Abstract
Objective
To describe outcomes and complications in the drowning subgroup from the Therapeutic Hypothermia After Pediatric Cardiac Arrest Out-of-Hospital (THAPCA-OH) Trial.
Design
Exploratory post hoc cohort analysis
Setting
Twenty-four PICUs
Patients
Pediatric drowning cases
Interventions
Therapeutic hypothermia versus therapeutic normothermia
Measurements and Main Results
An exploratory study of pediatric drowning from the THAPCA-OH Trial was conducted. Comatose patients >2 days and <18 years were randomized ≤6 hours following return-of-circulation to hypothermia (n=46) or normothermia (n=28). Outcomes assessed included 12-month survival with a Vineland Adaptive Behavior Scale (VABS-II) score ≥70, 1-year survival rate, change in VABS-II score pre-arrest to 12-months, and select safety measures. Seventy-four drowning cases were randomized. In patients with pre-arrest VABS-II ≥70 (n=65), there was no difference in 12-month survival with VABS-II score ≥70 between hypothermia and normothermia groups [29% vs. 17%; relative risk (RR) 1.74; 95% confidence interval (CI) 0.61 to 4.95; p=0.27]. Among all evaluable patients (n=68), the VABS-II score change from baseline to 12-months did not differ (p=0.46) and one-year survival was similar (49%, hypothermia vs. 42%, normothermia; RR 1.16; 95% CI 0.68 to 1.99; p=0.58). Hypothermia was associated with a higher incidence of positive bacterial culture (any blood, urine or respiratory sample) (67% vs. 43%; p=0.04), however, the rate per 100 days at risk did not differ (11.1 vs. 8.4; p=0.46). Cumulative incidence of blood product use, serious arrhythmias and 28-day mortality were not different. Among patients with CPR durations >30 minutes or epinephrine doses >4, none had favorable Pediatric Cerebral Performance Category (PCPC) outcomes (≤3).
Conclusions
In comatose survivors of out-of-hospital pediatric cardiac arrest due to drowning, hypothermia did not result in a statistically significant benefit in survival with good functional outcome or mortality at one year, as compared with normothermia. High-risk of culture-proven bacterial infection was observed in both groups.
Keywords: Drowning, pediatric, cardiac arrest, therapeutic hypothermia, clinical trial, mortality, functional outcome
Introduction
Out-of-hospital cardiac arrest (OHCA) in children commonly results in death or poor long-term functional outcome in survivors (1–3). In 2002, two trials in adults reported that therapeutic hypothermia (hypothermia) improved neurological outcomes in comatose survivors following OHCA with a shockable rhythm (4,5). A more recent trial in adults reported that hypothermia [targeted temperature management (TTM) 33°C] did not improve outcomes compared to actively maintained therapeutic normothermia (normothermia) (TTM-36°C) (6). The fundamental difference between the trials was the normothermia comparison group, which was an active intervention to prevent fever (4–6). Recently, the first trial of TTM after pediatric cardiac arrest was reported. Hypothermia (TTM-33°C) was not superior to normothermia (TTM-36.8°C) for survival with good neurological outcome or mortality (7). Newly published 2015 international resuscitation guidelines now recommend either hypothermia or normothermia for comatose adults and children with OHCA (8,9).
Drowning is an important cause of OHCA in children, and results in approximately 1,100 pediatric deaths in the US annually (10). One multicenter cohort study of children who incurred OHCA with return of circulation reported nearly one-third of cases attributable to drowning (43/138, 31%); drowning outcomes were better than outcomes in non-drowning cases (3). Yet, a recent Dutch retrospective cohort study reported no survivors of drowning with good functional outcome one year later [defined as Pediatric Cerebral Performance Category (PCPC) Scale Score ≤3] if temperature was <34°C on hospital presentation and more than 30 minutes of cardiopulmonary resuscitation (CPR) was required (11).
No randomized interventional clinical trials in children resuscitated after OHCA secondary to drowning have been published; information about interventions to improve outcome are limited to case reports or other observational studies (12–17). Recent expert reviews, however, cite hypothermia post-arrest as a potentially beneficial therapy (18,19). Additionally, international resuscitation guidelines for hypothermic comatose post-arrest drowning patients recommend rewarming to and maintaining temperature in a hypothermia range (32–34°C) (20). Reliance on case series reports may be misleading due to reporting and publication bias or chance observation. Therefore, larger multicenter prospective investigations with longer follow-up would significantly augment existing knowledge.
Sporadic case reports of ice-water drowning with prolonged cardiac arrest durations have described more favorable neurological outcomes than anticipated with (12–16) or without extra-corporeal membrane oxygenation (ECMO) treatment (17). Implementation of hypothermia after OHCA due to drowning was investigated over 25 years ago; in this case series no clinical benefit was described and major complications of hypothermia were neutropenia and severe septicemia (21). The goal temperature range described (30 to 33°C) was lower than in more recently conducted neonatal (22), adult (4–6) and pediatric (7) TTM trials; this may have contributed to the complications reported. Analysis of data from a recent neonatal encephalopathy hypothermia trial reported a trend of higher mortality with TTM-32°C vs. TTM-33.5°C (23).
