T. T. , Hu F. B., McCullough M. L., Newby P. K., Willett W. C., and Holmes M. D.. 2006. Diet quality is associated with the risk of estrogen receptor‐negative breast cancer in postmenopausal women. J. Nutr. 136:466–472. [DOI] [PubMed] [Google Scholar]
- 32. Whalen, K. A. , McCullough M., Flanders W. D., Hartman T. J., Judd S., and Bostick R. M.. 2014. Paleolithic and Mediterranean diet pattern scores and risk of incident, sporadic colorectal adenomas. Am. J. Epidemiol. 180:1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cottet, V. , Bonithon‐Kopp C., Kronborg O., Santos L., Andreatta R., Boutron‐Ruault M. C., et al. 2005. European Cancer Prevention Organisation Study G. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur. J. Cancer Prev. 14:21–29. [DOI] [PubMed] [Google Scholar]
- 34. Tognon, G. , Nilsson L. M., Lissner L., Johansson I., G. Hallmans , Lindahl B., et al. 2012. The Mediterranean diet score and mortality are inversely associated in adults living in the subarctic region. J. Nutr. 142:1547–1553. [DOI] [PubMed] [Google Scholar]
- 35. Xie, J. , Poole E. M., Terry K. L., Fung T. T., Rosner B. A., Willett W. C., et al. 2014. A prospective cohort study of dietary indices and incidence of epithelial ovarian cancer. J. Ovarian Res. 7:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grosso, G. , Biondi A., Galvano F., Mistretta A., Marventano S., Buscemi S., et al. 2014. Factors associated with colorectal cancer in the context of the Mediterranean diet: a case‐control study. Nutr. Cancer 66:558–565. [DOI] [PubMed] [Google Scholar]
- 37. Mourouti, N. , Kontogianni M. D., Papavagelis C., Plytzanopoulou P., Vassilakou T., Malamos N., et al. 2014. Adherence to the Mediterranean diet is associated with lower likelihood of breast cancer: a case‐control study. Nutr. Cancer 66:810–817. [DOI] [PubMed] [Google Scholar]
- 38. Panagiotakos, D. B. , Pitsavos C., Arvaniti F., and Stefanadis C.. 2007. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 44:335–340. [DOI] [PubMed] [Google Scholar]
- 39. Ax, E. , Garmo H., Grundmark B., Bill‐Axelson A., Holmberg L., Becker W., et al. 2014. Dietary patterns and prostate cancer risk: report from the population based ULSAM cohort study of Swedish men. Nutr. Cancer 66:77–87. [DOI] [PubMed] [Google Scholar]
- 40. Knoops, K. T. , de Groot L. C., Kromhout D., Perrin A. E., Moreiras‐Varela O., Menotti A., et al. 2004. Mediterranean diet, lifestyle factors, and 10‐year mortality in elderly European men and women: the HALE project. JAMA 292:1433–1439. [DOI] [PubMed] [Google Scholar]
- 41. Buckland, G. , Travier N., Cottet V., Gonzalez C. A., Lujan‐Barroso L., Agudo A., et al. 2013. Adherence to the Mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int. J. Cancer 132:2918–2927. [DOI] [PubMed] [Google Scholar]
- 42. Bessaoud, F. , Tretarre B., Daures J. P., and Gerber M.. 2012. Identification of dietary patterns using two statistical approaches and their association with breast cancer risk: a case‐control study in Southern France. Ann. Epidemiol. 22:499–510. [DOI] [PubMed] [Google Scholar]
- 43. Menotti, A. , Alberti‐Fidanza A., Fidanza F., Lanti M., and Fruttini D.. 2012. Factor analysis in the identification of dietary patterns and their predictive role in morbid and fatal events. Public Health Nutr. 15:1232–1239. [DOI] [PubMed] [Google Scholar]
- 44. Murtaugh, M. A. , Sweeney C., Giuliano A. R., Herrick J. S., Hines L., Byers T., et al. 2008. Diet patterns and breast cancer risk in Hispanic and non‐Hispanic white women: the Four‐Corners Breast Cancer Study. Am. J. Clin. Nutr. 87:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DerSimonian, R. , and Laird N.. 1986. Meta‐analysis in clinical trials. Control. Clin. Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- 46. Stovold, E. , Beecher D., Foxlee R., and Noel‐Storr A.. 2014. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Syst. Rev. 3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwingshackl, L. , and Hoffmann G.. 2014. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta‐analysis of intervention trials. Nutr. Metabol. Cardiovasc. Dis. 24:929–939. [DOI] [PubMed] [Google Scholar]
- 48. Singh, B. , Parsaik A. K., Mielke M. M., Erwin P. J., Knopman D. S., Petersen R. C., et al. 2014. Association of mediterranean diet with mild cognitive impairment and Alzheimer's disease: a systematic review and meta‐analysis. J. Alzheimer's Dis. 39:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwingshackl, L. , Missbach B., König J., and Hoffmann G.. 2014. Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta‐analysis. Public Health Nutr. 18:1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sofi, F. , Abbate R., Gensini G. F., and Casini A.. 2010. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta‐analysis. Am. J. Clin. Nutr. 92:1189–1196. [DOI] [PubMed] [Google Scholar]
- 51. Sofi, F. , Cesari F., Abbate R., Gensini G. F., and Casini A.. 2008. Adherence to Mediterranean diet and health status: meta‐analysis. BMJ 337:a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Centers for Disease C, Prevention . 2011. Cancer survivors–United States, 2007. MMWR Morbidity and mortality weekly report 60: 269–272. [PubMed] [Google Scholar]
- 53. Kroenke, C. H. , Fung T. T., Hu F. B., and M. D. Holmes . 2005. Dietary patterns and survival after breast cancer diagnosis. J. Clin. Oncol. 23:9295–9303. [DOI] [PubMed] [Google Scholar]