We conducted an exploratory subgroup analysis focused on the subgroup of drowning cases from our recently published Therapeutic Hypothermia After Pediatric Cardiac Arrest Out-of-Hospital (THAPCA-OH) Trial (7). First, we examined the efficacy and safety of hypothermia (TTM-33°C) versus normothermia (TTM-36.8°C) for the drowning cohort for the following outcomes: 1) survival with favorable 12-month outcome, defined as a Vineland Adaptive Behavior Scale, second edition, (VABS-II) score ≥70; 2) change in VABS-II score from pre-arrest to 12 months; 3) survival at 12 months; and 4) select prospectively monitored adverse outcomes (blood product use, serious arrhythmias, and culture proven infection). We additionally examined 5) outcomes in the drowning cases with CPR >30 minutes to determine if our North American experience was similar to a recent Dutch report that found no survivors with good functional outcome (defined as PCPC ≤3) if CPR duration was over 30 minutes (11). This report provides new and incremental information about outcomes of pediatric cardiac arrest due to drowning treated at North American intensive-care units (ICUs).
Materials and Methods
Overview and Study Design
The details of planning the THAPCA trials were published (24–26). The findings of the THAPCA-OH trial were recently reported and protocol details were provided in the supplementary appendix (7). This was a National Heart, Lung, and Blood Institute funded randomized clinical trial, conducted in pediatric ICUs at 36 children’s hospitals in the United States and Canada. The institutional review boards of all participating sites and the data-coordinating center (DCC) approved the protocol and informed consent documents. Site research coordinators collected all data, and statisticians at the DCC performed all analyses for both the initial trial results and the current report.
Patient Population and Randomization
Children older than 48 hours and <18 years of age who sustained OHCA required chest compressions for at least two minutes, and remained comatose and dependent on mechanical ventilation after return of circulation, met the original inclusion criteria. The only additional requirement in the current report was that the etiology of the cardiac arrest was drowning. Major exclusion criteria were inability to randomize within 6 hours of return of circulation, a score of 5 or 6 on the Glasgow Coma Scale motor response subscale (scores range from 1 to 6, lower scores indicate worse function), lack of commitment to aggressive care, association with major trauma, and drowning in ice-covered water; all exclusion criteria are listed in the trial report Supplementary Appendix (7). Written informed consent from a parent or legal guardian was required. Eligible subjects were randomized 1:1 to hypothermia or normothermia, using permuted blocks stratified by clinical center and age (<2 years, 2–11 years, ≥12 years).
Interventions
Targeted temperature management (TTM) was actively maintained for 120 hours in both groups. Children assigned to hypothermia were pharmacologically paralyzed, sedated and cooled (or warmed, if indicated) by surface cooling using a Blanketrol III cooling unit (Cincinnati SubZero, Cincinnati) with mattresses applied anteriorly and posteriorly, to achieve and maintain 33°C (32 to 34°C) core temperature for 48 hours. They were rewarmed over 16 hours or longer to target temperature 36.8°C (36 to 37.5°C); this temperature was actively maintained throughout the remaining 120 hour intervention period. Children randomized to normothermia received identical care except core temperature was actively maintained at 36.8°C (36 to 37.5°C) for 120 hours with the Blanketrol-III. Dual central temperature monitoring (esophageal, rectal, or bladder) and a servo-control mode were used. The probe connected to the Blanketrol was designated primary; the other was connected to the bedside monitor for backup. Clinical teams determined all other aspects of clinical care.
Outcomes
The primary outcome of THAPCA-OH trial was survival with favorable neurobehavioral outcome at 12 month follow-up, defined as an age-corrected VABS-II standard score ≥70 (27). The VABS-II has an age-corrected mean of 100 (standard deviation, 15); higher scores indicate better performance. VABS-II data were collected centrally (Kennedy-Krieger Institute, Baltimore, MD) via telephone by a trained interviewer blinded to treatment assignment. As pre-specified in the protocol, enrolled children whose reported pre-arrest VABS-II scores were <70 (based on data from formal parent interview at each site within 24 hours of randomization) were not included in the primary efficacy analysis. Subjects without a baseline VABS-II were eligible for the primary analysis if both baseline Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) scores (28) were in the normal or mild disability category (29). POPC and PCPC scores range from 1 to 6, with lower scores representing less disability; subjects with scores of 1 or 2 on both scales were eligible for the primary analysis. POPC and PCPC were scored at baseline and at 12 months.
Secondary efficacy outcomes were 12-month survival and change in VABS-II score from pre-arrest baseline to 12-month score; deceased patients and those with the lowest possible VABS-II score were assigned the worst possible outcomes. Safety outcomes included the incidences of blood product use, infection, and serious arrhythmias through seven days, and 28-day mortality. Complete blood counts were performed daily with values within 12 hours of 0, 24, 48, 72, 96, and 120 hours described.
Statistical Analyses
The efficacy analysis for the primary outcome used a pre-specified modified intention-to-treat approach, excluding children with poor pre-arrest neurobehavioral function. Secondary efficacy outcomes were analyzed among all children. Safety analyses were done by treatment received among treated patients only. The primary outcome and 12-month mortality were compared between assigned treatment groups using a Cochran-Mantel-Haenszel test stratified by categorized age. Change in VABS-II was analyzed using van Elteren’s modification of the Mann-Whitney test (30), stratifying by categorized age, treating death as the worst outcome and treating the lowest possible VABS-II score at one year as the second-worst outcome. The probability of one year survival was evaluated by comparing survival curves between groups using a log-rank test stratified by age category. All analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC). For these exploratory analyses, an alpha level of 0.05 was used with two-sided tests conducted. There was no adjustment for multiple statistical testing and no predetermined power calculation for the drowning cohort.
Results
Population
Between September 1, 2009 and December 31, 2012, 74 drowning cases from 24 of the 36 sites were randomized; 46 were assigned to hypothermia (TTM-33°C) and 28 to normothermia (TTM-36.8°C); 2 cases in the hypothermia group and 1 in the normothermia group were ineligible for the primary outcome analysis. At 12 months, vital status was unknown in 3 in hypothermia and 2 normothermia cases; one normothermia group survivor did not complete VABS-II testing. Thus, 65 drowning cases were evaluable for the primary outcome.
Baseline Characteristics and Temperature Intervention
Baseline characteristics (Table 1) included median age 2.9 years (IQR 1.6–8.0) with male gender 68% (50/74). Bystanders witnessed 17% (12/71) and performed CPR for 82% (58/71) of CA. The initial rhythm was asystole in 65% (48/74) and ventricular fibrillation or ventricular tachycardia in only 3% (2/74). Estimated median CPR duration for 69 cases (with accurate start and stop times or estimated start or stop time) was 25.0 minutes (IQR 15.0–35.0; in 42 of these cases with accurate start and stop times available median CPR duration was 24.5 minutes (IQR 15.0–35.0). CPR was ongoing at initial hospital arrival in 56% (40/71). The median number of epinephrine doses (total) was 3.5 (IQR 1.0–5.0) (available in 70 cases). The two groups were similar with respect to other baseline information. The median time (hours) from return of circulation to treatment initiation was 5.8 (IQR 5.1–6.7) in the hypothermia group and 5.7 (IQR 5.0–6.3) in the normothermia group. No subjects received extracorporeal life support.
Table 1.
Baseline Characteristics before Randomization.
| Characteristic | Overall N | Hypothermia Group (N = 46) |
Normothermia Group (N = 28) |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) – Median (Q1, Q3) | 74 | 2.4 (1.5, 5.9) | 3.6 (1.9, 8.2) |
| Male sex – no. (%) | 74 | 28 (61) | 22 (79) |
| Medical History – no. (%) | |||
| No preexisting medical condition | 74 | 32 (70) | 22 (79) |
| Characteristics of the cardiac arrest | |||
| Bystander witnessed cardiac arrest – no./total no. (%) |
71 | 8/44 (18) | 4/27 (15) |
| Bystander performed CPR – no./total no. (%) | 71 | 40/46 (87) | 18/25 (72) |
| Initial rhythm noted by EMS or hospital – no. (%) | 74 | ||
| Asystole | 29 (63) | 19 (68) | |
| Bradycardia | 2 (4) | 0 (0) | |
| Pulseless electrical activity | 7 (15) | 4 (14) | |
| Ventricular fibrillation or tachycardia | 0 (0) | 2 (7) | |
| Unknown | 8 (17) | 3 (11) | |
| Duration of CPR (minutes) – Median (Q1, Q3) | 42 | 21.0 (14.0, 34.0) | 32.0 (16.0, 55.0) |
| Estimated duration of CPR (minutes) – Median (Q1, Q3) |
69 | 25.0 (14.0, 35.0) | 32.0 (17.5, 55.0) |
| First hospital patient arrived at was the study hospital – no. (%) |
74 | 17 (37) | 9 (32) |
| Chest compressions still required at time of arrival at the first hospital – no./total no. (%) |
71 | 23/44 (52) | 17/27 (63) |
| No. of doses of epinephrine – Median (Q1, Q3) | |||
| Administered by EMS | 70 | 1.0 (0.0, 4.0) | 1.0 (0.0, 3.0) |
| Administered at hospital | 74 | 1.0 (0.0, 3.0) | 2.0 (0.0, 4.0) |
| All doses administered by EMS and at hospital |
70 | 4.0 (1.0, 6.0) | 3.0 (2.0, 5.0) |
| Baseline VABS-II score - Mean±SD | 67 | 98.2±13.0 | 97.2±19.8 |
| Baseline PCPC | 74 | ||
| Normal = 1 | 41 (89%) | 27 (96%) | |
| Mild disability = 2 | 3 (7%) | 0 (0%) | |
| Moderate disability = 3 | 2 (4%) | 0 (0%) | |
| Severe disability = 4 | 0 (0%) | 1 (4%) |
CPR denotes cardiopulmonary resuscitation, and EMS emergency medical services.