- 54. Kwan, M. L. , Weltzien E., Kushi L. H., Castillo A., Slattery M. L., and Caan B. J.. 2009. Dietary patterns and breast cancer recurrence and survival among women with early‐stage breast cancer. J. Clin. Oncol. 27:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meyerhardt, J. A. , Niedzwiecki D., Hollis D., Saltz L. B., Hu F. B., Mayer R. J., et al. 2007. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 298: 754–764. [DOI] [PubMed] [Google Scholar]
- 56. World, Cancer, Research, Fund;, American, Institute, for, Cancer, Research . 2007. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. AICR, Washington DC. [Google Scholar]
- 57. Nielsen, S. F. , Nordestgaard B. G., and Bojesen S. E.. 2012. Associations between first and second primary cancers: a population‐based study. CMAJ 184: E57–E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fortin, A. , Wang C. S., and Vigneault E.. 2009. Influence of smoking and alcohol drinking behaviors on treatment outcomes of patients with squamous cell carcinomas of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 74:1062–1069. [DOI] [PubMed] [Google Scholar]
- 59. Kwan, M. L. , Kushi L. H., Weltzien E., Tam E. K., Castillo A., Sweeney C., et al. 2010. Alcohol consumption and breast cancer recurrence and survival among women with early‐stage breast cancer: the life after cancer epidemiology study. J. Clini. Oncol. 28:4410–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li, C. I. , Daling J. R., Porter P. L., Tang M. T., and Malone K. E.. 2009. Relationship between potentially modifiable lifestyle factors and risk of second primary contralateral breast cancer among women diagnosed with estrogen receptor‐positive invasive breast cancer. J. Clin. Oncol. 27:5312–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reding, K. W. , Daling J. R., Doody D. R., O'Brien C. A., Peggy L. P., and Malone K. E.. 2008. The effect of pre‐diagnostic alcohol consumption on survival after breast cancer in young women. Cancer Epidemiol. Biomark. Prev. 17:1988–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Trentham‐Dietz, A. , Newcomb P. A., Nichols H. B., and Hampton J. M.. 2007. Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res. Treatment 105:195–207. [DOI] [PubMed] [Google Scholar]
- 63. Guasch‐Ferre, M. , Bullo M., Martinez‐Gonzalez M. A., Ros E., Corella D., Estruch R., et al. 2013. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guasch‐Ferre, M. , Hu F. B., Martinez‐Gonzalez M. A., Fito M., Bullo M., Estruch R., et al. 2014. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wells, G. A. , Shea B., O'Connell D., Peterson J., Welch V., and Losos M. P. T.. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (accessed 3 August 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Updated flow chart for meta‐analysis article selection process.
Figure S2. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of colorectal cancer for three cohort studies, and four case–control studies.
Figure S3. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of breast cancer for four cohort studies and eight case–control studies.
Figure S4. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of prostate cancer for three cohort studies and one case–control study.
Figure S5. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of gastric cancer for two cohort studies and one case–control study.
Figure S6. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of esophageal cancer for one cohort study and one case–control study.
Figure S7. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of endometrial cancer for one cohort study, and two case–control studies.
Figure S8. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of respiratory cancer for two cohort studies.
Figure S9. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of bladder for one cohort study.
Figure S10. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of pancreatic cancer for one cohort study and one case–control study.
Figure S11. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of liver cancer for one cohort study and one case–control study.
Figure S12. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of head and neck cancer for one cohort study and three case–control studies.
Figure S13. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of ovarian cancer for one cohort study and three case–control studies.
Figure S14. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of breast cancer for pre versus postmenopausal women.
Figure S15. Forest plot showing pooled risk ratio (RRs) with 95% CI for risk of breast cancer for breast cancer types.
Figure S16. Funnel plot showing study precision against the relative risk effect estimate with 95% CIs for cancer mortality. SE, standard error.
Figure S17. Funnel plot showing study precision against the relative risk effect estimate with 95% CIs for colorectal cancer. SE, standard error.
Figure S18. Funnel plot showing study precision against the relative risk effect estimate with 95% CIs for breast cancer. SE, standard error.
Figure S19. Bubble plot showing the association between sample size and cancer mortality (P = 0.045).
Figure S20. Bubble plot showing the association between years of age and cancer mortality (P = 0.000).