Outcomes
The proportion of survivors with VABS-II scores ≥70 at 12 months did not differ between groups [hypothermia and normothermia groups: 29% vs. 17%; relative risk 1.74; 95% confidence interval (CI) 0.61–4.95; p=0.27)] (Table 2). Change from baseline to 12-month VABS-II score also did not differ (p=0.46). The proportion of cases with 12-month VABS-II scores within 15 points (1 sd) of their baselines was similar (hypothermia, 23%; normothermia, 20%) (Table 2).
Table 2.
Primary and Secondary Outcomes.
| Outcome | Overall N | Hypothermia Group |
Normothermia Group |
Risk Difference (95% CI) |
Relative Risk (95% CI) |
P value |
|---|---|---|---|---|---|---|
| Primary Outcome | ||||||
| Alive and VABS-II score ≥ 70 at one year | 65 | 12/41 (29%) | 4/24 (17%) | 12.6% (−7.8%, 33.0%) | 1.74 (0.61, 4.95) | 0.27* |
| Secondary Outcomes | ||||||
| Alive at one year | 69 | 21/43 (49%) | 11/26 (42%) | 6.5% (−17.6%, 30.7%) | 1.16 (0.68, 1.99) | 0.58* |
| One year change in VABS-II score from baseline |
68 | n=43 | n=25 | 0.46‡ | ||
| Death | 22 (51%) | 15 (60%) | ||||
| Lowest possible VABS-II score | 0 (0%) | 0 (0%) | ||||
| VABS-II score decreased > 30 points | 8 (19%) | 4 (16%) | ||||
| VABS-II score decreased 16–30 points | 3 (7%) | 1 (4%) | ||||
| VABS-II score decreased no more than 15 points or improved | 10 (23%) | 5 (20%) |
P value reflects the Cochran-Mantel-Haenszel test, adjusted for age stratum.
P value reflects the Mann-Whitney test based on the continuous change in VABS score, stratified by age category. Deceased patients and those with the lowest possible VABS score are assigned ranks of −2000 and −1000, respectively (i.e., the worst possible scores).
Twelve month mortality was assessed for all randomized cases with known vital status (69/74, 93%). Survival did not differ [hypothermia, 49%; normothermia, 42%; relative risk 1.16; 95% CI 0.68–1.99; p=0.58] (Table 2); nor did survival time differ (log rank test p=0.52) (Figure 1). Appendix Table S1 summarizes the primary causes of death which were not statistically different between groups; brain death or withdrawal of technological support due to poor neurologic prognosis was reported in the majority of cases in both groups (91%, hypothermia; 73%, normothermia).
Figure 1.
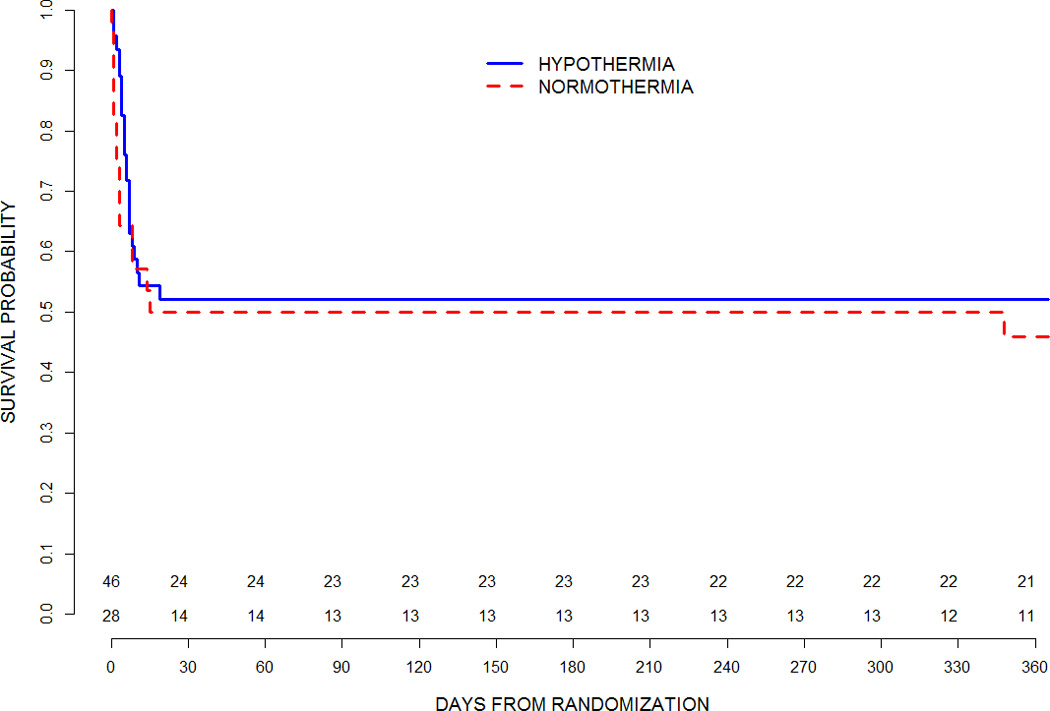
Survival Curve though 365 days for therapeutic hypothermia and therapeutic normothermia groups
Table 3 describes 12-month PCPC and VABS-II outcomes for all cases, overall and grouped by treatment and estimated chest compression duration (≤30 or >30 minutes). There were no survivors with PCPC ≤3 in either group among cases with CPR >30 minutes. Overall, in the ≤30 minute CPR group, median VABS-II score was 91 (IQR 51, 102) compared with 41 (IQR 37, 49) for group with CPR >30 minutes. There was one noteworthy discrepancy observed between the VABS-II and PCPC classification of one year outcome in a single drowning survivor, who was categorized with a PCPC of 4 and VABS of 80. This child had neurologically-based motor impairments and average communication and socialization skills. Although the correlation between PCPC and VABS is high (29), the VABS-II score is based on average functioning across several specific domains whereas the PCPC category is based on overall level of functioning and assistance needed. Table S2 depicts epinephrine dose received and outcomes. No one-year survivors received more than 11 doses, and no survivors with favorable outcome (one-year PCPC ≤3) received more than four doses. Similarly, no survivors with favorable outcome alternatively defined as one-year PCPC of 1 or 2, or no change if baseline was >2, received more than four epinephrine doses.
Table 3.
12-month PCPC and VABS-II Outcomes by duration of CPR.
| Estimated Duration of CPR | ||||
|---|---|---|---|---|
| Month 12 PCPC | ≤ 30 min. (N=44) |
> 30 min. (N=25) |
Unknown (N=5) |
|
| Missing | 5 (11%) | 0 (0%) | 1 (20%) | |
| Normal = 1 | 10 (23%) | 0 (0%) | 0 (0%) | |
| Mild disability = 2 | 2 (5%) | 0 (0%) | 0 (0%) | |
| Moderate disability = 3 | 3 (7%) | 0 (0%) | 0 (0%) | |
| Severe disability = 4 | 4 (9%) | 5 (20%) | 1 (20%) | |
| Coma or vegetative state = 5 | 3 (7%) | 3 (12%) | 0 (0%) | |
| Death = 6 | 17 (39%) | 17 (68%) | 3 (60%) | |
| Month 12 VABS-II | ||||
| N | 22 | 8 | 1 | |
| Mean±SD | 80±29 | 46±15 | 65 | |
| Median (IQR) | 91 (51, 102) | 41 (37, 49) | 65 (65, 65) | |
| Range | 27, 117 | 35, 80 | 65, 65 | |
Safety
Table 4 summarizes the incidence of prospectively monitored safety outcomes in all drowning cases. There was a trend for increased administration of blood products in the hypothermia group compared to the normothermia group [50% vs. 29%; p=0.08]. The frequency of severe arrhythmias within seven days of cardiac arrest was similar in both groups. Twenty-eight day mortality was not different (hypothermia 48% vs. normothermia 50%; p=0.86). Culture-proven bacterial infection from blood, respiratory and urine sites was more frequent in the hypothermia group [67% vs. 43%; p=0.04]. A broad range of gram-positive and gram-negative bacteria were cultured (Appendix Table S3).
Table 4.
Safety Outcomes within 7 Days after Randomization and 28-Day Mortality.
| Outcome | Hypothermia Group (N = 46) |
Normothermia Group (N = 28) |
P value* |
|---|---|---|---|
| Blood-product use | |||
| Overall (Any type) | 23 (50%) | 8 (29%) | 0.08 |
| Cryoprecipitate | 1 (2%) | 0 (0%) | 0.62 |
| Fresh frozen plasma | 15 (33%) | 5 (18%) | 0.18 |
| Packed red blood cells/whole blood | 15 (33%) | 4 (14%) | 0.09 |
| Platelets | 3 (7%) | 0 (0%) | 0.23 |
| Arrhythmias | |||
| Any serious arrhythmias | 3 (7%) | 3 (11%) | 0.55 |
| Asystole | 1 (2%) | 3 (11%) | 0.17 |
| Atrial (SVT, atrial flutter, JET) | 1 (2%) | 0 (0%) | 0.62 |
| Pulseless electrical activity (PEA) | 1 (2%) | 0 (0%) | 0.62 |
| Ventricular (sustained VT greater than 30 secs, VF, Torsades) |
0 (0%) | 0 (0%) | 1.00 |
| Other | 1 (2%) | 1 (4%) | 0.76 |
| Culture-proven infections** | |||
| Overall (Any) | 31 (67%) | 12 (43%) | 0.04 |
| Blood | 3 (7%) | 2 (7%) | 0.91 |
| Respiratory | 23 (50%) | 9 (32%) | 0.14 |
| Urine | 6 (13%) | 2 (7%) | 0.47 |
| Total number of infections | |||
| Overall | 36 | 14 | |
| Blood | 3 | 2 | |
| Respiratory | 26 | 10 | |
| Urine | 7 | 2 | |
| Number of days at risk | 323 | 166 | |
| Rate (95% CI) of infections per 100 days at risk† |
|||
| Overall | 11.1 (7.8, 15.4) | 8.4 (4.6, 14.2) | 0.46† |
| Blood | 0.9 (0.2, 2.7) | 1.2 (0.1, 4.4) | 1.00 |
| Respiratory | 8.0 (5.3, 11.8) | 6.0 (2.9, 11.1) | 0.49 |
| Urine | 2.2 (0.9, 4.5) | 1.2 (0.1, 4.4) | 0.73 |
| All cause 28-day mortality | 22 (48%) | 14 (50%) | 0.86 |
P values for all comparisons, except where noted, are 2-sided mid p-values, based on an exact likelihood ratio test.
Confidence intervals are exact two-sided 95% CIs, and p-values are based on an exact test of homogeneity of event rates between the Hypothermia arm and the Normothermia arm, assuming event data follow Poisson distributions.
For completeness: one eye culture with stenotrophomonas maltophilia andstaphylococcus coagulase negative (excluded); Respiratory culture isolation of Adenovirus; parainfluenza excluded; one blood culture with diphtheroids (excluded).
Appendix Table S4 provides blood count data. Statistically significant differences were observed with the following: hemoglobin was lower in the normothermia group at 24 and 48 hours; platelet counts were lower at 72, 96 and 120 hours for the hypothermia group; white blood counts were lower at 48 hours in the hypothermia group.
Discussion
We conducted this exploratory subgroup study of pediatric drowning and TTM because there are no randomized multicenter interventional study publications on this condition and because the drowning group represents a relatively homogenous group making up a relatively large proportion of cases in the THAPCA-OH trial population. Additionally, better outcomes have been reported among drowning cases compared to other causes of pediatric OHCA after adjusting for severity (3). As in the full THAPCA-OH trial (7), we found no difference for the drowning cohort in survival with favorable neurobehavioral outcome at 12 months between hypothermia and normothermia groups. Change in VABS-II score from baseline to one year or proportion of children with VABS-II scores within 15 points of their baselines (23% vs. 20%) also did not differ between groups. One-year all-cause mortality rates as well as corresponding survival analysis were similar. Culture-positive bacterial infection from combined sources of blood, urine and respiratory was high in both groups, and more common in the hypothermia than normothermia group; this trend has also been reported in adult OH-CA survivors (25–27).
The current report describes the largest cohort of pediatric drowning cases randomized for any intervention to date, and is the first multicenter pediatric drowning study that evaluated targeted temperature management, and that included assessments of function both at baseline and one year later, using standardized neurobehavioral measures (7). The study is limited by relatively small sample size (about ¼ of cases from the THAPCA-OH trial) and limited power to detect all but very large effect sizes. Nonetheless, this information will inform planning of future investigations and potentially contribute to future meta-analysis of pediatric drowning outcomes.
It should be noted that our results cannot be generalized to all drowning cases, as the THAPCA-OH trial had strict inclusion and exclusion criteria. For example, patients with initial Glasgow Coma Score motor score ≥ 5 (localizing or withdrawing to touch), indicating less severe neurological injury were excluded. Additionally, cases were excluded if the clinical teams determined patients were too hemodynamically unstable or implemented limitations to intensive care support in the first 6 hours after return of circulation.
Another limitation of this analysis shared by other studies, is the difficulty inherent in distinguishing infection from colonization for the bacterial respiratory culture results. Chest x-rays of drowning patients often demonstrate pulmonary aspiration infiltrates at hospital presentation, and since TTM in both groups prevented fever, this intervention made it difficult to diagnose pneumonia. Although hypothermia was associated with higher overall risk (combined blood, respiratory, urine) culture-proven bacterial infections, there was also a very high positive culture risk in the normothermia group. High-risk of pneumonia after adult OHCA has been reported (6, 31–33). In one large post-OHCA adult cohort study, high-risk of pneumonia (48%), but low risk of sepsis (4%) was described in cases treated with hypothermia (33); in a recent large adult clinical trial of TTM the risk of pneumonia was 52% (TTM-33°C) vs. 46% (TTM-36°C) and risk of sepsis 10% in both TTM groups (6). Prophylaxis or early antibiotic therapy for positive cultures should be considered. The wide range of organisms isolated in our study (Appendix Table S2) indicates broad-spectrum gram-positive and gram-negative antibiotic coverage would be needed if empiric treatment were utilized, a finding consistent with previous drowning reports (34–35). An observational report of hypothermia from over 25 years ago described a high incidence of severe sepsis (6/24 in the hypothermia group had blood stream infection) and neutropenia in pediatric drowning cases (21); however, the hypothermia intervention included both a lower targeted temperature goal and longer duration of hypothermia. Recent advances in ICU care such as prevention bundles for catheter associated–blood stream infection may also have contributed to the lower risk (7%) of blood stream infection in both groups in the current era report (Table 3).
A recent retrospective Dutch cohort study of pediatric drowning cases with temperature of <34°C on hospital admission reported no survivors with favorable functional outcomes (defined as PCPC ≤3) if CPR duration was over 30 minutes (11). Similarly, in our drowning cohort eight of 25 cases with CPR duration over 30 minutes survived, three in a persistent vegetative state (PCPC=5) and five with severe disability (PCPC=4). However, it should be noted that since only 25 [hypothermia (12) and normothermia (13)] drowning cases had CPR durations estimated over 30 minutes, the upper limit for the true population good outcome rate (using an exact 95% CI) within this subgroup is 14%, with zero good outcomes observed. Our trial did not collect temperature at the time of arrival to the first hospital; however, based on other reports, the majority of drowning cases are hypothermic on arrival to the hospital. One large cohort study with 1094 drowning cases reported duration of submersion, but not water temperature to be associated with drowning outcome (36).
Unanswered questions remain concerning the potential role of TTM for pediatric OHCA due to drowning. Alternative durations of TTM (longer or shorter), different depths of TTM control (higher or lower) and a different therapeutic window for initiating hypothermia (shorter) are modifications that might be considered for future study (7, 23). Modification of inclusion and exclusion criteria such as inclusion of cases with GCS motor score equal to 5 and exclusion of cases with greater than 30 minutes of CPR might be considered. Combining TTM with neuroprotective agents, might also be considered in future pediatric cardiac arrest drowning trials.
Conclusion
For comatose survivors of pediatric OHCA due to drowning from the THAPCA-OH trial, hypothermia could not be shown to result in a statistically significant benefit with respect to survival with good functional outcome at one year, as compared with normothermia. Mortality risk at 28 days and 12 months also did not differ between treatment groups. The hypothermia group had an overall higher proportion with positive bacterial cultures (any respiratory, blood, urine) compared to normothermia, however, both groups had high risk of positive cultures. For both groups, all 12 month survivors who had undergone more than 30 minutes of chest compressions had poor functional outcomes (PCPC≥4).
Supplementary Material
Acknowledgments
Support
Supported by the National Heart, Lung, and Blood Institute (NHLBI) grants HL094345 (to Dr. Moler) and HL094339 (to Dr. Dean).
Support in part from the following federal planning grants contributed to the planning of the THAPCA Trials: HD044955 (to Dr. Moler) and HD050531 (to Dr. Moler). Additional in part support from the following research networks: Pediatric Emergency Care Applied Research Network (PECARN) from cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008; and the Collaborative Pediatric Critical Care Research Network (CPCCRN) from cooperative agreements U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012 and U01HD049934. Site support from P30HD040677, UL1TR000003UL1, RR 024986, and UL1 TR 000433.
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Footnotes
Author Comment
Our work is important because it represents the first multicenter randomized study of therapeutic hypothermia and therapeutic normothermia for pediatric drowning. Mortality and functional outcomes at 1 year follow up, as well as, prospectively measured adverse event details are described. This report provides new and incremental information about outcomes of pediatric cardiac arrest due to drowning.
References
- 1.Young KD, Gausche-Hill M, McClung CD, Lewis RJ. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics. 2004;114:157–164. doi: 10.1542/peds.114.1.157. [DOI] [PubMed] [Google Scholar]
- 2.Donoghue AJ, Nadkarni V, Berg RA, et al. Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Moler FW, Donaldson AE, Meert K, et al. for the Pediatric Emergency Care Applied Research Network (PECARN) Multicenter Cohort Study of Out-of-Hospital Pediatric Cardiac Arrest. Crit Care Med. 2011;39:141–149. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. The Hypothermia After Cardiac Arrest Study Group. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 5.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 7.Moler FW, Silverstein FS, Holubkov R, et al. for the THAPCA Trial Investigators. Therapeutic Hypothermia after Out-of-Hospital Cardiac Arrest in Children. N Engl J Med. 2015;372:1898–18908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaway CW, Donnino MW, Fink EL, et al. Part 8: post–cardiac arrest care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(suppl 2):S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Caen AR, Maconochie IK, Aickin R, et al. on behalf of the Pediatric Basic Life Support and Pediatric Advanced Life Support Chapter Collaborators. Part 6: pediatric basic life support and pediatric advanced life support: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132(suppl 1):S177–S203. doi: 10.1161/CIR.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 10.Bowman SM, Aitken ME, Robbins JM, Baker SP. Trends in US pediatric drowning hospitalizations, 1993–2008. Pediatrics. 2012;129:275–281. doi: 10.1542/peds.2011-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieboom JK, Verkade HJ, Burgerhof JG, et al. Outcome after resuscitation beyond 30 minutes in drowned children with cardiac arrest and hypothermia: Dutch nationwide retrospective cohort study. BMJ. 2015;350:1–10. doi: 10.1136/bmj.h418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siebke H, Rod T, Breivik H, Link B. Survival after 40 minutes; submersion without cerebral sequeae. Lancet. 1975;7919:1275–1277. doi: 10.1016/s0140-6736(75)92554-4. [DOI] [PubMed] [Google Scholar]
- 13.Bolte RG, Black PG, Bowers RS, Thorne JK, Corneli HM. The Use of Extracorporeal Rewarming in a Child Submerged for 66 Minutes. JAMA. 1988;260:377–379. [PubMed] [Google Scholar]
- 14.Thalmann M, Trampitsch E, Haberfellner N, Eisendle E, Kraschl R, Kobinia G. Resuscitation in near drowning with extracorporeal membrane oxygenation. Ann Thorac Surg. 2001;72:607–608. doi: 10.1016/s0003-4975(00)02307-9. [DOI] [PubMed] [Google Scholar]
- 15.Letsou GV, Kopf GS, Elefteriades JA, Carter JE, Baldwin JC, Hammond GL. Is cardiopulmonary bypass effective for treatment of hypothermic arrest due to drowning or exposure? Arch Surg. 1992;127:525–528. doi: 10.1001/archsurg.1992.01420050045005. [DOI] [PubMed] [Google Scholar]
- 16.Romlin BS, Winberg H, Janson M, et al. Excellent Outcome With Extracorporeal Membrane Oxygenation After Accidental Profound Hypothermia (13.8°C) and Drowning. Crit Care Med. 2015;43:e521–e525. doi: 10.1097/CCM.0000000000001283. [DOI] [PubMed] [Google Scholar]
- 17.Hein O, Triltsch A, von Buch C, Kox WJ, Spies C. Mild hypothermia after near drowning in twin toddlers. Critical Care. 2004;8:R353–R357. doi: 10.1186/cc2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med. 2012;366:2102–2110. doi: 10.1056/NEJMra1013317. [DOI] [PubMed] [Google Scholar]
- 19.Topjian AA, Berg RA, Bierens JJ, et al. Brain resuscitation in the drowning victim. Neurocrit Care. 2012;17:441–467. doi: 10.1007/s12028-012-9747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S829–S861. doi: 10.1161/CIRCULATIONAHA.110.971069. [DOI] [PubMed] [Google Scholar]
- 21.Bohn DJ, Biggar WD, Smith CR, Conn AW, Barker GA. Influence of hypothermia, barbiturate therapy, and intracranial pressure monitoring on morbidity and mortality after near-drowning. Crit Care Med. 1986;14:529–534. doi: 10.1097/00003246-198606000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Shankaran S, Laptook AR, Ehrenkranz RA, et al. for the National Institute of Health and Human Development Neonatal Research Network. Whole-Body Hypothermia for Neonates with Hypoxic-Ischemic Encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 23.Shankaran S, Laptook AR, Pappas A, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312:2629–2639. doi: 10.1001/jama.2014.16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pemberton VL, Browning B, Webster A, Dean JM, Moler FW. Therapeutic Hypothermia after Pediatric Cardiac Arrest Trial: The Vanguard Phase Experience and Implications for other Trials. Pediatr Critical Care Med. 2013;14:19–26. doi: 10.1097/PCC.0b013e31825b860b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moler FW, Silverstein FS, Meert KL, et al. Rationale, Timeline, Study Design and Protocol Overview of the Therapeutic Hypothermia After Pediatric Cardiac Arrest Trials. Pediatr Critical Care Med. 2013;14:e304–e315. doi: 10.1097/PCC.0b013e31828a863a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holubkov R, Clark AE, Moler FW, et al. Efficacy outcome selection in the Therapeutic Hypothermia After Pediatric Cardiac Arrest (THAPCA) Trials. Pediatr Crit Care Med. 2015;16:1–10. doi: 10.1097/PCC.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparrow S, Cicchetti D, Balla D. Vineland Adaptive Behavior Scales. 2nd. Minneapolis, MN: Pearson Assessment; 2005. [Google Scholar]
- 28.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 29.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow- up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 30.van Elteren PH. On the combination of independent two sample tests of Wilcoxon. Bulletin of the Institute of International Statistics. 1960;37:351–361. [Google Scholar]
- 31.Perbet S, Mongardon N, Dumas F, et al. Early-onset pneumonia after cardiac arrest: characteristics, risk factors and influence on prognosis. Am J Respir Crit Care Med. 2011;184:1048–1054. doi: 10.1164/rccm.201102-0331OC. [DOI] [PubMed] [Google Scholar]
- 32.Tadié JM, Heming N, Serve E, et al. Drowning associated pneumonia: A descriptive cohort. Resuscitation. 2012;83:399–401. doi: 10.1016/j.resuscitation.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen N, Sunde K, Hovdenes J, et al. Hypothermia Network. Adverse events and their relation to mortality in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med. 2011;39:57–64. doi: 10.1097/CCM.0b013e3181fa4301. [DOI] [PubMed] [Google Scholar]
- 34.Ender PT, Dolan MJ. Pneumonia associated with near-drowning. Clin Infect Dis. 1997;25:896–907. doi: 10.1086/515532. [DOI] [PubMed] [Google Scholar]
- 35.Kakavas S, Mongardon N, Cariou A, Gulati A, Xanthos T. Early-onset pneumonia after out-of-hospital cardiac arrest. J Infect. 2015;70:553–562. doi: 10.1016/j.jinf.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Quan L, Mack CD, Schiff MA. Association of water temperature and submersion duration and drowning outcome. Resuscitation. 2014;85:790–794. doi: 10.1016/j.resuscitation.2014.02.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


